Abstract
Abstract 4291
The Belgian series included 107 patients (It remains unclear whether demographic, socioeconomic and adherence-related variables that influence outcome in childhood acute lymphoblastic leukemia (ALL) impact outcomes in adults. Registry-based studies of adults cannot investigate differences in treatment, adherence or the most modern cytogenetic and molecular classifications. Furthermore, landmark prospective trials in adults have not reported data on outcomes among minority populations. However, registry studies of children have demonstrated inferior survival among minorities, and analyses of children enrolled in prospective studies have associated both host genomic differences and rates of adherence to maintenance therapy with relapse and survival. To generate hypotheses relevant to disparate outcomes among diverse adult populations with ALL, we conducted a retrospective cohort study of consecutive adolescent and adult ALL patients in a large single institution with an ethnically diverse patient population.
Consecutive patients with ALL over age 15 years who were diagnosed between 2000 and 2010 were identified from the institutional tumor registry. Diagnoses were confirmed by review of pathology reports, immunophenotyping and cytogenetics. Burkitt leukemia patients were excluded. Clinical, pathologic and demographic variables were derived from the medical records. Treatment variables included initial chemotherapy regimen, duration of maintenance therapy (≥ or < 12 months for patients not relapsing or proceeding to transplant before 12 months) and receipt of allogeneic stem cell transplant. Pearson's exact chi-square test, and the Kruskal-Wallis exact test, were used to test for differences between the three racial/ethnic groups of non-Hispanic white, African-American, and Hispanic, for the respective categorical and continuous variables of interest. The Kaplan-Meier method and Cox regression were used to investigate the association between variables of interest and progression-free survival (PFS).
Baseline demographic and disease characteristics varied considerably among the racial/ethnic subgroups (table).
| . | Non-Hispanic White . | African American . | Hispanic . | p-value . |
|---|---|---|---|---|
| n (%) | 50 (58%) | 13 (15%) | 22 (26%) | – |
| Sex (% male) | 58% | 62% | 73% | .54 |
| age in years (median, IQR*) | 29 (21, 56) | 41 (34, 57) | 25.5 (21, 33) | .04 |
| % low SES** | 13% | 23% | 86% | <.0001 |
| % B-cell ALL | 68% | 38% | 91% | .005 |
| % Unfavorable karyotype | 48% | 71% | 20% | .04 |
| t(9;22) | 20% | 18% | 6% | .42 |
| WBC at presentation (median, IQR) | 12.4 (4.9, 59.4) | 13.3 (6.8, 17.9) | 38 (9.9, 87.2) | .43 |
| Percent CD20 expression (median, IQR) | 13 (2, 50) | 45 (44, 83) | 48 (8, 90) | .14 |
| Duration of maintenance therapy ≥12 mos. | 68% | 22% | 47% | .04 |
| % receiving allogeneic SCT | 30% | 15% | 5% | .045 |
| . | Non-Hispanic White . | African American . | Hispanic . | p-value . |
|---|---|---|---|---|
| n (%) | 50 (58%) | 13 (15%) | 22 (26%) | – |
| Sex (% male) | 58% | 62% | 73% | .54 |
| age in years (median, IQR*) | 29 (21, 56) | 41 (34, 57) | 25.5 (21, 33) | .04 |
| % low SES** | 13% | 23% | 86% | <.0001 |
| % B-cell ALL | 68% | 38% | 91% | .005 |
| % Unfavorable karyotype | 48% | 71% | 20% | .04 |
| t(9;22) | 20% | 18% | 6% | .42 |
| WBC at presentation (median, IQR) | 12.4 (4.9, 59.4) | 13.3 (6.8, 17.9) | 38 (9.9, 87.2) | .43 |
| Percent CD20 expression (median, IQR) | 13 (2, 50) | 45 (44, 83) | 48 (8, 90) | .14 |
| Duration of maintenance therapy ≥12 mos. | 68% | 22% | 47% | .04 |
| % receiving allogeneic SCT | 30% | 15% | 5% | .045 |
Inter-Quartile Range.
Low socioeconomic status (SES) defined as enrollment in Medicaid or hospital charity program.
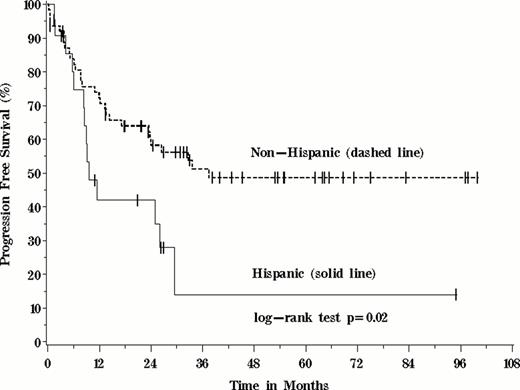
Due to the wide variation in induction, consolidation and intensification regimens employed (n=16), treatment variables (table) included in time-to-event models were limited to treatment with allogeneic stem cell transplant and duration of maintenance chemotherapy. The median follow-up for survivors was 32 months. PFS was significantly worse for Hispanic patients (p=0.02). Hispanics were 2.4 times more likely to either progress or die than Non-Hispanics (95%CI: 1.2 to 4.7). Among the other variables of interest, only age greater than 35 and the receipt of <12 months of maintenance therapy were associated with inferior PFS (p=0.004 and p<0.0001, respectively). Having an unfavorable karyotype did not appear to be significantly associated with PFS in these patients (p=0.44).
In a diverse single-center population, significant disease and demographic differences between racial and ethnic minority patients with ALL existed at baseline. Patients of Hispanic ethnicity had inferior PFS, seemingly not explained by measurable treatment differences or adverse karyotypes. Despite the constraints of a single-center, retrospective analysis, the current results should guide future hypothesis-testing studies of such disparities and their underpinnings, including disease genomic differences, pharmacogenomic polymorphisms, adherence to prescribed therapies and access to care.
Foster:Sanofi: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal