Abstract
Abstract 4600
Targeted intervention against driver mutations is beginning to transform cancer treatment. A particular activating mutation of the BRAF serine/threonine protein kinase, BRAF V600E, is found in virtually all cases of hairy-cell leukemia (HCL), suggesting disease-specific oncogene dependence.
Here, we present the extended follow up of a patient with chemotherapy refractory HCL who was treated with a short course of vemurafenib, a specific BRAF inhibitor. Before vemurafenib treatment, the patient had an almost complete bone marrow (BM) infiltration by hairy cells and massive splenomegaly (24.8×8.3 cm) leading to severe cytopenias (leukocytes, 680/μl; hemoglobin, 10 g/dl; platelets, 36,000/μl). No objective response could be achieved by three lines of purine analogue based treatment regimens (Cladribine, Pentostatin and R-Cladribine).
We demonstrated the presence of the BRAFV600E mutation with a mutation specific antibody and 454 sequencing. In order to investigate if recurrent mutations may have contributed to refractoriness to purine analogues, a panel of genes commonly mutated across lymphoid malignancies were analysed (EZH2, KRAS, MYD88, NOTCH1, NRAS, PIK3CA, SF3B1, or TP53). No mutations were demonstrated
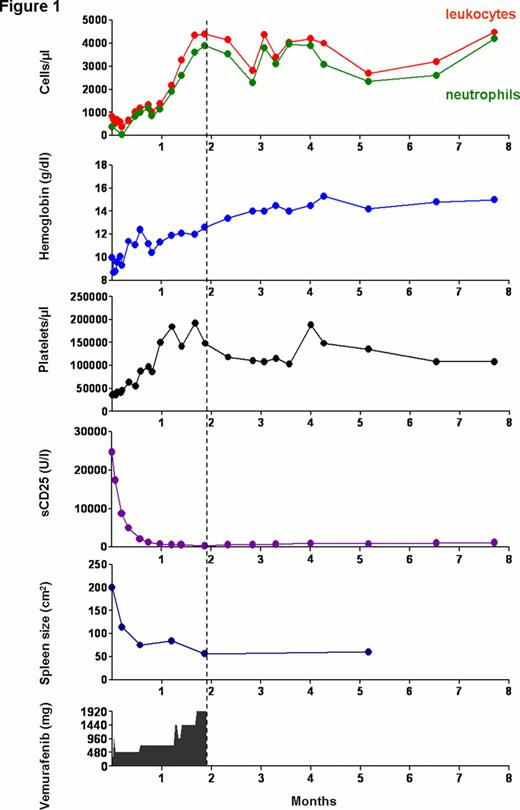
Because of limited treatment options and recent success with vemurafenib in BRAF mutated melanoma we decided to use experimental treatment with vemurafenib after intensive counseling and started treatment with 240 mg twice daily after a single loading dose of 960 mg. The dose was slowly escalated to 1,920 mg/d which is the standard dose used in melanoma. After 6 and 16 days of 240mg bid the spleen size had shrunk to 18.8 × 5.8 and 14×5 cm, respectively. Blood counts rapidly recovered and sCD25 which is considered a reliable marker of HCL cell load dropped quickly to normal levels already at the lowest dose of 240 mg vemurafenib bid (Figure 1 ). There was no evidence of tumor lysis. Response was further evaluated by repeated trephine biopsies on days -1, 6, 17 and 36. After only 6 days of vemurafenib treatment p-ERK signaling was almost completely abolished in HCL cells in vivo, followed by apoptosis of HCL cells as shown by Tunnel staining and finally complete clearance of hairy cells on day 36. CR criteria were achieved on day 43. Because of the excellent disease control and the risk of short-latency non-melanoma skin cancers during therapy with vemurafenib, we discontinued vemurafenib after 56 days. CR continues to persist in the absence of drug exposure for more than 6 months at the time of abstract preparation (Figure 1 ).
Massively parallel DNA sequencing was used to detect remaining mutant BRAF alleles in peripheral blood leukocytes on day 36. Among over 105 sequencing reads, the BRAF V600E mutation was not detectable above background (<0.3% of variant reads). Minimal residual disease (MRD by FACS) assessment of the peripheral blood revealed an approximately 100-fold reduction of hairy cells by day 22 of treatment and a complete eradication from day 36, which continues to persist for more than 6 months.
Our observations show that targeting of a single mutated oncogene can provide durable disease control in this leukemia. Trials exploring chemotherapy-free treatment approaches with BRAF inhibitors in HCL are highly warranted.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal