Abstract
Abstract 4754
The current standard of care for secondary stroke prevention in children and young adults with sickle cell disease and cerebral infarction is chronic simple transfusion (ST). Published data indicate that at least 18% of patients treated with chronic ST will experience a second overt infarct and at least 28% will experience additional silent infarcts. Since 1996, we have used chronic erythrocytapheresis (RCE) instead of chronic ST in our hospital to treat all patients with either overt infarction or abnormal transcranial doppler (TCD) with silent infarction. Here we present clinical, radiographic, and laboratory data from this group of patients treated with chronic RCE for secondary stroke prevention.
This was a retrospective study of all patients treated with chronic RCE for either overt infarction or for an abnormal TCD with silent infarction at Arkansas Children's Hospital from 1996 through 2011. We reviewed clinical records and serial MRI/MRA scans and determined the time to progression from the time of the initial diagnosis of an overt or silent infarct to the time of the second overt or silent infarct. Events were classified as overt infarcts if the MRI demonstrated acute cerebral ischemia, based on increased signal intensity on T2-weighted images and restricted diffusion on diffusion-weighted images, and abnormal neurologic findings correlated with the abnormalities identified on MRI. Events were classified as silent infarcts if the MRI demonstrated new lesions 3 mm or greater in a single dimension with increased signal intensity on T2-weighted images and there were no corresponding abnormal neurologic findings. We also studied the pre-procedure hemoglobin S concentration (%S), pre-procedure ferritin levels, volume of blood transfused per kilogram, necessity for chelation medication, and presence of end-organ damage.
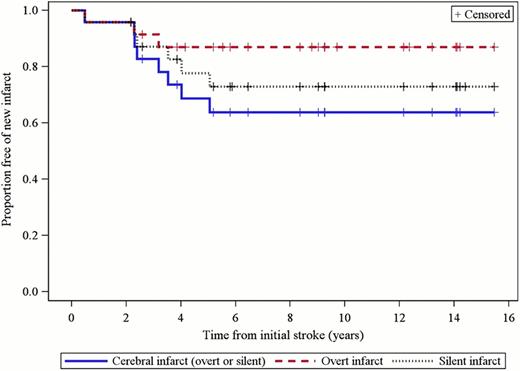
We identified 24 patients, ranging in age from 2 to 18 years at the initiation of chronic RCE, who were treated with 2539 RCE procedures during the study period. These patients were treated with RCE every two to six weeks with the goal of maintaining their pre-RCE %S at less than 30%. Progressive cerebral infarcts occurred in 42% (10 of 24) of the patients while receiving chronic RCE (Figure 1): 3 were overt (13%) and 7 were silent (29%). There were no additional infarcts observed after patients had been on chronic RCE for greater than 5 years. Eight patients (33%) experienced increased vasculopathy and 3 patients (13%) had an improvement in vasculopathy while on therapy. The mean pre-procedure %S concentration was 29%. The mean pre-procedure ferritin was 1188 ng/ml but approximately 60% of the patients had ferritin levels under 1000 ng/ml and only three patients required chelation. Patients received a mean of 45.5 ml/kg of packed red blood cells per procedure. There was no evidence of end-organ damage secondary to iron overload.
Survival free of new cerebral infarcts. The solid line represents the proportion of patients free of either a second overt or silent infarct. The dashed line represents the proportion of patients free of a second overt infarct. The dotted line represents the proportion of patients free of a second silent infarct. Plus signs represent censored cases.
Survival free of new cerebral infarcts. The solid line represents the proportion of patients free of either a second overt or silent infarct. The dashed line represents the proportion of patients free of a second overt infarct. The dotted line represents the proportion of patients free of a second silent infarct. Plus signs represent censored cases.
We determined that children with sickle cell disease and cerebral infarction experience additional silent and overt strokes despite intensive treatment with chronic RCE. The proportion of patients developing new overt infarcts in our study (13%) was slightly lower than that in a recent multi-institution study (18%; Hulbert et al, Blood 117:772, 2011) but the proportion of patients developing new silent infarcts in our study (29%) was no different (28%). Although patients receiving RCE have increased blood product utilization as compared with patients receiving ST, only three patients required chelation medication and none experienced end-organ damage secondary to iron overload. We conclude that chronic RCE is no more effective than chronic ST for secondary stroke prevention, that chronic RCE prevents the iron overload and need for chelation that is common with chronic ST, and that other forms of therapy are needed to prevent the progressive accumulation of cerebral infarcts in these patients.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal