Abstract
Abstract 4903
Use of BCR-ABL TKIs (Tyrosine-Kinase inhibitors) is the standard therapy for chronic myeloid leukemia. Dasatinib at the dosage of 100 mg once daily (QD) has been registered as first-line treatment for chronic phase chronic myeloid leukemia (CML) because it has been shown to be able to elicit faster and deeper cytogenetic and molecular responses than imatinib 400 QD, preventing also some events of progression to advanced phases of the disease, that can still occur during the first 2–3 years of treatment with TKIs. This is likely to be related to the strength of BCR-ABL in inhibiting the BCR-ABL TK activity and to its capacity to inhibit many of the BCR-ABL mutant forms than can escape inhibition by imatinib. However, very little is at present known about the relationship existing between the plasma level of dasatinib and its intracellular concentration, as well as about the factors that can influence drug intracellular penetration. This could help in understanding the relationship existing between drug level and its effectiveness in individual patients, as well as it may potentially help to modulate drug level by dose adjustment, useful to prevent toxicity and unwanted side-effects that could limit the efficacy of this drug in CML treatment.
Aim of our study were to perform quantification of dasatinib in human peripheral blood mononuclear cells (PBMC) and evaluated whether single-nucleotide polymorphisms (SNPs) in ABCB1 gene may work as predictors of dasatinib exposure.
Patients administered with dasatinib since at least 1 month were considered in the study. Sampling was performed after written informed consent was obtained in accordance with local ethics committee indications. Main inclusion criteria were, no concomitant interacting drugs, no hepatic or renal function impairment, self-reported adherence > 95%. Dasatinib PBMC and plasma Ctrough was measured in samples by a validated High Pressure Liquid Chromatography tandem MS detection method. We evaluated the following ABCB1 SNPs: rs1128503, rs2032582 and rs1045642. Genotyping was conducted by real time PCR based allelic discrimination using standard methodology. Statistical analysis was conducted by Mann Whitney or Spearman Rank to assess the effects of weight, age, gender, and dosage on dasatinib intracellular Ctrough. Median value of individual measurements was considered (±SD). Values were expressed as ng/mL.
Twelve patients met the inclusion criteria. No associations between weight, age, gender, dosage and dasatinib PBMC Ctrough were observed.
Despite the low number of patients, significant correlations between PBMC concentrations and PBMC concentrations/dose and ABCB1 SNPs 3435C>T and 1236C>T were observed.
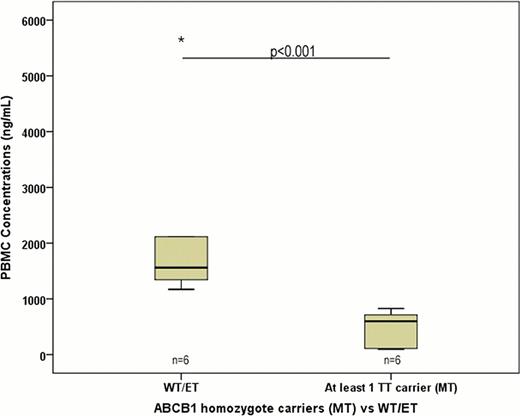
Specifically, the median intracellular dasatinib Ctrough in individuals with homozygote mutation allele (TT, n=4) for 1236C>T was lower as compared to patients with wild-type(WT)/heterozygote(ET) genotype (CC/CT, n=8) [599 (±271) vs 1436 (±1670), respectively, p=0. 042]. Similarly, intracellular dasatinib concentrations in individuals with homozygote mutation allele for 3435TT (n=4) and 2677TT (n=2) were lower as compared to patients with WT/ET genotype, with no significant p-value [673 (±324) vs 1436 (±1692), p=0. 089; 673 (±54) vs 1256 (±1599), p=0. 390, respectively], but with a clear trend. This data were significant when intracellular concentrations were corrected with each patient dosage of dasatinib. When we considered patient carriers of at least a homozygote mutation (TT carrier) on SNPs examined (n = 6), intracellular concentrations have been shown to be significantly lower than WT/ET patients [599 (±313) vs 1559 (±1706), p<0. 001, respectively].
These findings suggest that ABCB1 SNPs may actually determine an important influence on PBMC exposure. Since the drug fraction reaching the intracellular compartment is the one exerting therapeutic action, these factors could of course exert an influence on the response to therapy as well as on the onset of some toxicity effects. Further clinico-pharmacological studies, with an higher number of patients, are now required to confirm this association and to establish a relationship between this single-nucleotide polymorphisms of the ABCB1 gene and response and side effects observed in individual patients during dasatinib therapy.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal