Abstract
Abstract 4919
Bendamustine, a unique alkylating agent that leads to cancer cell death via several pathways, and rituximab, an anti-CD20 monoclonal antibody, are effective as combination therapy for non-Hodgkin's lymphoma (NHL) and mantle cell lymphoma (MCL). Because bendamustine and rituximab are molecularly unique, drug-drug interactions may be unlikely. Bendamustine is primarily metabolized via hydrolysis, also reducing the likelihood of drug-drug interactions. A recently reported analysis confirmed that rituximab does not affect systemic exposure to bendamustine, but the potential impact of bendamustine on rituximab has not been reported. Moreover, because bendamustine is a CYP1A2 substrate, the potential may exist for drug-drug interactions with CYP1A2 inhibitors and inducers. This analysis graphically explored the potential for drug-drug interactions by examining (1) the effect of bendamustine on rituximab pharmacokinetics (PK) and (2) bendamustine metabolism in the presence of cytochrome P450 (CYP) 1A2 inhibitors.
Data from a phase 3 study of combination bendamustine-rituximab in patients with advanced indolent NHL or MCL were assessed to evaluate the potential of bendamustine to affect systemic rituximab exposure. Rituximab 375 mg/m2 was administered on day 1 followed by bendamustine 90 mg/m2 30-min infusion on days 1 and 2 of 6 to 8 consecutive 28-day cycles. Serum rituximab concentrations at end of rituximab infusion (ie, rituximab monotherapy), 24 hours and 7 days postinfusion (ie, after bendamustine exposure) were measured. Data were compared with published literature on PK of rituximab administered without bendamustine (data on methods used in the published reports were limited). To evaluate effect of CYP1A2 inhibitors on bendamustine PK, graphical displays of observed bendamustine concentrations versus time since previous dose stratified by presence or absence of CYP1A2 inhibitors were generated.
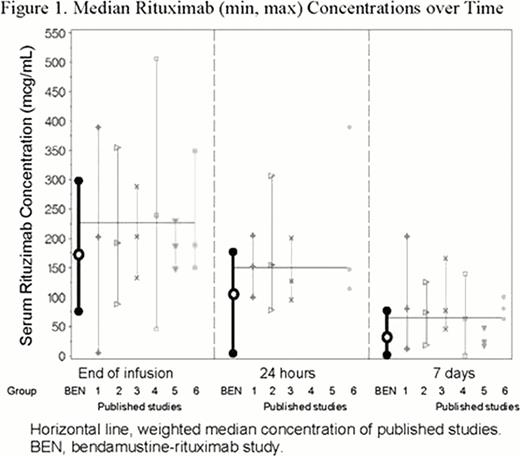
The full analysis population included 49 patients (median age, 63. 1 years; range 37–84). To analyze the effect of bendamustine on rituximab PK, 77 rituximab PK sample records from a subset of 19 patients were available. Minimum, median, and maximum observed serum rituximab concentrations in the absence (at end of rituximab infusion) and presence (at 24 hours and 7 days after rituximab infusion) of bendamustine are shown in Figure 1. The slope of rituximab concentrations from end of infusion to 24 hours and 7 days was similar to that seen in rituximab studies without bendamustine. In this study, rituximab concentrations with and without bendamustine were consistently lower than those in the literature (24% at end of infusion, 30% at 24 hours, and 53% at 7 days).
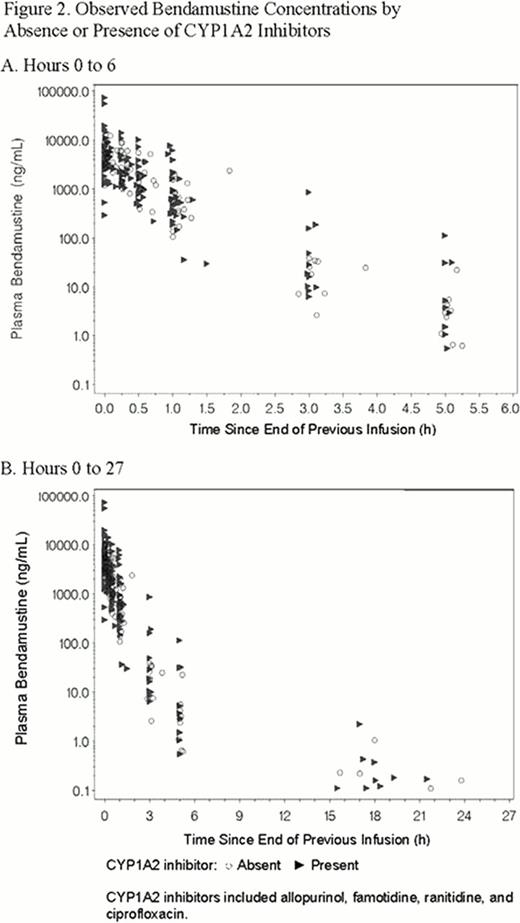
To analyze the effect of CYP1A2 inhibitors on bendamustine PK, 141 samples from 29/49 (59%) patients were taken in the presence of CYP1A2 inhibitors, and 102 samples from the remaining 20/49 patients were CYP1A2 inhibitor-free. Bendamustine concentrations were similar in observed time plots stratified by presence or absence of CYP1A2 inhibitors (Figure 2).
Serious adverse events considered related to bendamustine plus rituximab were neutropenia, transient acantholytic dermatosis, bacteremia, pyrexia, and febrile neutropenia, which is consistent with the serious adverse event profile for this combination in larger studies.
This graphical exploration suggests that no drug-drug interactions were demonstrated between bendamustine and rituximab or CYP1A2 inhibitors. Just as rituximab does not affect bendamustine PK, bendamustine did not appear to affect rituximab PK over time. Differences in observed rituximab concentration could be due to potential differences in assay methods/sensitivity or the length of rituximab infusion. Analysis of graphical displays of bendamustine concentrations in the presence and absence of CYP1A2 inhibitors suggests that CYP1A2 inhibitors do not increase systemic exposure to bendamustine, a result that is consistent with the near instantaneous hydrolytic metabolism of bendamustine. These results suggest that CYP1A2 is not a substantial metabolic pathway for bendamustine; however, whether CYP1A2 inducers have clinically relevant drug-drug interactions was not analyzed in this study. Based on results from this study, the likelihood of drug-drug interactions with bendamustine appears to be low.
Support: Teva Pharmaceutical Industries Ltd.
Darwish:Teva Pharmaceuticals: Employment. Bond:Teva Pharmaceuticals: Employment. Burke:Spectrum Pharmaceuticals: Consultancy. Hellriegel:Teva Pharmaceutical Industries Ltd: Employment. Robertson:Teva Pharmaceutical Industries Ltd: Employment. Phillips:Cognigen Corporation, which received research funding from Teva Pharmaceuticals: Employment, Research Funding. Grasela:Cognigen Corporation, which received research funding from Teva Pharmaceuticals: Employment, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal