Abstract
Abstract 5033
Renal impairment (RI) is a relatively common feature of multiple myeloma (MM) and it has been shown in several studies that RI at diagnosis correlates to inferior survival, significant morbidity and increased early death rate.
To understand the impact of RI on survival in the era of novel agents and to evaluate the efficacy of bortezomib-based treatment in patients presenting with RI. The primary endpoint of the study was overall survival (OS) in the whole population and renal response (RR) in 1st, 2nd and 3rd line treatment in a selected center (Karolinska). Time to progression (TTP), time to next treatment (TTNT), overall survival (OS) and MM response were the secondary endpoints.
The study population included all patients diagnosed with MM since earliest January 2000 until latest June 2011 at 6 university clinics, 3 regional centers and 4 local hospitals in Sweden. A S-Creatinine limit of 177 μmol/L was chosen as cutoff for OS calculations. For a detailed analysis on RR all patients with MM with a S-Creatinine >=130 μmol/L that were diagnosed in clinical practice between January 2000 and July 2010 at Karolinska Huddinge and between January 2005 and July 2010 at Karolinska Solna were selected. This S-Creatinine limit was selected in an attempt to cover all MM patients with GFR <50 mL/min. The study population was divided into those receiving bortezomib and those receiving other drug combinations (control group).
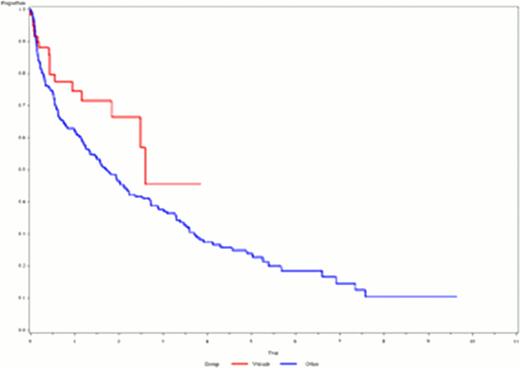
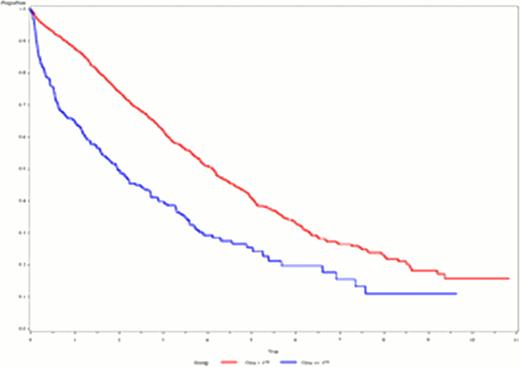
The population consisted of 1642 patients, but S-Creatinine values were missing for 100 patients resulting in a study population of 1542 patients. Patients with S-Creatinine >=177 μmol/L (n=267) had a significantly worse median OS of 1. 9 years 95% CI[1. 5;2. 6] compared to S-Creatinine <177 μmol/L (n=1275), with a median OS of 4. 1 years 95% CI[3. 8;4. 4]; (p=<0. 001). Patients with >=177 in S-Creatinine receiving bortezomib (n=60) had a median OS of 2. 6 years compared to 1. 7 years in the control group (n=206) (p=0. 05).
Of the 1542 patients, 556 were diagnosed and treated at our center. Ninety-five of these patients had a S-Creatinine >=130 μmol/L at diagnosis. Of the 95 patients 52 also had RI in 2nd line and 25 in 3rd line treatment resulting in a total of 172 treatment occasions where anti-myeloma treatment was given to patients with RI. There was no significant difference regarding age, sex, hemoglobin, β2-microglobulin, calcium, or albumin between the bortezomib-group and the control group. In the bortezomib-group, in 1st line treatment, 11 of 12 patients (92%) improved their GFR compared to 57 of 83 (69%) in the control group (p=0. 049). In the 2nd and 3rd treatment line the overall RR was 19% and 43% in the bortezomib treated compared to 23% and 28% in the control group (p=0. 749, and p=0. 257). When analyzing all treatment lines together the MM response was better in the bortezomib-group with significantly higher overall response rate of 80%, compared to 55% (p=0. 026). Median TTP in 1st, 2nd and 3rd line in the bortezomib-group was 18, 6 and 10 months and in the control group 11, 10 and 8 months. Median survival time was 3. 4 years for the control group, whereas 62% of the bortezomib treated patients still were alive at median time of follow up.
RI is still an important prognostic marker in MM despite modern treatment with a proteasome inhibitor. Bortezomib-based regimens can partly overcome the negative impact of RI by a higher frequency of RR and MM responses, as well as an improved median OS in comparison to other treatment regimens for MM patients with RI.
OS in patients with S-Creatinine >=177 μmol/L receiving bortezomib based treatment compared to the control group
OS in patients with S-Creatinine >=177 μmol/L receiving bortezomib based treatment compared to the control group
OS in patients with S-Creatinine >=177 μmol/L compared to those with S-Creatinine <177 μmol/L
OS in patients with S-Creatinine >=177 μmol/L compared to those with S-Creatinine <177 μmol/L
Liwing:Janssen-Cilag: Employment, Equity Ownership. Näsman:Janssen-Cilag: Consultancy. Aschan:Janssen-Cilag: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal