Abstract
Abstract 678
BCL-2 activation plays a role in the progression of MDS to AML and BCL 2 inhibition may represent a therapeutic target in such patients. Using our double transgenic mouse model MRP8[NRASD12/BCL2], in which the transgenes induce MDS progressing to AML with dysplasia (Omidvar Cancer Res, 2007), we assessed the effect of ABT-737, a small molecule and mimetic inhibitor binding the BH3 domain of the BCL-2 family of proteins, on survival and leukemia initiating cells (LIC) in this mouse model.
In this MRP8[NRASD12/hBCL2] double transgenic mouse model 2-week old mice have MDS with a mean of 6% bone marrow (BM) blasts (compared with 3% in the normal wild type littermates), while adult mice have AML, with a mean of 60% marrow blasts. In the present study, double transgenic mice were treated just after weaning and genotyping at 3 weeks of age with 75 mg/kg dose of ABT-737 (MDA & Abbott) 3 times weekly for 30 days. A cohort of mice (60 untreated and 35 treated) was followed for survival. Mice were sacrificed and BM harvested after treatment and Giemsa stained for BM analysis by microscopy (n=6 in each group), for LICs characterized as part of the lineage negative (Lin-)/Sca1+/cKit+ (LSK) population by flow cytometry (in 8 treated and 12 untreated mice), and for progenitor assays (n=4 in each group). Hematoxylin and eosin stained liver sections were examined and apoptosis assessed by the terminal deoxynucleotidyl transferase (TdT)-mediated dUTP nick end-labeling (TUNEL) assays of liver sections (n=4 per group). RNA was extracted from Sca+ enriched spleen cells from untreated and treated mice (n=3 from each group) and assayed for gene expression profiling using exon specific arrays (Affymetrix).
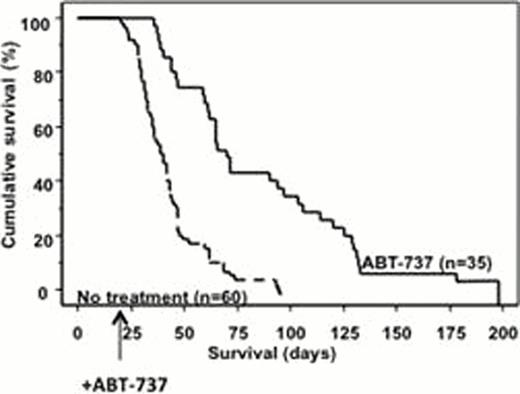
Survival from birth of 35 treated mice was significantly longer than for 60 untreated mice (p<0.0001) underscoring drug efficacy and tolerance (Fig. 1). This correlated with a reduction of bone marrow blasts (19%±7% in treated versus 60%±6% in untreated mice, p<0.0001). After ABT-737 treatment, the proportion of BM LSK cell population decreased to nearly normal levels (normal wild type littermates mean of 3%, n=4) (12.9±1.4 in untreated versus 6.4±1.1 in treated, p<0.005)) with a complete restoration of colony growth to normal range (40±10 in wild type normal mice, 79.6±7.0 in untreated versus 48.6±13.5 in treated, p<0.01). Decreased invasion of the liver and spleen was observed due to increased apoptosis (3±2 in untreated to 50±12% in treated, p<0.001). Exon specific gene expression arrays with Sca1+ enriched splenocytes showed that 997 genes were differentially expressed between the treated and the untreated mice; 764 and 233 genes were upregulated and downregulated respectively amongst which were upregulated genes important for stem cell development, maintenance and differentiation such as TCF712 (or TCF4), PIWII2, BRMPR1a and Spp1. This may reflect the partial restoration of normal stem cell function, which is consistent with the reduced LSK and progenitor numbers. Downregulation of anti-apoptotic genes such as BCL-2a1b or upregulation of pro-apoptotic genes such as PARP4, CALPAIN2, TNFR, and CARD was observed, consistent with the TUNEL data. Restoration of normal hematopoiesis was confirmed by the upregulation of myeloid differentiation genes (CD14, CSF1, RARalpha) and down regulation of genes implicated in cell cycle (Hsp60, MYC and E2F1).
ABT-737 extends lifespan in NRASD12/BCL2 transgenic mice, a preclinical model of high risk MDS/AML. ABT-737 targets the leukemia initiating cell, and regulates, amongst several, cell cycle (proliferation), differentiation and apoptosis pathways. These data suggest that clinical trials in high risk MDS and AML patients are warranted.
Kaplan-Meier survival curves showing prolonged survival of AML-Like mice of treated with ABT-737 compared with untreated mice from birth (p<0.0001).
Kaplan-Meier survival curves showing prolonged survival of AML-Like mice of treated with ABT-737 compared with untreated mice from birth (p<0.0001).
Grange:Genosplice Technology: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal