Abstract
Abstract 76
We measured proliferation in MM cells transfected with 13,984 small interfering RNAs in the absence/presence of increasing concentrations of bortezomib (BTZ) and identified 37 non lethal genes as reproducible BTZ sensitizers. The strongest hit was CDK5 a gene expressed at high levels in MM and neural tissues with relatively low expression in other organs suggesting a large therapeutic window. The small molecule CDK5 inhibitor Dinaciclib is a novel, potent, small molecule inhibitor of cyclin dependent kinases (CDK). It selectively inhibits CDK1, CDK 2, CDK 5 and CDK9 with 50% inhibitory concentrations (IC50) in the low nanomolar concentration. Based on these data, we undertook the current trial to evaluate the maximally tolerated dose of Dinaciclib in this patient population as well as assess the efficacy of single agent Dinaciclib in relapsed MM.
Patients with relapsed MM and measurable disease were enrolled on this phase 1/2 trial provided they had not more than 4 prior lines of therapy for MM, had adequate performance status and organ and hematological function. Patients receiving strong inhibitors/inducers of CYP3A4 were not allowed unless the drugs could be discontinued. The primary objectives were to (i) to determine the MTD of dinaciclib in subjects with relapsed MM (phase 1) and (ii) to evaluate the confirmed response rate with dinaciclib in patients with relapsed MM (phase 2). Dinaciclib was administered on day 1 of a 21-day cycle at doses of 30–50 mg/m2. Dose escalation was done using a 3+3 design and MTD was defined as one dose level below that resulted in >=2 DLTs among 6 patients.
Overall, twenty-nine patients were accrued to this study (19 phase I, 10 phase II) from July 2009 to December 2011. Two patients were replaced for study violations and are not included in analysis. The Phase II analysis included the 6 patients treated at the Phase I dose level 50mg/m2 as well as the first 9 patients accrued to the Phase II portion (15 evaluable patients). The median age of the 27 evaluable patients was 66 (range, 49–81); 52% were male. The median duration from diagnosis was 42 months (range, 7–207 months); median number of prior therapies was 4 (range, 1–5). Overall, 11 (41%) patients were considered high-risk by FISH and 56% were ISS stage III at study entry. Patients have received a median of 2 cycles (range: 1–12) and all patients have discontinued treatment for disease progression (18), alternative Treatment (2), adverse Event (4), or other reasons (3).
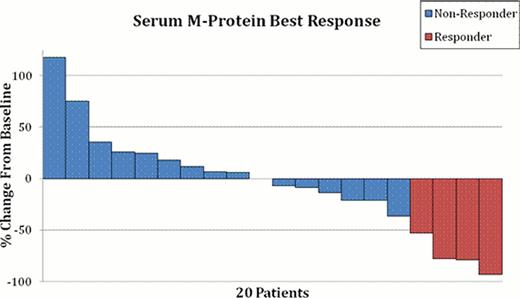
One dose limiting toxicity was seen over all dose levels. One patient at 40 mg/m2 experienced grade 3 constipation possibly related to treatment. The dose level of 50 mg/m2 was determined to be the MTD for the Phase II portion. The overall confirmed response rate was 3 of 27 (11%); including two patients at 40 mg/m2 dose (1 VGPR, 1 PR) and one patient at 50 mg/m2 dose (1 VGPR) with a PR or better. In addition, two patients at 50 mg/mg2 dose have achieved an MR; translating to an overall response rate of 18.5% (5 of 27). Figure 1 shows a waterfall plot of the M protein responses among patients with a measurable M spike. Overall, 21 patients had disease progression and 14 patients have died. Median follow-up for patients still alive is 11.8 months (range: 6.5–19.3). Leukopenia and thrombocytopenia were the most common hematological AEs, and gastrointestinal symptoms, alopecia, and fatigue, were the most common non-hematological AEs seen in the study.
The current study demonstrates potential single agent anti-myeloma activity of dinaciclib in a relapsed MM patient population with 2 patients achieving a deep response (VGPR) and many patients obtaining some degree of M protein stabilization or decrease. Ongoing studies are examining dinaciclib in combination with proteasome inhibitors, exploring both weekly and every three-week dosing schedules.
Zonder:Millennium Pharmaceuticals, Inc: Consultancy, Research Funding; Celgene: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal