Abstract
Abstract  807
807
The discovery of JAK2 (JAK2V617F) mutation in polycythemia vera (PV) and essential thrombocythemia (ET) has revolutionized our understanding of PV and ET. However, JAK2V617F is not the disease-initiating mutation and constitutes only part of the clone. Pegylated interferona (PegInfα), a form of interferon-alpha with a longer half-life and more tolerable toxicity, can induce not only hematological remission but also reduction of JAK2V617F allelic burden and suppression of the pre-JAK2V617F PV clone, as demonstrated by the return of polyclonal hematopoiesis (Liu Blood, 03). It is not clear whether this salutary effect is achieved by stimulation of an immune response against the PV clone, preferential activation of normal dormant stem cells (HSCs), or suppression of the PV clone by PegInfα. Using X-chromosome based clonality (qTCA) and JAK2V617F allelic burden quantitative assays, we analyzed selected biomarkers in HSCs, granulocytes and platelets of 18 patients treated with PegInfα, and evaluated cell cycle activity in HSCs. Previous data have indicated that PegInfα modulates the number of CD4+FOXP3+ regulatory T cells (Tregs) in the periphery. This is perhaps an indicator that PegInfα alters immunity against the JAK2V617F-bearing clone. We hypothesized that PegInfα mobilizes marrow Tregs to the periphery, thus decreasing their immunosuppressive and tumor promoting influence on the marrow microenvironment. Therefore, we correlated the number of Tregs before, during and after treatment with JAK2 allelic burden in peripheral blood and marrow samples in ongoing studies.
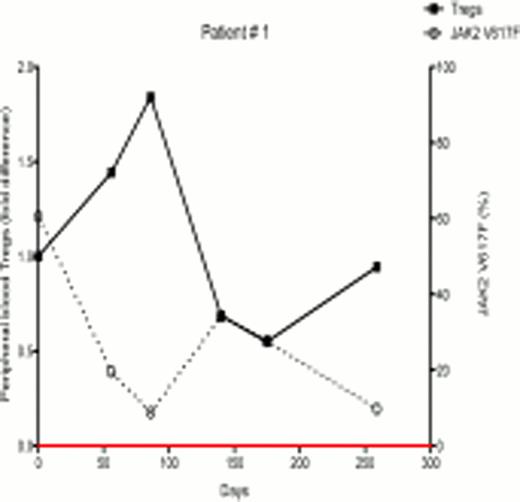
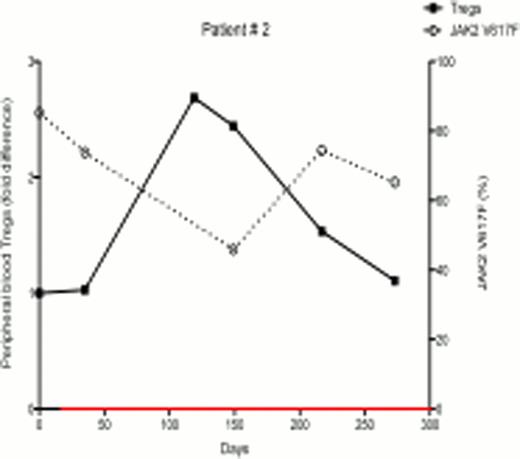
In ongoing studies, 18 patients (7 females and 11 males) with PV and ET were treated with PegInfα, with a median follow-up of 6 months. There were variable JAK2V617F allelic burdens at enrollment (1.2% – 99.8%). Following treatment, 7 of 18 patients demonstrated a decrease in JAK2V617F allelic burden, indicating that PegInfα had an effect on the JAK2V617F-positive clone, while 11 had no response or had an increase in JAK2V617F levels. Six out of 7 female patients were informative for at least one exonic polymorphism out of 5 X-chromosome genes, and all 6 had clonal hematopoiesis as determined by qTCA at the start of treatment. Two of these 6 patients developed polyclonal hematopoiesis during therapy, but in one the JAK2V617F allelic burden was unchanged, indicating that, in this patient, PegInfα had a selective salutary effect on a pre-JAK2V617F clone, while in other patients PegInfα clearly decreased the JAK2V617F-positive clone. In the 7 patients who demonstrated decreased JAK2V617F levels, there was an inverse relationship of JAK2V617F level with the number of circulating peripheral blood Tregs (examples shown in Figs 1 and 2). Statistical analysis of 18 patients showed a significant negative relationship between the number of circulating Tregs and JAK2V617F allelic burden (Spearman's correlation coefficient -0.4308, p<0.001). This suggests that an immunomodulatory effect by PegInfα involving Tregs may, at least in part, be associated with suppression of pre-JAK2V617F and JAK2V617F-positive clonal hematopoiesis.
We assessed total colony forming ability in methylcellulose culture media as a measure of hematopoietic progenitor abundance. In 5 patients treated with Pegasys, all 5 demonstrated an increase in colony-forming units. Furthermore, when cell cycle status of CD34+ HSCs was ascertained, we found a decrease in the percentage of HSCs in G0 (quiescence) in 4 out of 6 samples. These findings suggest that PegInfα therapy promotes normal HSC proliferation.
For the first time we directly demonstrate that PegInfα therapy in some patients recruits dormant pre-clonal PV HSCs to active hematopoiesis, thereby resulting in production of polyclonal granulocytes and platelets. PegInfα also appears to mobilize Tregs to the circulation, an effect that may be associated with changes in the marrow microenvironment. We conclude that PegInfα can suppress both pre-JAK2V617F and JAK2V617F PV clones in some PV patients, as demonstrated by evaluation of HSCs, granulocytes and platelets, but this often occurs asynchronously. Our preliminary data suggest that PegInfα therapy is associated with an increase in cell cycle activity in HSCs and mobilization of Tregs to the circulation. This provides a possible mechanism by which polyclonal hematopoiesis is restored after PegInfα treatment.
No relevant conflicts of interest to declare.
First and second authors contributed equally
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal