Abstract
Abstract 83
Protein quality control (PQC) pathways, including the ubiquitin-proteasome system, play critical roles in tissue development, including erythropoiesis. PQC mechanisms also regulate mitosis by facilitating the degradation of cell cycle stage-specific proteins. During erythropoiesis, committed progenitors undergo specialized cell divisions that facilitate terminal maturation and culminate in nuclear extrusion. We identified the tri partite motif-containing protein family member Trim58 as a putative ubiquitin ligase that is specifically expressed during late erythroid maturation as cells undergo their final round of division. Genome-wide ChIP-Seq and transcriptome analyses indicate that the Trim58 gene is activated by the essential erythroid nuclear proteins GATA1 and SCL/TAL1 (Cheng et al. Genome Research 2009). In human genome-wide association studies (GWAS), TRIM58 gene polymorphisms correlate with altered erythrocyte size (Ganesh et al. Nature Genetics 2009; Kamatani et al. Nature Genetics 2010). To study the function of Trim58, we used shRNAs to inhibit its expression in primary erythroid cultures of murine fetal liver hematopoietic progenitors. Four Trim58 shRNAs knocked down mRNA and protein expression from 60–90%. Compared to controls, Trim58 knockdown had no effect on erythroblast proliferation, survival, hemoglobinization, or expression of the maturation markers Ter119 and CD44. However, Trim58-deficient erythroblasts exhibited defective enucleation to the reticulocyte stage after 48 hours in culture (54% reticulocytes vs. 69% in controls, p<0.05, n= 4 replicates) (Figure 1). Additionally, Trim58 knockdown caused late-stage erythroblasts to acquire 2 or more nuclei (15.1% vs. 1.7% in controls, p<0.01, n= 3 experiments). Similar results were observed using 4 different Trim58 shRNAs compared to 3 controls (mock infection, scrambled shRNA, or anti-luciferase shRNA). Immunofluorescence showed that endogenous Trim58 or ectopically expressed mCherry-Trim58 fusion protein both localized to mitotic structures including centrosomes, which participate in chromosomal organization during metaphase, and the midbody, which connects dividing cells in late telophase. Immunoprecipitation-mass spectrometry and GST-pulldown studies demonstrated that Trim58 interacts with dynein and Nup153, two proteins that are known to participate in cell division. Based on these findings, we hypothesize that Trim58 is an E3 ubiquitin ligase that regulates terminal erythroid cell cycles and enucleation by coordinating nuclear division (karyokinesis) with cytokinesis. Our findings provide new insights into mechanisms of normal erythropoiesis and are relevant to the pathophysiology of numerous disorders associated with erythroid precursor multinuclearity, including myelodysplastic syndrome and the congenital dyserythropoietic anemias.
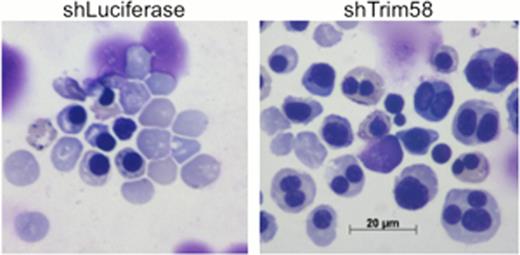
Compared to control shRNA treatment (shLuciferase), Trim58 knockdown (shTrim58) in differentiating primary murine erythroblasts causes a late maturation block with reduced reticulocytes and accumulation of multinuclear erythroblasts.
Compared to control shRNA treatment (shLuciferase), Trim58 knockdown (shTrim58) in differentiating primary murine erythroblasts causes a late maturation block with reduced reticulocytes and accumulation of multinuclear erythroblasts.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal