Abstract
Nonhematopoietic stromal cells of secondary lymphoid organs form important scaffold and fluid transport structures, such as lymph node (LN) trabeculae, lymph vessels, and conduits. Furthermore, through the production of chemokines and cytokines, these cells generate a particular microenvironment that determines lymphocyte positioning and supports lymphocyte homeostasis. IL-7 is an important stromal cell-derived cytokine that has been considered to be derived mainly from T-cell zone fibroblastic reticular cells. We show here that lymphatic endothelial cells (LECs) are a prominent source of IL-7 both in human and murine LNs. Using bacterial artificial chromosome transgenic IL-7–Cre mice, we found that fibroblastic reticular cells and LECs strongly up-regulated IL-7 expression during LN remodeling after viral infection and LN reconstruction after avascular transplantation. Furthermore, IL-7–producing stromal cells contributed to de novo formation of LyveI-positive lymphatic structures connecting reconstructed LNs with the surrounding tissue. Importantly, diphtheria toxin–mediated depletion of IL-7–producing stromal cells completely abolished LN reconstruction. Taken together, this study identifies LN LECs as a major source of IL-7 and shows that IL-7–producing stromal cells are critical for reconstruction and remodeling of the distinct LN microenvironment.
Introduction
IL-7 is an important cytokine that controls development and activation of different immune cells.1 The broad expression of this cytokine in primary and secondary lymphoid organs is indicative for its multiple functions. In bone marrow, IL-7 acts on the development of B cells by determining B-cell lineage commitment2 and regulating immunoglobulin gene arrangement.3,4 In the thymus, IL-7 serves as a key factor for thymocyte survival and maturation.5,6 Likewise, IL-7 provides antiapoptotic and proliferative signals to T cells within secondary lymphoid organs and is hence critical for peripheral T-cell homeostasis.7,8 Furthermore, some intrinsic functions of marginal zone B cells and structural organization of the splenic marginal zone microenvironment depend, at least partially, on IL-7.9 Besides these effects on T and B lymphocytes, IL-7 can impact on the development of dendritic cells10 and NK T cells.11 Hence, because of its pleiotropic functions, IL-7 can be regarded as one of the central regulators of immune cell homeostasis.
Besides its direct impact on immune cells, IL-7 acts also on the formation of secondary lymphoid organs. During lymph node (LN) development, for example, IL-7 is produced by VCAM1+ICAM1+ mesenchymal cells, also known as stromal organizer cells.12 Stromal cell-derived IL-7 promotes survival of lymphoid tissue inducer (LTi) cells13 that initiate lymphotoxin-β receptor-dependent formation of the LN environment.14 The importance of IL-7 in LN development and maturation is illustrated by the absence of most peripheral LNs in IL-7Rα–deficient mice.15,16 Furthermore, overexpression of IL-7 results in the formation of ectopic lymphoid tissues,17 suggesting that IL-7 critically contributes to the adaptation of lymphoid organ structure during immune reactions.
IL-7 production is tightly regulated and detection of both IL-7 protein and mRNA in situ requires highly sensitive detection systems.18 It has been suggested that IL-7 produced by stromal cells in secondary lymphoid organs is locally consumed by IL-7Rα–expressing cells and that a production-consumption equilibrium regulates lymphocyte homeostasis.19 Indeed, a recent study suggested that T-cell homeostasis is controlled by T-cell zone fibroblastic reticular cells (FRCs), which exhibited higher IL-7 expression compared with bulk endothelial cell preparations.20 However, not all IL-7Rα–expressing cells reside in the T-cell zone. For example, IL-7Rα+ γδ T cells preferentially traffic through interfollicular regions of secondary lymphoid organ.21,22 Likewise, LTi cells reside preferentially at the T-B border zone and in interfollicular regions.23 Thus, provided that cells can receive signals via their IL-7R only in the vicinity of IL-7–producing stromal cells,19 it is probable that stromal cells in different LN subcompartments, such as interfollicular regions and the medulla, produce IL-7 to support homeostasis of various IL-7R–expressing cells.
We show here that lymphatic endothelial cells (LECs) are an important source of IL-7 in murine and human LNs. Assessment of IL-7 regulation in bacterial artificial chromosome transgenic IL-7–Cre mice revealed that IL-7 promoter-dependent transgene expression was significantly up-regulated both in FRCs and LECs during LN remodeling. Furthermore, depletion experiments showed that IL-7–producing stromal cells were required for LN reconstruction after avascular transplantation, indicating that IL-7–producing stromal cells critically contribute to adaptive LN remodeling.
Methods
Ethics statement
Experiments were performed in accordance with federal and cantonal guidelines under permission numbers SG09/83 and SG09/87 after review and approval by the Cantonal Veterinary Office (St Gallen, Switzerland) or performed under A(SP)A Home Office license. Use of human fetal tissues and human adult tissue was approved by the Medical Ethical Committee of the Erasmus MC and the Kantonale Ethikkommission St Gallen, respectively.
Mice
Bacterial artificial chromosome-transgenic IL-7–Cre and IL-7.hCD25 mice24 were crossed with R26-enhanced yellow fluorescent protein (EYFP) reporter25 and IL-7–Cre mice were crossed with R26-iDTR mice.26 C57BL/6 mice were obtained from Charles River. All animals were kept under conventional conditions in individually ventilated cages.
Cell lines and viruses
Primary human LECs were obtained from ScienCell. HUVECs were obtained from Promocell. Cells were cultured in endothelial basal medium (Lonza Walkersville) supplemented with 20% FBS (Invitrogen), antibiotic and antimycotic solution, hydrocortisone (10 μg/mL) and N6,2′-O-dibutyryladenosine 3′,5′-cyclic monophosphate sodium salt (all Fluka). Cells were grown at 37°C in 5% CO2 and cultured for up to 8 passages. Lymphocytic choriomeningitis virus (LCMV) WE strain, originally obtained from Dr R. Zinkernagel (University of Zürich, Zürich, Switzerland), was propagated on L929 cells at a low MOI and quantified as previously described.27 Mice were infected with 200 pfu LCMV WE strain intravenously and analyzed at the indicated time points after infection.
T-cell survival assay
Human LECs (ScienCell) or HUVECs were seeded in 96-well plates and 2 × 105 MACS-sorted naive CD4+ T cells (MACS selection kit; Miltenyi Biotec) added to LEC and HUVEC cultures. Alternatively, naive CD4+ T cells were exposed to supernatant collected from LEC cultures. IL-7 was neutralized using a commercially available anti–IL-7 antibody (R&D Systems). After 48 hours of incubation, T-cell survival was assessed by flow cytometry using anti-CD4 (BD Biosciences) and 7-amino-actinomycin D.
LN transplantation
Two or 3 inguinal LNs from IL-7–CrexR26-EYFP or IL-7–Cre/iDTR donor mice were transplanted under the kidney capsule of C57BL/6 recipients. Recipients were anesthetized with isoflurane, and surgery was performed as described previously.28
Preparation of stromal cells
Murine LNs were dissected into small pieces and transferred into a 24-well dish filled with RPMI 1640 medium containing 2% FCS, 20mM HEPES (all from Lonza Walkersville), 1 mg/mL Collagenase Type IV (Sigma-Aldrich) and 25 μg/mL DNaseI (Applichem). Dissociated LNs were incubated at 37°C for 30 minutes. After enzymatic digestion, cell suspensions were passed through a 45-μm gauze filter (BD Biosciences) and washed with PBS containing 0.5% FCS and 10mM EDTA (Sigma-Aldrich). The cell pellet was resuspended in 5 mL MACS buffer. To further enrich the stromal cell fraction, lymphocytes were depleted by incubating the cell suspension with MACS anti-CD45 Microbeads and passing over a MACS LS column (Miltenyi Biotec). Endothelial vessels (arterial and venal vessels) were microdissected from neonatal skin and sorted based on the expression of CD31 and LyveI. For embryonic anlagen, matings were setup between IL-7–Cre+ males and Rosa26-EYFP females and plugs recorded as day 0.5. The mesenterium was isolated from the embryos at different stages in development. The developing mesenteric fetal anlagen were dissected and digested using liberase/DNASE I for 30 minutes at 37°C on a shaking heat block.
Human fetal mesenteric LNs were obtained after elective abortion and contingent on informed consent. Human LNs were digested with Collagenase D (Roche Diagnostics) to produce single-cell suspension. Cells were stained with antibodies directed to human CD45 (eBioscience) and podoplanin (Pdpn) and CD31 (both BioLegend) for FACS-based sorting.
Antibodies, flow cytometry, and cell sorting
Stromal cell suspensions were incubated for 20 minutes at 4°C in PBS containing 1% FCS and 10mM EDTA with the following antibodies: anti-CD45, anti-gp38/Pdpn (all BD Biosciences), anti-CD31, anti–human CD25 (all from eBioscience). Cells were acquired with a FACSCanto (BD Biosciences) or a Cyan (Beckman Coulter) flow cytometer and analyzed using FlowJo Version 7.6.5 (TreeStar) or Summit (Beckman Coulter). Cell sorting was performed using a FACSAria cell sorter (BD Biosciences) or a MoFlo cell sorter (Beckman Coulter).
Quantitative real-time PCR
Total cellular RNA was extracted from cultivated cells, homogenized tissues, and sorted cells using TRIZOL reagent (Invitrogen) following the manufacturer's protocol. cDNA was prepared using cDNA archive kit (Applied Biosystems). Quantitative RT-PCR for IL-7 was performed using the Light Cycler-FastStart DNA Master SYBR Green I kit (Roche Diagnostics) on a LightCycler machine (Roche Diagnostics). Expression levels of murine Il7 were measured using the following primers: forward, GTGCCACATTAAAGACAAAGAAG; and reverse, GTTCATTATTCGGGCAATTACTATC Normalization was performed with the expression of TATA-box binding protein: forward primer, CCTTCACCAATGACTCCTATGAC; and reverse primer, CAAGTTTACAGCCAAGATTCAC. Expression levels of human Il7 were examined on a 7900HT Fast Real-Time PCR System (Applied Biosystems). The following sets of primers (1μM final concentration) were used for the comparison of Il7 mRNA levels in LECs and HUVECs: Il7 (QIAGEN, Hs_IL7_1_SG, ID:QT00094185) and β actin (QIAGEN, Hs_ACTB_2_SG, ID:QT01680476).
ELISA
Human IL-7 levels in cell culture supernatants were measured using a multiplexed particle-based flow cytometric cytokine assay29 allowing detection limit of 1 pg/mL, following the instruction of the manufacturer (Millipore). The analysis was conducted using a conventional Guava EasyCyte Plus flow cytometer (Millipore).
Immunohistochemistry
Lymphoid tissues were fixed overnight in freshly prepared 4% paraformaldehyde (Merck). Fixed tissues were embedded in 4% low melting agarose (Invitrogen) in PBS and sectioned with a vibratome (Leica VT-1200). Sections 20- to 30-μm thick were blocked in PBS containing 10% FCS, 1 mg/mL anti-Fcγ receptor (BD Biosciences), and 0.1% Triton X-100. Sections were incubated overnight at 4°C with the following monoclonal antibodies: anti-gp38/Pdpn (clone 8.1.1, BD Biosciences), anti-B220 (BD Biosciences), anti-eYFP (Clontech), anti-GFP (Invitrogen), anti-CD31, anti-LyveI, anti-LTβR, anti-CD169 (all eBioscience), anti-Ki67 (BD Biosciences) and anti-RANKL (R&D Systems). Unconjugated antibodies were detected with the following secondary antibodies: Dylight649-conjugated anti–rat IgG, Alexa488-conjugated anti–rabbit IgG and Dylight549-conjugated anti–syrian hamster IgG (all purchased from Jackson ImmunoResearch Laboratories). Fluorescence signals of RANKL and LTβR were enhanced using a tyramide-based signal-enhancer kit (Invitrogen). Imaging was performed using a confocal microscope (Zeiss LSM-710 or Zeiss LSM-780), and images were processed with ZEN 2009 software (Carl Zeiss) or Volocity 6.0 (PerkinElmer Life and Analytical Sciences).
Statistical analysis
All statistical analyses were performed with Prism Version 4.0 (GraphPad Software). Data were analyzed with the nonpaired Student t test. P < .05 was considered significant.
Results
IL-7 production by lymphatic endothelial cells
In murine LNs, LECs are identified by the expression of lymphatic vessel endothelial hyaluron receptor 1 (LyveI) and their location in subcapsular sinus (SCS) and medulla. To assess the phenotype of human LECs, we histologically analyzed fetal and adult human LNs for the expression of LyveI and other stromal cell markers. We found that LyveI+ LECs in adult human LNs were located in medullary regions (Figure 1A-B asterisk) and at the SCS (Figure 1A-B arrows). Human LN LECs in the SCS were located adjacent to VCAM1+MadCAM1+ marginal reticular cells. To assess whether LECs contribute to IL-7 production in LNs, we determined IL-7 gene expression in sorted LECs, FRCs, and blood endothelial cells (BECs) from fetal mesenteric LNs. As shown in Figure 1C, substantial expression of Il7 mRNA could be detected both in freshly isolated LECs and FRCs, but not in BECs. Furthermore, when LECs derived from human LNs were cultured in vitro, expression of Il7 mRNA and secretion of IL-7 protein was readily detectable in contrast to HUVECs (Figure 1D). Using an in vitro T-cell survival assay, we found that coculture of naive T cells with human LECs or exposure of naive T cells to supernatants from human LEC cultures significantly increased T-cell survival (Figure 1E). Importantly, antibody-mediated neutralization of IL-7 abolished this effect (Figure 1E), indicating that LEC-derived IL-7 is functionally active and provides critical survival signals to T cells.
Assessment of IL-7 expression in human LN LECs. (A) Fetal LN sections were stained with fluorescently labeled antibodies against LyveI, MadCAM-1, and CD3. (B) Adult LN sections were stained with antibodies against LyveI, VCAM-1, MadCAM-1, and Pdpn. LyveI+Pdpn+ LECs (blue in left panel and green in right panel) are indicated in the subcapsular sinus (arrows) and LN medulla (*). (C) CD31+Pdpn+ LECs, CD31−Pdpn+ FRCs, and CD31+Pdpn− BECs from human fetal mesenteric LNs were sorted by flow cytometry. IL-7 expression levels were determined by quantitative RT-PCR. Values indicate mean ± SEM from triplicates, representative results from 1 of 2 independent sorting experiments. (D) Human LN LECs were cultivated and analyzed for IL-7 mRNA expression by quantitative RT-PCR. Cell culture supernatants were collected after 48 hours and analyzed for IL-7 protein by ELISA (right graph). Measurements were carried out in triplicates (mean ± SEM). (E) MACS-isolated human naive CD4+ T cells (2 × 105) were cocultured with human LECs, HUVECs, or supernatant from human LEC cultures in the presence and absence of neutralizing anti–IL-7 antibody. After 48 hours, T-cell survival was analyzed by flow cytometry and is displayed as difference (Δ) to medium control. Values indicate mean ± SEM from triplicates, representative results from 1 of 2 independent experiments; *P < .05.
Assessment of IL-7 expression in human LN LECs. (A) Fetal LN sections were stained with fluorescently labeled antibodies against LyveI, MadCAM-1, and CD3. (B) Adult LN sections were stained with antibodies against LyveI, VCAM-1, MadCAM-1, and Pdpn. LyveI+Pdpn+ LECs (blue in left panel and green in right panel) are indicated in the subcapsular sinus (arrows) and LN medulla (*). (C) CD31+Pdpn+ LECs, CD31−Pdpn+ FRCs, and CD31+Pdpn− BECs from human fetal mesenteric LNs were sorted by flow cytometry. IL-7 expression levels were determined by quantitative RT-PCR. Values indicate mean ± SEM from triplicates, representative results from 1 of 2 independent sorting experiments. (D) Human LN LECs were cultivated and analyzed for IL-7 mRNA expression by quantitative RT-PCR. Cell culture supernatants were collected after 48 hours and analyzed for IL-7 protein by ELISA (right graph). Measurements were carried out in triplicates (mean ± SEM). (E) MACS-isolated human naive CD4+ T cells (2 × 105) were cocultured with human LECs, HUVECs, or supernatant from human LEC cultures in the presence and absence of neutralizing anti–IL-7 antibody. After 48 hours, T-cell survival was analyzed by flow cytometry and is displayed as difference (Δ) to medium control. Values indicate mean ± SEM from triplicates, representative results from 1 of 2 independent experiments; *P < .05.
Pdpn and CD31 (platelet/endothelial cell adhesion molecule 1) double-positive LECs constitute a prominent fraction of stromal cells in murine LNs (Figure 2A). To assess whether murine LECs express considerable amounts of Il7 mRNA, LN stromal cells were sorted to high purity (supplemental Figure 1A-D, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Comparable with our findings for human LECs, murine LN and skin draining LECs displayed a considerable Il7 mRNA signal, whereas Il7 mRNA was not detectable in BECs (Figure 2B; supplemental Figure 1E). To assess the IL-7 expression pattern in vivo and to define in situ localization of IL-7–expressing cells, we used a bacterial artificial chromosome transgenic mouse with Cre-recombinase expression under the control of the IL-7 promoter.24 Crossing IL-7–Cre mice with the R26-EYFP reporter strain25 revealed that the Pdpn/CD31 double-positive LEC fraction contained the highest percentage of transgene-positive cells (Figure 2C). Confocal laser scanning microscopic (CLSM) analysis of LN sections confirmed that not only Pdpn-positive T-cell zone stromal cells (Figure 2D-E asterisks), but also LyveI-positive LECs showed significant transgene activity (Figure 2D-E arrows). Because the density of EYFP+ cells was highest in the region of the subcapsular sinus, LN sections were also stained for the expression of the metallophilic macrophage marker CD169. As shown in Figure 2F, transgene expression in the subcapsular sinus was only found in CD169-negative cells, hence excluding transgene activity in this myeloid cell population. Because histologic detection of IL-7 protein production in vivo is cumbersome,18 we used the production of human CD25 from the IL-7–hCD25 reporter construct24 as a surrogate for active protein production driven by the IL-7 promoter in EYFP+ cells. This analysis revealed that the production of the IL-7 protein surrogate was almost exclusively confined to the EYFP+ fraction (Figure 2G), suggesting that both murine FRCs and LECs produce IL-7 in vivo.
IL-7–Cre transgene expression in adult murine LNs. (A) Inguinal LN cell suspensions were depleted of CD45+ cells, and CD45− stromal cells were analyzed by flow cytometry for CD31 and Pdpn expression. Stromal cell subpopulations are designated in the respective quadrant. DN indicates double-negative cells. (B) FACS-sorted LN stromal cells were analyzed for IL-7 expression by quantitative RT-PCR (n = 4 from 2 independent sorts). (C) Histograms displaying EYFP expression within LEC and FRC subpopulations, IL-7–CrexR26-EYFP (solid line), and C57BL/6 (gray shading) LNs. Values indicate mean percentage ± SEM of EYFP+ cells (n = 6 in independent preparations). (D) Confocal laser scanning microscopic analysis of inguinal LNs from IL-7–CrexR26-EYFP mice. Scale bar represents 200 μm. (E) High magnification of boxed area in panel D; indicated are transgene-expressing LECs (arrows) and FRCs (asterisks). Scale bar represents 50 μm. (F) Anti-CD169 staining in the subcapsular sinus region of an IL-7–CrexR26-EYFP inguinal LN. Scale bar represents 20 μm. (G) Flow cytometric analysis of human CD25 expression on EYFP+ (black line) and EYFP− (gray line) LN stromal cells from triple transgenic IL-7–hCD25xIL-7CrexR26-EYFP mice.
IL-7–Cre transgene expression in adult murine LNs. (A) Inguinal LN cell suspensions were depleted of CD45+ cells, and CD45− stromal cells were analyzed by flow cytometry for CD31 and Pdpn expression. Stromal cell subpopulations are designated in the respective quadrant. DN indicates double-negative cells. (B) FACS-sorted LN stromal cells were analyzed for IL-7 expression by quantitative RT-PCR (n = 4 from 2 independent sorts). (C) Histograms displaying EYFP expression within LEC and FRC subpopulations, IL-7–CrexR26-EYFP (solid line), and C57BL/6 (gray shading) LNs. Values indicate mean percentage ± SEM of EYFP+ cells (n = 6 in independent preparations). (D) Confocal laser scanning microscopic analysis of inguinal LNs from IL-7–CrexR26-EYFP mice. Scale bar represents 200 μm. (E) High magnification of boxed area in panel D; indicated are transgene-expressing LECs (arrows) and FRCs (asterisks). Scale bar represents 50 μm. (F) Anti-CD169 staining in the subcapsular sinus region of an IL-7–CrexR26-EYFP inguinal LN. Scale bar represents 20 μm. (G) Flow cytometric analysis of human CD25 expression on EYFP+ (black line) and EYFP− (gray line) LN stromal cells from triple transgenic IL-7–hCD25xIL-7CrexR26-EYFP mice.
IL-7–Cre transgene activity during embryonic LN development
Because IL-7R–mediated stimulation of LTi cells during embryogenesis is crucial for LN development,17 we analyzed Cre-recombinase activity in developing LNs of IL-7–CrexR26-EYFP mice. As shown in Figure 3A, IL-7–Cre-positive cells could be detected already at E13.5. At the time point of LN anlagen formation (ie, at E16.5), IL-7 promoter activity had strongly increased (Figure 3A). To determine the identity and localization of IL-7–Cre-expressing cells in developing LNs, we histologically analyzed embryonic and neonatal LNs from IL-7–CrexR26-EYFP mice and found that IL-7–Cre-positive cells were localized in the endothelium lining the jugular lymph sac (jls) at E14.5 (Figure 3B arrows) and in the subcapsular sinus region of the neonatal inguinal LN (Figure 3C). High-resolution CLSM analysis confirmed that mainly LyveI+ LECs exhibited IL-7 promoter activity in the developing LN (Figure 3D arrows), whereas Pdpn+ stromal cells in the center of the developing LN were almost completely IL-7–Cre-negative. These data suggest that LECs represent an important source of IL-7 during LN development.
IL-7–Cre transgene activity during embryonic LN development. (A) IL-7–CrexR26-EYFP mouse embryos at different developmental stages were harvested, and dissected LN anlagen were analyzed for transgene-expressing (EYFP+) stromal cells. (B) E14.5 embryonic sections were stained with antibodies against EYFP, CD31, and Pdpn and analyzed by CLSM. EYFP+CD31+ cells (arrows) were found in the jugular lymph sac (jls) lining endothelium. (C) Neonatal inguinal LNs from IL-7–CrexR26-EYFP mice stained for EYFP (green), LyveI+ lymphatic endothelium (blue), and Pdpn+ parenchymal stroma (red). Scale bar represents 50 μm. (D) Higher magnification of boxed area in panel B. EYFP+LyveI+ LECs are indicated by arrows. Scale bar represents 20 μm.
IL-7–Cre transgene activity during embryonic LN development. (A) IL-7–CrexR26-EYFP mouse embryos at different developmental stages were harvested, and dissected LN anlagen were analyzed for transgene-expressing (EYFP+) stromal cells. (B) E14.5 embryonic sections were stained with antibodies against EYFP, CD31, and Pdpn and analyzed by CLSM. EYFP+CD31+ cells (arrows) were found in the jugular lymph sac (jls) lining endothelium. (C) Neonatal inguinal LNs from IL-7–CrexR26-EYFP mice stained for EYFP (green), LyveI+ lymphatic endothelium (blue), and Pdpn+ parenchymal stroma (red). Scale bar represents 50 μm. (D) Higher magnification of boxed area in panel B. EYFP+LyveI+ LECs are indicated by arrows. Scale bar represents 20 μm.
IL-7–Cre activity during virus-induced LN remodeling
To track the changes of IL-7 expression in the course of immune reactions that impact profoundly on the global LN structure and stromal cell composition, we used a well-established model for virus-induced LN remodeling.30,31 During low-dose infection with the WE strain of the LCMV, secondary lymphoid organ structure is destroyed because of loss of myeloid and stromal cells.30,32 After clearance of the virus, secondary lymphoid organ structure is reestablished through a LTβR-mediated cross-talk between LTi and stromal cells.33 Here, LNs from IL-7–CrexR26-EYFP mice were examined on day 20 after infection (ie, after successful reestablishment of LN structure). In situ analysis revealed an increase of IL-7–Cre+ cells in the T-cell area (Figure 4A arrowheads) and in the LEC-rich interfollicular regions (Figure 4A-B arrows). Flow cytometric analysis confirmed a significant increase in IL-7–Cre+ FRCs (EYFP+ cells in Figure 4C) and LECs (EYFP+ cells in Figure 4D) on day 20 after LCMV infection. In contrast, IL-7–Cre− FRC and LEC numbers (EYFP− in Figure 4C-D) were not significantly increased, indicating a specific expansion of IL-7–producing stromal cells during virus-induced LN remodeling. To further analyze the changes in the spatial organization of IL-7–Cre-expressing stromal cells, we determined EYFP and LyveI expression levels in cortical, paracortical, and medullary sections from infected and uninfected IL-7–CrexR26-EYFP LNs (Figure 4E; supplemental Figure 2). EYFP and LyveI expression levels were recorded as mean fluorescence intensity values on whole LN sections by confocal microscopy. As shown in Figure 4F, EYFP expression, together with LyveI expression, was significantly up-regulated in the cortical LN layers, indicating a pronounced remodeling activity in the LEC fraction of the subcapsular sinus. The increase of IL-7 promoter–dependent EYFP expression in cortical sections and the increased proportion of IL-7–Cre-positive cortical areas (Figure 4G) suggest that the elevated IL-7 expression in cortical LECs might be an important adaptation mechanism during LN remodeling.
Expansion of IL-7–expressing stromal cells during virus-induced LN remodeling. IL-7–CrexR26-EYFP mice were infected with 200 pfu LCMV-WE, and transgene activity was determined on day 20 after infection. (A) In situ analysis of naive (upper panels) and infected (lower panel) IL-7–CrexR26-EYFP inguinal LNs (blue represents B220; red, Pdpn; and green, EYFP). Scale bar represents 200 μm. (B) Higher magnification of day 20 IL-7–CrexR26-EYFP inguinal LNs (red represents LYVEI; blue, Pdpn; and green, EYFP). Scale bar represents 100 μm. (C-D) Enumeration of EYFP+ and EYFP− FRCs (C) and LECs (D) in LNs from naive and LCMV-infected mice (mean ± SEM; n = 6 mice). (E) Flow cytometric FACS analysis of EYFP+ FRCs (C) and LECs (D) in LNs taken from naive and infected IL-7–CrexR26-EYFP mice. (E) Evaluation of transgene activity in LECs in LNs of naive (left column) and LCMV-infected mice (right column). Cortical, paracortical, and medullary sections were stained for lymphatic endothelium (LyveI, red) and transgene activity (EYFP, green). (F) Quantification of EYFP and LyveI mean fluorescence intensity (MFI) in the different LN regions (mean ± SEM; n = 6 mice). (G) Proportion of EYFP+ areas in cortical LN sections from naive and infected mice. *P < .05; **P < .01; and n.s. indicates not significant.
Expansion of IL-7–expressing stromal cells during virus-induced LN remodeling. IL-7–CrexR26-EYFP mice were infected with 200 pfu LCMV-WE, and transgene activity was determined on day 20 after infection. (A) In situ analysis of naive (upper panels) and infected (lower panel) IL-7–CrexR26-EYFP inguinal LNs (blue represents B220; red, Pdpn; and green, EYFP). Scale bar represents 200 μm. (B) Higher magnification of day 20 IL-7–CrexR26-EYFP inguinal LNs (red represents LYVEI; blue, Pdpn; and green, EYFP). Scale bar represents 100 μm. (C-D) Enumeration of EYFP+ and EYFP− FRCs (C) and LECs (D) in LNs from naive and LCMV-infected mice (mean ± SEM; n = 6 mice). (E) Flow cytometric FACS analysis of EYFP+ FRCs (C) and LECs (D) in LNs taken from naive and infected IL-7–CrexR26-EYFP mice. (E) Evaluation of transgene activity in LECs in LNs of naive (left column) and LCMV-infected mice (right column). Cortical, paracortical, and medullary sections were stained for lymphatic endothelium (LyveI, red) and transgene activity (EYFP, green). (F) Quantification of EYFP and LyveI mean fluorescence intensity (MFI) in the different LN regions (mean ± SEM; n = 6 mice). (G) Proportion of EYFP+ areas in cortical LN sections from naive and infected mice. *P < .05; **P < .01; and n.s. indicates not significant.
Importance of IL-7–producing stromal cells during LN reconstruction
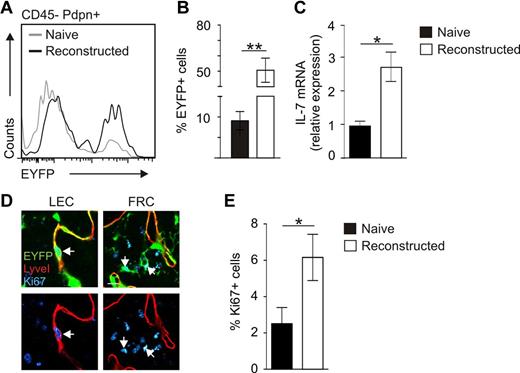
To determine the impact of IL-7–producing stromal cells on LN remodeling in more detail, we used an experimental approach of profound LN remodeling after avascular transplantation.34 Here, LNs from IL-7–CrexR26-EYFP mice were transplanted under the renal capsule of adult C57BL/6 mice. LNs became necrotic and had lost their structural integrity, but subsequent reconstruction processes led to restoration of LN structure, including full vascularization (supplemental Figure 3). CLSM analysis using antibodies against Pdpn, B220, and EYFP showed high activity of the IL-7–Cre transgene in the completely reconstructed LN (Figure 5A). Transplanted LNs at 8 weeks after transplantation were pervaded by EYFP+LyveI+ lymphatic sinuses (Figure 5B arrows) and had formed an EYFP+Pdpn+ FRC network (Figure 5C arrows). In addition, newly developed LyveI+Pdpn+ lymphatic vessels expressed EYFP (Figure 5D arrowheads). These lymphatic vessels were connected to the EYFP-expressing cortical sinus of the engrafted LNs (Figure 5E three-dimensional rendering of boxed area in panel D). Flow cytometric quantification of the transgene expression revealed a 5-fold increase in EYFP-expressing stromal cells during LN reconstruction (Figure 6A-B). The elevated EYFP expression in the reconstructed LN was accompanied by a substantial increase in Il7 mRNA expression (Figure 6C). To assess whether increasing numbers of IL-7–producing stromal cells and higher expression of endogenous IL-7 in regenerating tissues were the result of proliferation of IL-7–expressing cells, we stained sections of naive and transplanted LNs for Ki67 to label proliferating cells (Figure 6D). Quantification of Ki67+EYFP+ cells in regenerating LNs revealed a significant increase of proliferating, IL-7–expressing stromal cells (Figure 6E). Furthermore, LECs in reconstructed LNs expressed RANKL and LTβR (supplemental Figure 4), indicating that adaptive LN remodeling is not only associated with an expansion of IL-7–producing LECs and FRCs but that IL-7–expressing stromal cells also produce cell surface molecules that regulate LN formation and structural organization.
Transgenic IL-7–expression during LN reconstruction after avascular transplantation. LNs from IL-7–CrexR26-EYFP donor mice were transplanted under the kidney capsule of C57BL/6 mice and harvested 8 weeks after transplantation. (A) Mosaic scan of a regenerated LN (tLN) developing on the kidney (Ki; blue represents B220; red, Pdpn; and green, EYFP). Scale bar represents 200 μm. (B) Three-dimensional reconstruction of subcapsular sinus region (red represents LyveI; and green, EYFP). Scale bar represents 50 μm. Arrows indicate transgene-positive LECs. (C) Three-dimensional reconstruction of T-cell zone region (red represents Pdpn; and green, EYFP). Scale bar represents 50 μm. Arrows indicate transgene-positive FRCs. (D) Lymphatic vessel connecting tLN with surrounding tissue (blue represents Pdpn; red, LyveI; and green, EYFP). Scale bar represents 100 μm. (E) Three-dimensional rendering of boxed region in panel D showing lymph vessel connection to the SCS (red represents LyveI; and green, EYFP). Scale bar represents 20 μm.
Transgenic IL-7–expression during LN reconstruction after avascular transplantation. LNs from IL-7–CrexR26-EYFP donor mice were transplanted under the kidney capsule of C57BL/6 mice and harvested 8 weeks after transplantation. (A) Mosaic scan of a regenerated LN (tLN) developing on the kidney (Ki; blue represents B220; red, Pdpn; and green, EYFP). Scale bar represents 200 μm. (B) Three-dimensional reconstruction of subcapsular sinus region (red represents LyveI; and green, EYFP). Scale bar represents 50 μm. Arrows indicate transgene-positive LECs. (C) Three-dimensional reconstruction of T-cell zone region (red represents Pdpn; and green, EYFP). Scale bar represents 50 μm. Arrows indicate transgene-positive FRCs. (D) Lymphatic vessel connecting tLN with surrounding tissue (blue represents Pdpn; red, LyveI; and green, EYFP). Scale bar represents 100 μm. (E) Three-dimensional rendering of boxed region in panel D showing lymph vessel connection to the SCS (red represents LyveI; and green, EYFP). Scale bar represents 20 μm.
Assessment of stromal cell proliferation during posttransplantation LN reconstruction. (A) Stromal cells from pretransplantation (naive, gray line) inguinal LNs and reconstructed LNs (black line) were analyzed for EYFP expression on CD45−Pdpn+ stromal cells by flow cytometry. (B) Percentage of EYFP-expressing stromal cells within CD45− cells (mean ± SEM; n = 6 LNs). **P < .01. (C) IL-7 mRNA expression as determined by RT-PCR analysis from CD45− cells (mean ± SEM; n = 3 LNs). *P < .05. (D) Ki67 expression in transplanted IL-7–CrexR26-EYFP LNs. Left panels: Ki67+ LECs (arrow). Right panels: Ki67+ FRCs (blue represents Ki67; red, LyveI; and green, EYFP). Scale bar represents 10 μm. (E) Percentage of Ki67+ transgene-expressing cells in naive and reconstructed LNs. Evaluation of 10 high power fields per LN (mean ± SEM; n = 4 LNs). *P < .05.
Assessment of stromal cell proliferation during posttransplantation LN reconstruction. (A) Stromal cells from pretransplantation (naive, gray line) inguinal LNs and reconstructed LNs (black line) were analyzed for EYFP expression on CD45−Pdpn+ stromal cells by flow cytometry. (B) Percentage of EYFP-expressing stromal cells within CD45− cells (mean ± SEM; n = 6 LNs). **P < .01. (C) IL-7 mRNA expression as determined by RT-PCR analysis from CD45− cells (mean ± SEM; n = 3 LNs). *P < .05. (D) Ki67 expression in transplanted IL-7–CrexR26-EYFP LNs. Left panels: Ki67+ LECs (arrow). Right panels: Ki67+ FRCs (blue represents Ki67; red, LyveI; and green, EYFP). Scale bar represents 10 μm. (E) Percentage of Ki67+ transgene-expressing cells in naive and reconstructed LNs. Evaluation of 10 high power fields per LN (mean ± SEM; n = 4 LNs). *P < .05.
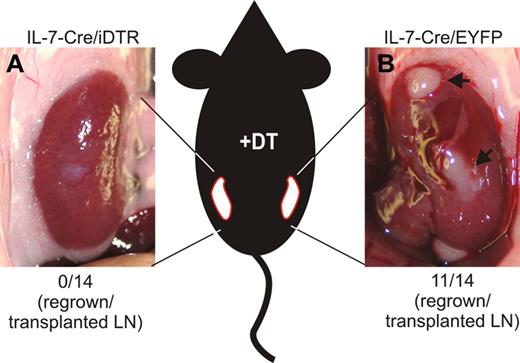
To assess whether IL-7–expressing stromal cells play a critical role during LN remodeling, IL-7–Cre mice were crossed with inducible diphtheria toxin receptor (iDTR) mice26 that permit DT-mediated ablation of cells in a Cre recombinase–dependent manner. Inguinal LNs from IL-7–Cre/iDTR mice were transplanted under the kidney capsule at one side of C57BL/6 recipients, and IL-7–CrexR26-EYFP control LNs were transplanted under the kidney capsule of the contralateral side (Figure 7). From weeks 4-8 after transplantation, recipients received DT twice weekly, hence permitting ablation of IL-7–expressing stromal cells. None of the 14 transplanted IL-7–Cre/iDTR LN grew (Figure 7A), whereas 11 of 14 IL-7–CrexR26-EYFP LNs successfully regenerated (Figure 7B). These results indicate that IL-7–expressing stromal cells critically contribute to adaptive LN remodeling.
Importance of IL-7–expressing stromal cells for LN regeneration. Inguinal LNs of IL-7–CrexR26-iDTR and IL-7–CrexR26-EYFP mice were transplanted under the kidney capsule of C57BL/6 mice. Between weeks 4 and 8 after transplantation, recipients were treated with DT (20 ng/g body weight) twice weekly. (A) LN remnants of IL-7–CrexR26-iDTR LNs. None of the 14 transplanted LNs had regenerated. (B) Fully regenerated IL-7–CrexR26-EYFP LNs from the contralateral kidney. Eleven of 14 transplanted LNs had regenerated.
Importance of IL-7–expressing stromal cells for LN regeneration. Inguinal LNs of IL-7–CrexR26-iDTR and IL-7–CrexR26-EYFP mice were transplanted under the kidney capsule of C57BL/6 mice. Between weeks 4 and 8 after transplantation, recipients were treated with DT (20 ng/g body weight) twice weekly. (A) LN remnants of IL-7–CrexR26-iDTR LNs. None of the 14 transplanted LNs had regenerated. (B) Fully regenerated IL-7–CrexR26-EYFP LNs from the contralateral kidney. Eleven of 14 transplanted LNs had regenerated.
Discussion
The present analysis revealed substantial Il7 mRNA expression in LN LECs and showed biologically relevant IL-7 protein production in human LECs together with the murine IL-7 surrogate marker expression in vivo. Our data indicate that IL-7–producing LECs, together with T-cell zone FRCs, participate in the structural adaptation of the LN microenvironment during virus-induced LN remodeling. Furthermore, the presence of IL-7–producing stromal cells was mandatory for successful reestablishment of LN structure after avascular transplantation.
During embryonic LN development, IL-7 directly acts on the survival of LTi cells and their fetal liver progenitors.13,17 LTi cells recruit and stimulate VCAM-1+ mesenchymal lymphoid tissue organizer cells via LTβR and thereby foster LN development and organization.14 In embryos of IL-7–Cre mice, transgene expression was observed as early as E13.5-E15.5 (ie, the time frame of first cluster formation between LTi cells and mesenchymal organizer cells). Early LyveI+ lymph sac cells give rise to both mesenchymal organizer cells and LECs during later LN development.35 The stromal cell progenitor fate-mapping analysis in embryonic and neonatal LNs, as revealed by the IL-7–Cre transgene, showed that IL-7 promoter activity was almost exclusively restricted to the LEC differentiation pathway. We therefore conclude that IL-7 expression in FRCs, as shown here (and by Link et al20 ) appears to be a process that is restricted to later stages of LN development (ie, during colonization of LNs by lymphocytes and during adaptive remodeling processes). It is interesting to note that the IL-7 expression pattern in adult homeostatic LNs revealed by our IL-7–Cre reporter is comparable with that obtained with a very recently developed IL-7–GFP knock-in reporter36 (ie, pronounced IL-7 promoter activity in LN LECs). In the combination of the 2 models, a fate-mapping analysis will permit a better resolution of the population dynamics of IL-7–producing stromal cells during LN development and adaptive remodeling.
Within adult LNs, LECs form subcapsular, cortical, and medullary sinuses.37 The LN LEC network extends into tissues, such as the skin, where LECs form specialized portals for leukocyte entry.38 Leukocyte migration within lymphatic vessels is regulated by LEC-expressed CCL21 and, in larger lymph vessels, by the lymph flow itself.39 Likewise, LN LECs in the cortical sinuses regulate intranodal lymphocyte trafficking by collecting lymphocytes for further transit to medullary sinuses and eventual LN egress.40 Importantly, there is also frequent transit of lymphocytes from the lymphatic sinuses back to the LN parenchyma.40 Along these lines, a recent study has shown that recirculating, lymph-borne lymphocytes enter the LN parenchyma mainly via medullary sinuses.41 Thus, IL-7Rα+ lymphocytes are in frequent contact with LECs during entrance to the LN and during their intranodal migration. Our finding that LECs represent a prominent source of IL-7 thus supports the concept that IL-7 is provided locally and “on demand” to best facilitate regulation of lymphocyte homeostasis.19 Likewise, other leukocyte populations, such as γδ T cells21,22 or LTi cells, which reside in the LEC-rich interfollicular regions of the LN,23 probably also use LEC-derived IL-7 and thereby receive antiapoptotic and stimulatory signals to maintain their survival.
Reorganization of lymphatic sinus structures during inflammatory processes impinges on intranodal lymphocyte dynamics.40 We found that IL-7–expressing cortical sinus LECs contribute significantly to virus-induced LN remodeling, supporting the notion that the subcapsular sinus region is of particular importance during the acute phase of adaptive antiviral immune responses.42,43 Thus, IL-7Rα+ lymphocytes that arrive to the subcapsular sinus and/or are primed in this region may benefit in particular from LEC-derived IL-7.
With the entrance of pathogens into the LN, the interplay between nonhematopoietic stromal cells and hematopoietic immune cells changes dramatically. For example, viral infection can lead to down-regulation of the stromal cell-derived chemokines CCL19 and CCL21,30,44 resulting in disorganization of the T-cell zone and altered T-DC interaction. Such infection-associated immunopathologic reactions can severely damage the stromal cell network, leading to a transient loss of immunocompetence against secondary infections.30,45 However, during such immunopathologic LN alterations, potent reorganization processes are initiated, including expansion of LTi cells and up-regulation of “LN organizing” genes, such as lymphotoxin, LTβR, and IL-7.30 We found that LECs of reorganizing LNs not only up-regulated IL-7 but also expressed RANKL and LTβR, indicating that LECs provide the appropriate molecular equipment for the maintenance of LN structural integrity.
In conclusion, this study has revealed novel insight into the biology of IL-7–expressing stromal cells: (1) embryonic LN LECs appear to be a key source of IL-7; (2) adult LECs produce substantial amounts of biologically active IL-7; and (3) IL-7–producing stromal cells (FRCs and/or LECs) are critical for adaptive LN remodeling. Future studies with truly LEC- and FRC-specific Cre recombinase-targeting will reveal to which extent the different IL-7–expressing stromal cell populations contribute to LN remodeling and regulation of immune responsiveness. Such mechanistic studies can provide important clinical insight because HIV infection-associated lymphoid tissue fibrosis and impaired IL-7 supply for T cells have been identified as major pathogenic principles in this disease.46 Furthermore, the feasibility of IL-7 treatment to foster T cell recovery in HIV patients47 warrants further investigations on IL-7–producing stromal cells in LNs and other secondary lymphoid organs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Rita de Giuli and Eva Allgäuer for expert assistance, the cell sorting core facility of the University of Zürich, and Peter O'Toole in the Technology Facility at the University of York for technical assistance.
This work was supported by the Swiss National Science Foundation (CRS113_125447 and 130823/1; B.L.), the Human Frontiers Science Program (RGP0006/2009; M.C. and T.C.), and the Medical Research Council (United Kingdom; G0601156).
Authorship
Contribution: L.O. and E.S. performed research and wrote the paper; Q.C., M.I., P.N., and K.H. performed research; E.R. provided mice; C.H., P.K., J.W., and T.C. discussed data; and M.C. and B.L. designed the study and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Burkhard Ludewig, Institute of Immunobiology, Kantonsspital St Gallen, 9007 St Gallen, Switzerland; e-mail: burkhard.ludewig@kssg.ch; and Mark Coles, Centre for Immunology and Infection, Department of Biology, Wentworth Way, University of York, York YO10 5DD, United Kingdom; e-mail: mark.coles@york.ac.uk.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal