Abstract
Inorganic polyphosphates are linear polymers of orthophosphate that modulate blood clotting and inflammation. Polyphosphate accumulates in infectious microorganisms and is secreted by activated platelets; long-chain polyphosphate in particular is an extremely potent initiator of the contact pathway, a limb of the clotting cascade important for thrombosis but dispensable for hemostasis. Polyphosphate inhibitors therefore might act as novel antithrombotic/anti-inflammatory agents with reduced bleeding side effects. Antipolyphosphate antibodies are unlikely because of polyphosphate's ubiquity and simple structure; and although phosphatases such as alkaline phosphatase can digest polyphosphate, they take time and may degrade other biologically active molecules. We now identify a panel of polyphosphate inhibitors, including cationic proteins, polymers, and small molecules, and report their effectiveness in vitro and in vivo. We also compare their effectiveness against the procoagulant activity of RNA. Polyphosphate inhibitors were antithrombotic in mouse models of venous and arterial thrombosis and blocked the inflammatory effect of polyphosphate injected intradermally in mice. This study provides proof of principle for polyphosphate inhibitors as antithrombotic/anti-inflammatory agents in vitro and in vivo, with a novel mode of action compared with conventional anticoagulants.
Introduction
Polyphosphate (polyP) is a linear polymer of inorganic phosphate residues that is widely present in biology.1 Of particular interest to hematology, polyP accumulates in many infectious microorganisms2 and is secreted by activated human platelets3 and mast cells.4 Work from our laboratory and others has shown that polyP is a potent procoagulant, prothrombotic, and pro-inflammatory molecule,5-7 acting at 4 points in the clotting cascade: it triggers clotting via the contact pathway,5,6,8 it accelerates factor V activation,5 it enhances fibrin clot structure,9,10 and it accelerates factor XI back-activation by thrombin.11
The ability of polyP (especially, long-chain polyP of the type found in microorganisms8 ) to trigger clotting via the contact pathway is interesting in light of an elegant series of studies that have shown that the contact pathway is important for thrombosis but dispensable for hemostasis.12-15 We therefore hypothesized that polyP inhibitors might act as novel antithrombotic/anti-inflammatory agents with reduced bleeding side effects. Raising antibodies against polyP is unlikely to be successful because of the ubiquity of polyP and its simple structure. Phosphatases, such as alkaline phosphatase, can digest polyP,6,11 but they take time to act and may degrade other phosphate-containing molecules in addition to polyP. In this study, we identify a panel of polyP inhibitors including cationic proteins, polymers, and small molecules. We report their effectiveness as anticoagulants in vitro and as antithrombotic and anti-inflammatory agents in vivo using mouse models. We also compare the effectiveness of these polyP inhibitors against the procoagulant activity of RNA16 and the anticoagulant activity of heparin. This study therefore provides proof of principle for polyP inhibitors as novel antithrombotic/anti-inflammatory agents that are directed against a unique target in the blood clotting system.
Methods
Materials
Reagents were from Sigma-Aldrich unless otherwise noted. Long-chain synthetic polyP (marketed by Sigma-Aldrich as “phosphate glass, water insoluble”) was differentially solubilized as previously described.8 Its polymer lengths ranged from ∼ 50-1500 phosphates, with a modal length of ∼ 650 phosphates,8 and its endotoxin content was 1.6 × 10−3 units/μg polyP (by Limulus assay; Charles River Laboratories). Biotinylated long-chain polyP was prepared as described.17 All polyP concentrations in this paper are given in terms of the concentration of phosphate monomers (monomer formula: NaPO3).
Other supplies included human platelet factor 4, antithrombin, plasma kallikrein, factor Xa, and α-thrombin (Enzyme Research Laboratories); human factor XI (Haematologic Technologies); pooled normal plasma (George King Bio-Medical); and Sar-Pro-Arg-p-nitroanilide (Bachem). Recombinant polyP-binding domain from Escherichia coli exopolyphosphatase (PPXbd) was produced as described.11 Liposomes made by sonication had 10% phosphatidylserine, 40% phosphatidylethanolamine, and 50% phosphatidylcholine (Avanti Polar Lipids). Recombinant human tissue factor was relipidated as described.18
Inhibition of polyP binding to thrombin
Other than the high-throughput screens, thrombin binding to immobilized biotinylated polyP in streptavidin-coated, 96-well microplates was performed essentially as previously described.17 Briefly, 35nM human α-thrombin was incubated with candidate inhibitor in 20mM HEPES NaOH, pH 7.4, 50mM NaCl, 0.1% BSA, 0.05% Tween-20, 0.05% NaN3 for 1 hour in wells containing biotin-polyP. After washing, thrombin was quantified by cleavage of 400μM Sar-Pro-Arg-p-nitroanilide (Bachem).
High-throughput screening assay
High-throughput screens were conducted at the High-Throughput Screening Facility (HTSF) at the University of Illinois. High-binding 384-well plates (Corning) were coated overnight at room temperature with 50 μL/well of 10 μg/mL avidin (Invitrogen) in water, then washed twice with 100 μL/well of TBS (50mM Tris-HCl buffer, pH 7.4, 100mM NaCl, 0.05% NaN3) containing 0.05% Tween-20. Biotinylated polyP was then immobilized on the wells (and, in the process, the wells were simultaneously blocked with BSA) by incubating the wells for 3 hours at room temperature with 50 μL/well of 20μM biotin-polyP in TBS plus 1% BSA and 0.05% Tween-20. The wells were washed twice with 100 μL/well of 1M LiCl, followed by 2 water washes. Each well then received 60 μL of storage buffer (50mM Tris-HCl buffer, pH 7.4 + 0.05% NaN3), and the plates were stored at room temperature until needed. Thrombin-binding assays were performed by removing the storage buffer and dispensing 50 μL/well of 40nM bovine thrombin (BioPharm Laboratories) in 20mM HEPES-NaOH buffer, pH 7.4, 50mM NaCl, 1.4mM CaCl2, 0.5mM MgCl2, 0.05% Tween-20, 0.05% NaN3, 0.1% BSA. The wells then received 100-nL aliquots of compounds to be tested. (To decrease the number of plates screened, 5-7 compounds were pooled [always within libraries] and added per well, at 100 nL of each compound per well. Final concentrations in test wells were 1 μg/mL of each compound for the Chembridge compounds, or 2μM of each compound for the NCI/Marvel/HTSF compounds.) Some wells received no compounds, which served as reference wells for the level of thrombin bound in the absence of any inhibitor. The plates were incubated for a minimum of 30 minutes (maximum of 3 hours) at room temperature, after which they were washed thrice. Each well then received 50 μL/well of 0.4mM chromogenic thrombin substrate (Sar-Pro-Arg-pNA) diluted in 20mM HEPES-NaOH buffer, pH 7.4, 0.05% NaN3. The wells were incubated for 1.8 hours at room temperature, after which the reaction was quenched with 25 μL/well of 0.1N HCl, and the absorbance at 405 nm was quantified. (Control experiments indicated that the rate of chromogenic substrate hydrolysis remained linear over the 1.8-hour time course, under the conditions tested.)
Compound libraries screened
Detailed descriptions of all 4 of the libraries screened in this study are available at HTSF web site (http://scs.illinois.edu/htsf/compound_collections.html), including comprehensive structural information files for the compounds in each library, readable by the ChemBioFinder program. The libraries screened in this study, which together included ∼ 175 000 compounds, were: (1) ChemBridge MicroFormat Library (∼ 150 000 compounds); (2) HTSF House Library (∼ 4700 compounds); (3) Marvel Library (∼ 10 000 compounds); and (4) NCI Library, composed of (a) Open Set (∼ 8000 compounds), (b) Diversity Set (∼ 2000 compounds), and (c) Natural Products and Challenge Set (∼ 300 compounds).
Inhibition of heparin-catalyzed inactivation of factor Xa by antithrombin
Antithrombin (120nM), unfractionated heparin (1.5 × 10−2 units/mL), and candidate inhibitor were incubated at room temperature for 2 minutes with 4.6nM human factor Xa in 30mM HEPES NaOH, pH 7.4, 100mM NaCl, 0.1% BSA. Factor Xa activity was quantified by hydrolysis of 250μM Spectrozyme Xa substrate (American Diagnostica) and converted to percent heparin activity by reference to a standard curve.
Plasma clotting assays
Plasma clotting times were quantified at 37°C using a STart4 coagulometer (Diagnostica Stago). Contact pathway tests used final concentrations of 33% plasma, 25μM liposomes, 41.7mM imidazole, pH 7.0, and 8.33mM CaCl2. Contact activator, inhibitor, and liposomes were mixed with prewarmed plasma for 3 minutes; then clotting was initiated with CaCl2. Activator concentrations were selected to give 80- to 100-second clotting times: 10 μg/mL long-chain polyP, 10 μg/mL kaolin, 100 μg/mL diatomaceous earth, or 100 μg/mL polyguanylic acid (RNA). Tissue factor clotting tests used 30pM relipidated tissue factor.
Whole blood thromboelastometry
Human blood studies, conducted in accordance with the Declaration of Helsinki, were approved by the University of Illinois Institutional Review Board. Thromboelastometry was performed using ROTEM (Pentapharm) with supplied software (ROTEM Gamma Version 1.1). Nonanticoagulated whole blood was collected via venipuncture (discarding the initial 3 mL) from normal human donors, then immediately transferred to the supplied plastic cups (280 μL per cup) and thoroughly mixed with 20 μL candidate inhibitor in TBS plus either 20 μL 1.7mM long-chain polyP in TBS or tissue factor reagent (Ex-tem, Pentapharm). Final concentrations were 82% whole blood, 0 or 100μM polyP, and inhibitor as indicated.
In vivo thrombus formation
Animal studies were approved by the Institutional Animal Care and Use Committee of the Medical College of Wisconsin (venous thrombosis) or the University of Illinois (arterial thrombosis). For venous thrombosis, electrolytic injuries were induced on exposed femoral veins of pentobarbital-anesthetized C57BL/6 mice, as described.19 Three to 5 minutes before thrombus induction, rhodamine 6G-labeled platelets (up to 1 × 107 per mouse) and Alexa-647–labeled antifibrin antibodies (10-20 μg per mouse) were injected into the jugular vein at volumes up to 100 μL, followed by inhibitor, unfractionated heparin (APP Pharmaceuticals) or vehicle; fluorescence imaging of the thrombus induction site was then recorded for 60 minutes. Data among groups were analyzed by 1-way ANOVA, with between-group comparisons using P values calculated from posthoc Tukey test. For arterial thrombosis, C57BL/6 male mice (6-8 weeks old) were anesthetized using isoflurane, polyP inhibitors were injected retro-orbitally, the left carotid artery was exposed, and blood flow monitored with a Doppler vascular flow probe (Transonic, 0.5 PSB) connected to a perivascular flow meter (Transonic, TS420). To induce thrombosis, 2 pieces of 1 × 2-mm filter paper (Whatman GB003) saturated with freshly prepared 5% anhydrous FeCl3 in 0.9% saline were applied to the deep and superficial surfaces of the artery. After 5 minutes, the filter papers were removed and the vessel irrigated with saline. Blood flow was monitored from FeCl3 application for 30 minutes or until occlusion, defined as no detectable flow for 1 minute. Flow data were interpreted with LabScribe2 (iWorx Systems).
In vivo vascular leakage
Vascular leakage assays were used to quantify polyP-induced extravasation of Evans blue dye in animal studies approved by the University of Illinois Institutional Animal Care and Use Committee. Wild-type ICR mice (Harlan Laboratories) anesthetized with isoflurane were injected retro-orbitally with 4% Evans blue in saline (1 μL/g body weight). PolyP inhibitors or saline were administered retro-orbitally (contralateral eye). After 40 minutes, 3 dorsal skin locations were injected intradermally with 25 μL of saline (negative control), 100μM bradykinin (positive control), or 20mM long-chain polyP. After 30 minutes, animals were killed, skins removed for punch biopsy (12-mm diameter), and Evans blue quantified as described.6 Data were compared between groups by Mann-Whitney rank-sum test.
Results
High-throughput screening for polyP inhibitors
We previously showed that polyP binds tightly to thrombin,20 a central protease in blood clotting. We used this interaction to develop a high-throughput screen for polyP inhibitors by first immobilizing biotinylated polyP on avidin-coated multiwell plates, then incubating wells with mixtures of thrombin and potential inhibitors, washing, and quantifying relative amounts of bound thrombin by chromogenic substrate hydrolysis. Candidate inhibitors were identified from high-throughput screening of a library of ∼ 175 000 small molecules as well as a panel of 42 additional cationic compounds, polymers and proteins chosen for their possible association with a polyanion like polyP. For the latter panel (listed in Table 1), we hypothesized that cationic substances would bind to polyP, thereby competitively inhibiting its interaction with clotting proteins.
Panel of cationic compounds, polymers, and proteins
| Compound . | No.* . | IC50 (or % inhibition)* . |
|---|---|---|
| Low MW polyethyleneimine | 1 | 10 ng/mL |
| Polybrene | 2 | 19 ng/mL |
| High MW polyethyleneimine | 3 | 35 ng/mL |
| Generation 3.0 dendrimer | 40 ng/mL | |
| Generation 2.0 dendrimer | 47 ng/mL | |
| Norspermine | 52 ng/mL | |
| Spermine | 4 | 57 ng/mL |
| Generation 1.0 dendrimer | 5 | 58 ng/mL |
| Histone H1 | 6 | 59 ng/mL |
| Poly-D-lysine | 79 ng/mL | |
| Poly-L-arginine | 81 ng/mL | |
| Protamine sulfate | 8 | 0.11 μg/mL |
| Poly-L-lysine | 0.14 μg/mL | |
| Generation 0.0 dendrimer | 0.14 μg/mL | |
| Heparin | 0.27 μg/mL | |
| Polymyxin B | 9 | 0.38 μg/mL |
| Histone H2A/H2B dimer | 0.38 μg/mL | |
| Histone H2B | 0.46 μg/mL | |
| Histone H3.1/H4 tetramer | 0.53 μg/mL | |
| Histone H4 | 0.84 μg/mL | |
| Colistin | 0.91 μg/mL | |
| Histones (mixed) | 0.93 μg/mL | |
| Histone H2A | 1.3 μg/mL | |
| Spermidine | 1.7 μg/mL | |
| Histone H3.1 | 2.5 μg/mL | |
| Human platelet factor 4 | 10 | 3.8 μg/mL |
| PPXbd | 11 | 8.5 μg/mL |
| Lon protease polyP-binding domain | 72 μg/mL | |
| 1,3-diaminopropane | 0.47 mg/mL | |
| Ethylenediamine | 0.89 mg/mL | |
| Norspermidine | (62% inhibition at 200 μg/mL) | |
| 1,2-bis(3-aminopropylamino)ethane | (53% inhibition at 200 μg/mL) | |
| Kanamycin | (48% inhibition at 200 μg/mL) | |
| DNase I | (36% inhibition at 200 μg/mL) | |
| RNase A | (23% inhibition at 100 μg/mL) | |
| Bacitracin | (12% inhibition at 200 μg/mL) | |
| Methylenediamine | (73% inhibition at 6 mg/mL) | |
| Ammonium chloride | (44% inhibition at 2.7 mg/mL) | |
| Melamine | (0% inhibition at 200 μg/mL) | |
| Cystamine | (0% inhibition at 200 μg/mL) | |
| Histamine | (0% inhibition at 200 μg/mL) | |
| Histidine | (0% inhibition at 200 μg/mL) |
| Compound . | No.* . | IC50 (or % inhibition)* . |
|---|---|---|
| Low MW polyethyleneimine | 1 | 10 ng/mL |
| Polybrene | 2 | 19 ng/mL |
| High MW polyethyleneimine | 3 | 35 ng/mL |
| Generation 3.0 dendrimer | 40 ng/mL | |
| Generation 2.0 dendrimer | 47 ng/mL | |
| Norspermine | 52 ng/mL | |
| Spermine | 4 | 57 ng/mL |
| Generation 1.0 dendrimer | 5 | 58 ng/mL |
| Histone H1 | 6 | 59 ng/mL |
| Poly-D-lysine | 79 ng/mL | |
| Poly-L-arginine | 81 ng/mL | |
| Protamine sulfate | 8 | 0.11 μg/mL |
| Poly-L-lysine | 0.14 μg/mL | |
| Generation 0.0 dendrimer | 0.14 μg/mL | |
| Heparin | 0.27 μg/mL | |
| Polymyxin B | 9 | 0.38 μg/mL |
| Histone H2A/H2B dimer | 0.38 μg/mL | |
| Histone H2B | 0.46 μg/mL | |
| Histone H3.1/H4 tetramer | 0.53 μg/mL | |
| Histone H4 | 0.84 μg/mL | |
| Colistin | 0.91 μg/mL | |
| Histones (mixed) | 0.93 μg/mL | |
| Histone H2A | 1.3 μg/mL | |
| Spermidine | 1.7 μg/mL | |
| Histone H3.1 | 2.5 μg/mL | |
| Human platelet factor 4 | 10 | 3.8 μg/mL |
| PPXbd | 11 | 8.5 μg/mL |
| Lon protease polyP-binding domain | 72 μg/mL | |
| 1,3-diaminopropane | 0.47 mg/mL | |
| Ethylenediamine | 0.89 mg/mL | |
| Norspermidine | (62% inhibition at 200 μg/mL) | |
| 1,2-bis(3-aminopropylamino)ethane | (53% inhibition at 200 μg/mL) | |
| Kanamycin | (48% inhibition at 200 μg/mL) | |
| DNase I | (36% inhibition at 200 μg/mL) | |
| RNase A | (23% inhibition at 100 μg/mL) | |
| Bacitracin | (12% inhibition at 200 μg/mL) | |
| Methylenediamine | (73% inhibition at 6 mg/mL) | |
| Ammonium chloride | (44% inhibition at 2.7 mg/mL) | |
| Melamine | (0% inhibition at 200 μg/mL) | |
| Cystamine | (0% inhibition at 200 μg/mL) | |
| Histamine | (0% inhibition at 200 μg/mL) | |
| Histidine | (0% inhibition at 200 μg/mL) |
Each substance was tested at 200 μg/mL (or indicated concentrations) for inhibition of thrombin binding to immobilized biotin-polyP. Those exhibiting > 70% inhibition were retested at various inhibitor concentrations, from which IC50 values were derived (listed here in order of decreasing potency).
Numbers are the compound numbers used in Figure 2.
Nine of the tested wells from the initial high-throughput screen of ∼ 175 000 compounds exhibited a ≥ 30% decrease in the rate of chromogenic substrate hydrolysis and were therefore flagged as containing potential inhibitors of thrombin-polyP binding. The 46 individual compounds in these 9 wells were retested singly in secondary screens to identify the actual inhibitors responsible for the reduction in thrombin binding. The secondary screens consisted of: (1) a repeat of the thrombin-polyP binding assay; (2) a kallikrein-polyP binding assay in which 100nM human plasma kallikrein (Enzyme Research Laboratories) was substituted for thrombin and H-D-Pro-Phe-Arg-pNA substrate (Bachem) was used to detect bound kallikrein; (3) a test of direct thrombin inhibition, in which the compound was added directly to 1nM thrombin in solution, and the rate of chromogenic substrate hydrolysis was quantified; and (4) a test of direct kallikrein inhibition, in which the compound was added directly to kallikrein in solution, and the rate of chromogenic substrate hydrolysis was quantified. The purpose of the parallel assays of both thrombin-polyP and kallikrein-polyP binding was to identify compounds that could inhibit the association of polyP with 2 different polyP-binding proteins, the idea being that this would identify compounds with a general ability to inhibit polyP-protein interactions (presumably via the compound binding to polyP). The purpose of the test of direct inhibition of thrombin and kallikrein was to identify compounds that decreased the measured signal, not by blocking polyP-thrombin or polyP-kallikrein binding, but by inhibiting the enzymatic activity of these proteases, even in the absence of polyP.
Eight of the tested compounds showed reduced signal in one or more of these secondary assays, the results of which are summarized in Table 2. Four of the compounds (numbered A, B, D, and G) exhibited < 20% inhibition in the thrombin-polyP binding assay and were therefore not promising leads to explore further. The other 4 compounds in Table 2 (numbered C, E, F, and H) exhibited > 90% inhibition in the thrombin-polyP binding assay. Of these latter 4 compounds, 3 (C, E, and F) inhibited the enzymatic activity of free thrombin by > 98%, one of which (C) also inhibited the enzymatic activity of free kallikrein by 97%. Thus, these 3 compounds are direct protease inhibitors and apparently did not decrease the signal in the thrombin-polyP binding assay by actually interfering with thrombin binding to polyP. One of the compounds, surfen (compound H), decreased the signal in the thrombin-polyP binding assay by 95% and the signal in the kallikrein-polyP binding assay by 55%, while having a modest effect on the enzymatic activity of free thrombin. Thus surfen, which is also reported to be a small-molecule antagonist of heparin and heparan sulfate,21 was chosen for further analysis.
Secondary screening of 8 compounds identified from the high-throughput screen of ∼ 175 000 compounds
| No. . | Chemical name . | Library . | Concentration tested . | Percent inhibition of: . | |||
|---|---|---|---|---|---|---|---|
| Thrombin binding to polyP . | Kallikrein binding to polyP . | Free thrombin activity . | Free kallikrein activity . | ||||
| A | N-benzyl-2,2,6,6-tetramethyl-4-piperidinamine dihydrochloride | Chembridge | 3 μg/mL | 0 | 0 | 36.1 | NT |
| B | [(6-nitro-1,3-benzodioxol-5-yl)methylene]malononitrile | Chembridge | 10 μg/mL | 17.5 | 0 | 11.1 | 2.5 |
| C | 2-(allylthio)-1-(1,3-benzodioxol-5-ylcarbonyl)-1H-benzimidazole | Chembridge | 10 μg/mL | 95.1 | 81.4 | 99.6 | 97.4 |
| D | N-(2,4-dichlorophenyl)-N′-{5-[(4-nitrophenoxy) methyl]-1,3,4-thiadiazol-2-yl}urea | Chembridge | 10 μg/mL | 0 | 0 | 5.3 | 1.5 |
| E | 1-(3,4-dimethoxybenzoyl)-3-(2-furyl)-1H-1,2,4-triazol-5-amine | Chembridge | 10 μg/mL | 98.6 | 18.8 | 98.9 | 39.3 |
| F | 5-(benzylthio)-1-butyryl-3-phenyl-1H-1,2,4-triazole | Chembridge | 10 μg/mL | 92.8 | 9.6 | 99.5 | 12.3 |
| G | 2-(3-nitrophenyl)-3,1-benzoxazin-4(4H)-one | NCI Open Plate Set | 2μM | 2.0 | 0 | 3.8 | 0.6 |
| H | Surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide) | NCI Diversity Set | 2μM | 95.6 | 55.3 | 32.2 | NT |
| No. . | Chemical name . | Library . | Concentration tested . | Percent inhibition of: . | |||
|---|---|---|---|---|---|---|---|
| Thrombin binding to polyP . | Kallikrein binding to polyP . | Free thrombin activity . | Free kallikrein activity . | ||||
| A | N-benzyl-2,2,6,6-tetramethyl-4-piperidinamine dihydrochloride | Chembridge | 3 μg/mL | 0 | 0 | 36.1 | NT |
| B | [(6-nitro-1,3-benzodioxol-5-yl)methylene]malononitrile | Chembridge | 10 μg/mL | 17.5 | 0 | 11.1 | 2.5 |
| C | 2-(allylthio)-1-(1,3-benzodioxol-5-ylcarbonyl)-1H-benzimidazole | Chembridge | 10 μg/mL | 95.1 | 81.4 | 99.6 | 97.4 |
| D | N-(2,4-dichlorophenyl)-N′-{5-[(4-nitrophenoxy) methyl]-1,3,4-thiadiazol-2-yl}urea | Chembridge | 10 μg/mL | 0 | 0 | 5.3 | 1.5 |
| E | 1-(3,4-dimethoxybenzoyl)-3-(2-furyl)-1H-1,2,4-triazol-5-amine | Chembridge | 10 μg/mL | 98.6 | 18.8 | 98.9 | 39.3 |
| F | 5-(benzylthio)-1-butyryl-3-phenyl-1H-1,2,4-triazole | Chembridge | 10 μg/mL | 92.8 | 9.6 | 99.5 | 12.3 |
| G | 2-(3-nitrophenyl)-3,1-benzoxazin-4(4H)-one | NCI Open Plate Set | 2μM | 2.0 | 0 | 3.8 | 0.6 |
| H | Surfen (bis-2-methyl-4-amino-quinolyl-6-carbamide) | NCI Diversity Set | 2μM | 95.6 | 55.3 | 32.2 | NT |
NT indicates not tested.
It was perhaps surprising that this relatively large screen identified just a single compound (surfen) that inhibited the binding of both thrombin and kallikrein to immobilized polyP under the conditions tested. A possible explanation is that the screen was performed at rather low concentrations of test compounds (typically, 1 μg/mL), so that only very potent inhibitors could be identified. In addition, the libraries used in this study might not include simple polyamines; and in any case, they did not include polymers or proteins. Given the simple, repeating structure of polyP and its highly anionic nature, cationic polymers and proteins might be expected to bind tightly to polyP and thereby abrogate its procoagulant functions. Accordingly, we screened the additional panel of 42 cationic proteins, polymers, and small molecules for ability to inhibit polyP-thrombin interactions. The majority of the cationic substances in this panel inhibited thrombin binding to polyP, with the results summarized in Table 1.
In vitro potency of polyP inhibitors
Potencies of prospective polyP inhibitors were determined using thrombin-polyP binding assays (example inhibition curves in Figure 1). IC50 values for the 21 most potent inhibitors are plotted in Figure 2A on the basis of both mass and molarity. Of these, surfen was identified from the high-throughput screen and the rest came from the panel of 42 cationic compounds, polymers and proteins. On a mass basis, low molecular weight (MW) polyethyleneimine was the most potent (IC50, 10 ng/mL), whereas the recombinant, isolated PPXbd22 was the least potent (IC50, 8.5 μg/mL). On a molar basis, poly-L-lysine was the most potent (IC50, 0.49nM), whereas spermidine was the least potent (IC50, 11.7μM). Four generations of cationic poly(amido amine) (PAMAM) dendrimers, with ethylenediamine cores and terminal NH2 groups, all inhibited polyP binding to thrombin.
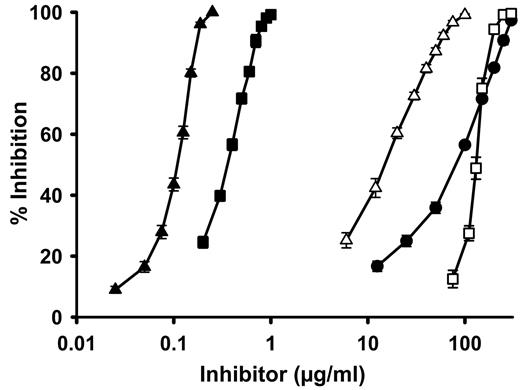
Examples of plots of inhibition of thrombin binding to immobilized polyP for 4 selected inhibitors. The percent inhibition of thrombin binding to polyP is plotted for the following inhibitors that encompassed a range of IC50 values: low MW polyethyleneimine (▿), generation 1.0 PAMAM dendrimer (■), polymyxin B (□), and spermidine (▴). The dotted line represents 50% inhibition. Data are mean ± SE (n = 3).
Examples of plots of inhibition of thrombin binding to immobilized polyP for 4 selected inhibitors. The percent inhibition of thrombin binding to polyP is plotted for the following inhibitors that encompassed a range of IC50 values: low MW polyethyleneimine (▿), generation 1.0 PAMAM dendrimer (■), polymyxin B (□), and spermidine (▴). The dotted line represents 50% inhibition. Data are mean ± SE (n = 3).
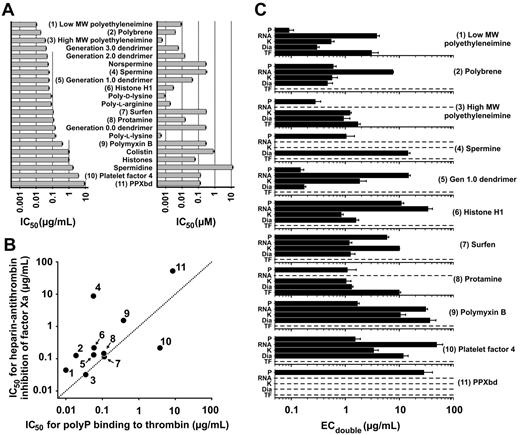
Relative potencies of polyP inhibitors. (A) Inhibitor concentrations resulting in 50% reduction of thrombin binding to immobilized polyP (IC50) are plotted for the 21 most potent substances tested, expressed in terms of mass (left) and molarity (right). Inhibitors that were also used in panels B and C are numbered in parentheses. Data are mean ± SE (n = 3). (B) Plot of IC50 values of the 11 numbered substances from panel A for inhibition of heparin-mediated inactivation of factor Xa by antithrombin (y-axis) versus inhibition of thrombin binding to immobilized polyP (x-axis). Dotted line represents equivalent potency. Data are mean ± bidirectional SE (although error bars are within the symbols; n = 3). (C) Effectiveness of polyP inhibitors in prolonging clotting. Clotting of human plasma was initiated by long-chain polyP (P), polyguanylic acid (RNA), kaolin (K), diatomaceous earth (Dia), or tissue factor (TF). Data are mean inhibitor concentrations that doubled the clotting time relative to no inhibitor (ECdouble) ± SE (n = 4). Horizontal dotted lines indicate that the clotting time with that initiator was either unaffected by the inhibitor or was not prolonged sufficiently to reach a doubling point, even at 100 μg/mL inhibitor.
Relative potencies of polyP inhibitors. (A) Inhibitor concentrations resulting in 50% reduction of thrombin binding to immobilized polyP (IC50) are plotted for the 21 most potent substances tested, expressed in terms of mass (left) and molarity (right). Inhibitors that were also used in panels B and C are numbered in parentheses. Data are mean ± SE (n = 3). (B) Plot of IC50 values of the 11 numbered substances from panel A for inhibition of heparin-mediated inactivation of factor Xa by antithrombin (y-axis) versus inhibition of thrombin binding to immobilized polyP (x-axis). Dotted line represents equivalent potency. Data are mean ± bidirectional SE (although error bars are within the symbols; n = 3). (C) Effectiveness of polyP inhibitors in prolonging clotting. Clotting of human plasma was initiated by long-chain polyP (P), polyguanylic acid (RNA), kaolin (K), diatomaceous earth (Dia), or tissue factor (TF). Data are mean inhibitor concentrations that doubled the clotting time relative to no inhibitor (ECdouble) ± SE (n = 4). Horizontal dotted lines indicate that the clotting time with that initiator was either unaffected by the inhibitor or was not prolonged sufficiently to reach a doubling point, even at 100 μg/mL inhibitor.
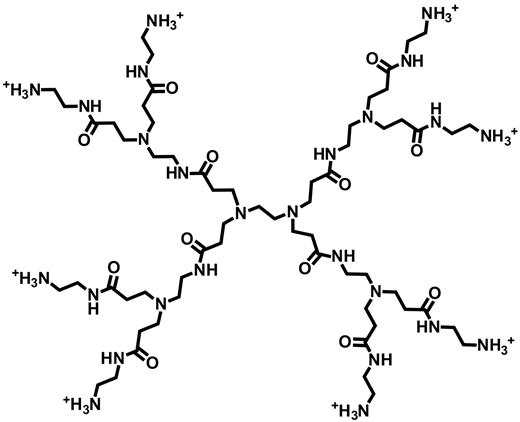
We chose 11 of the inhibitors for further scrutiny for the following reasons (with the compound numbers here in parentheses being the same as those in Figure 2A): low (inhibitor 1) and high (inhibitor 3) MW polyethyleneimine are among the most potent inhibitors; polybrene (inhibitor 2) is commonly included in prothrombin time clotting tests to inactivate heparin; spermine (inhibitor 4) is an endogenous polyamine that might modulate polyP function; polyP interacts with histone H1 (inhibitor 6) to modulate its procoagulant activities23 ; surfen (inhibitor 7) is a small-molecule antagonist of heparin and heparan sulfate21 ; intravenous protamine (inhibitor 8) is used clinically to reverse heparin anticoagulation24 ; polymyxin B (inhibitor 9) is a clinical antibiotic that targets bacterial lipopolysaccharide (but perhaps polyP is an additional target of this drug, and furthermore, Salmonella lacking polyP kinase exhibit increased polymyxin B sensitivity25 ); platelet factor 4 (inhibitor 10) is secreted from activated platelets and neutralizes heparin26 ; and PPXbd (inhibitor 11) potently blocks polyP-mediated factor XI activation by thrombin.11 In addition, cationic PAMAM dendrimers have received much recent attention for nanoparticle formation and drug delivery; we focused on generation 1.0 dendrimer (inhibitor 5; structure given in Figure 3) because lower generation cationic dendrimers are reportedly less toxic than higher generation dendrimers or amine-functionalized linear polymers.27
Chemical structure of the generation 1.0 cationic PAMAM dendrimer used in this study.
Chemical structure of the generation 1.0 cationic PAMAM dendrimer used in this study.
Heparin, like polyP, is a highly anionic linear polymer (although unlike polyP, heparin is strongly anticoagulant via binding to antithrombin). Cationic inhibitors of polyP might also bind to anionic glycosaminoglycans and thus exhibit complex in vivo activities. On the other hand, heparin and polyP have different anionic groups (sulfates vs phosphates) with different spacing and charge densities, so a given inhibitor might bind preferentially to one or the other polyanion. Figure 2B compares IC50 values of the 11 selected polyP inhibitors, plotted on the y-axis for potency for abrogating factor Xa inactivation by a mixture of heparin and antithrombin, and on the x-axis for abrogating thrombin binding to polyP. Compounds interacting more potently with polyP than heparin lie above the dotted line, with the most potent and specific polyP inhibitors in the upper left. Spermine was 152 times more potent against polyP than against heparin/antithrombin, whereas polymyxin B, histone H1, polybrene, low MW polyethyleneimine, and PPXbd were 4-7 times more potent against polyP than against heparin/antithrombin. On the other hand, platelet factor 4 was 18 times less potent against polyP than against heparin/antithrombin.
Potency and specificity of polyP inhibitors in clotting assays
We next evaluated the ability of these 11 inhibitors to block polyP-initiated clotting of human plasma. We expected at least some of these inhibitors to be considerably less effective in plasma clotting assays compared with assays using purified proteins because plasma has many proteins and substances that might compete for binding to these candidate inhibitors. In addition, to initiate plasma clotting, polyP must interact with multiple proteins in the contact pathway, which differ in their affinities for polyP.11,17 To investigate inhibitor potency in human plasma, we therefore quantified the concentrations of inhibitors necessary to double the clotting time of plasma triggered by long-chain polyP, compared with clotting times triggered by other agents known to activate the contact pathway, including RNA,16 powdered kaolin, and diatomaceous earth. Cationic polyP inhibitors might also interfere with downstream clotting reactions (for example, by binding to the anionic lipid, phosphatidylserine); accordingly, we quantified the inhibitor concentration necessary to double the plasma clotting time triggered by relipidated tissue factor, the protein that initiates clotting in normal hemostasis.28 Figure 2C shows that, although low and high MW polyethyleneimine and protamine (inhibitors 1, 3, and 8) inhibited polyP-induced clotting, they also significantly prolonged the tissue factor clotting time (confirming the reported anticoagulant effect of protamine29 ). The other 8 inhibitors tested did not significantly prolong tissue factor clotting times at concentrations up to 100 μg/mL (Figure 2C); of these, generation 1.0 PAMAM dendrimer (inhibitor 5) was the most potent inhibitor of polyP-initiated clotting. Over the concentration range tested, PPXbd (inhibitor 11) inhibited clotting triggered by polyP but not by RNA, kaolin, diatomaceous earth, or tissue factor. Spermine (inhibitor 4) was also a highly specific inhibitor of polyP-triggered plasma clotting, although it had some ability to attenuate clotting triggered by diatomaceous earth. However, because spermine is reported to attenuate platelet aggregation (see “Discussion” for details), we chose not to pursue it as a polyP inhibitor in our in vivo experiments in this study.
Efficacy of polyP inhibitors in whole-blood thromboelastometry
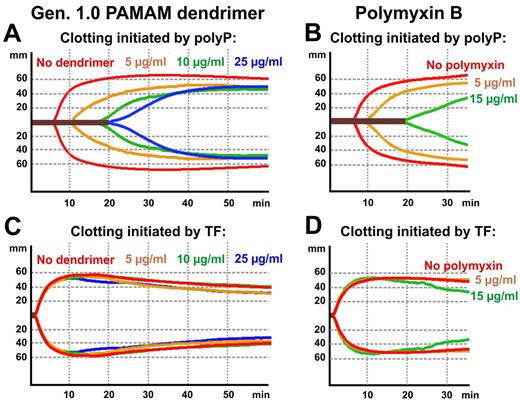
Coagulation of whole blood is more complex than plasma clotting, as it includes contributions from blood cells, including platelets. Consequently, we selected 2 polyP inhibitors (generation 1.0 dendrimer and the antibiotic, polymyxin B) to investigate their effects on polyP-triggered coagulation of whole blood in vitro, by measuring the viscoelastic properties of the developing clot using thromboelastometry. When clotting was initiated by adding long-chain polyP to whole blood, both generation1.0 dendrimer (Figure 4A) and polymyxin B (Figure 4B) prolonged clot formation in a concentration-dependent manner. On the other hand, neither inhibitor, over the same concentration ranges, significantly altered the thromboelastometry profiles of whole blood clotting initiated by tissue factor (Figure 4C-D).
Generation 1.0 dendrimer and polymyxin B inhibit clotting of whole human blood initiated by polyP but not by tissue factor. Thromboelastometry (ROTEM) profiles are given for clotting of freshly drawn, nonanticoagulated whole human blood initiated by long-chain polyP (A-B) or tissue factor (C-D), in the presence of generation 1.0 dendrimer (A,C) or polymyxin B (B,D). The x-axis represents time from addition of clotting trigger; and y-axis, amplitude of clot strength.
Generation 1.0 dendrimer and polymyxin B inhibit clotting of whole human blood initiated by polyP but not by tissue factor. Thromboelastometry (ROTEM) profiles are given for clotting of freshly drawn, nonanticoagulated whole human blood initiated by long-chain polyP (A-B) or tissue factor (C-D), in the presence of generation 1.0 dendrimer (A,C) or polymyxin B (B,D). The x-axis represents time from addition of clotting trigger; and y-axis, amplitude of clot strength.
PolyP inhibitors abrogate the procoagulant activity of platelet polyP
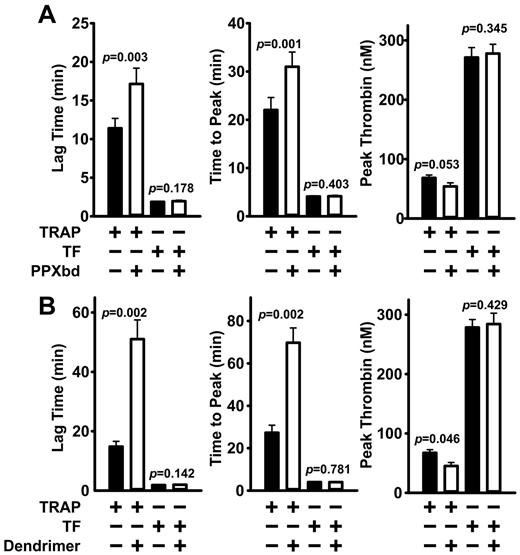
We next investigated the ability of polyP inhibitors to diminish the procoagulant effect of platelet polyP because polyP is known to be secreted by activated platelets.3,6 In the first approach, we added polyP inhibitors to freshly drawn human blood, from which we prepared platelet-rich plasma. We then activated the platelets using a thrombin receptor agonist peptide (TRAP) and quantified thrombin generation in real time. In parallel experiments, we triggered clotting by adding tissue factor to the platelet-rich plasma instead of TRAP. Both PPXbd (Figure 5A) and generation 1.0 dendrimer (Figure 5B) significantly delayed the onset of thrombin generation (lag time) as well as the time to peak thrombin, with only marginal effects on the peak thrombin levels themselves. In contrast, neither polyP inhibitor significantly altered thrombin generation when clotting was triggered with tissue factor.
Thrombin generation. (A) PPXbd and (B) generation 1.0 dendrimer delay thrombin generation in human plasma containing activated platelets. Real-time thrombin generation in plasma was quantified using calibrated automated thrombogram assays (Thrombinoscope; Diagnostica Stago). PPXbd (500 μg/mL), 20 μg/mL dendrimer, or saline was added to freshly drawn, citrated human blood, from which platelet-rich plasma was prepared and the platelet concentration adjusted to 150 000/μL. To some platelet-rich plasmas, TRAP was added at 10μM to activate platelets. After 5 minutes, FluCa reagent (fluorogenic substrate + CaCl2) was added and thrombin generation was quantified. Parallel assays were performed on the same platelet-rich plasmas not pretreated with TRAP, but in which clotting was triggered using FluCa reagent that also contained 5pM tissue factor (TF). Thrombin generation parameters are plotted as mean ± SE (for 5 donors assayed in triplicate). Indicated P values are from paired t tests with and without inhibitor.
Thrombin generation. (A) PPXbd and (B) generation 1.0 dendrimer delay thrombin generation in human plasma containing activated platelets. Real-time thrombin generation in plasma was quantified using calibrated automated thrombogram assays (Thrombinoscope; Diagnostica Stago). PPXbd (500 μg/mL), 20 μg/mL dendrimer, or saline was added to freshly drawn, citrated human blood, from which platelet-rich plasma was prepared and the platelet concentration adjusted to 150 000/μL. To some platelet-rich plasmas, TRAP was added at 10μM to activate platelets. After 5 minutes, FluCa reagent (fluorogenic substrate + CaCl2) was added and thrombin generation was quantified. Parallel assays were performed on the same platelet-rich plasmas not pretreated with TRAP, but in which clotting was triggered using FluCa reagent that also contained 5pM tissue factor (TF). Thrombin generation parameters are plotted as mean ± SE (for 5 donors assayed in triplicate). Indicated P values are from paired t tests with and without inhibitor.
In the second approach, we examined the ability of releasates from activated human platelets to accelerate factor XI activation by thrombin, which we recently showed was entirely the result of the presence of polyP.11 All 5 of the tested polyP inhibitors abrogated platelet releasate-dependent factor XI activation by thrombin (Figure 6); IC50 values were: low MW polyethyleneimine, 107 ± 1.7 ng/mL; generation 1.0 dendrimer, 360 ± 13 ng/mL; spermine, 17.1 ± 1.4 μg/mL; PPXbd, 91.7 ± 2.5 μg/mL; and polymyxin B, 128 ± 2.8 μg/mL (mean ± SE; n = 4).
PolyP inhibitors reverse the ability of platelet releasates to accelerate factor XI activation by thrombin. Initial rates of activation of 30nM human factor XI by 20nM human α-thrombin were determined in the presence of releasate prepared from TRAP-stimulated human platelets as described,11 normalized to the rate of factor XI activation without any added polyP inhibitor. Percent inhibition is plotted versus inhibitor concentration for the following: low MW polyethyleneimine (▴); generation 1.0 dendrimer (■); spermine (▵); PPXbd (●); or polymyxin B (□). Data are mean ± SE (n = 4). IC50 values calculated from these curves are given in the text. In the second stage of the assay, factor XIa levels were quantified, as previously described,11 by monitoring the rate of cleavage of the chromogenic substrate, L-Pyr-Pro-Arg-p-nitroanilide. At the concentrations used, none of the inhibitors altered the rate of hydrolysis of this substrate by factor XIa.
PolyP inhibitors reverse the ability of platelet releasates to accelerate factor XI activation by thrombin. Initial rates of activation of 30nM human factor XI by 20nM human α-thrombin were determined in the presence of releasate prepared from TRAP-stimulated human platelets as described,11 normalized to the rate of factor XI activation without any added polyP inhibitor. Percent inhibition is plotted versus inhibitor concentration for the following: low MW polyethyleneimine (▴); generation 1.0 dendrimer (■); spermine (▵); PPXbd (●); or polymyxin B (□). Data are mean ± SE (n = 4). IC50 values calculated from these curves are given in the text. In the second stage of the assay, factor XIa levels were quantified, as previously described,11 by monitoring the rate of cleavage of the chromogenic substrate, L-Pyr-Pro-Arg-p-nitroanilide. At the concentrations used, none of the inhibitors altered the rate of hydrolysis of this substrate by factor XIa.
Efficacy of polyP inhibitors in a mouse models of venous and arterial thrombosis
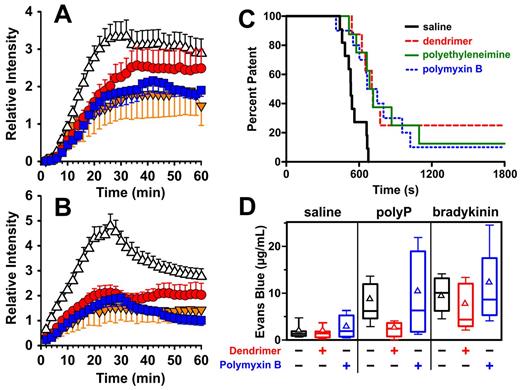
PolyP contributes to thrombosis in mouse models.6 We therefore examined polyP inhibitors in a mouse venous thrombosis model driven by electrolytic injury to the femoral vein.19 Intravenous administration of generation 1.0 dendrimer or polymyxin B inhibited the accumulation of fibrin (Figure 7A) and platelets (Figure 7B) in the developing thrombus (as did heparin, provided for comparison). Peak accumulation of these thrombus markers (comparing mean relative intensities 26 minutes after injury) was significantly (P < .05) lower for animals treated with dendrimer, polymyxin B, or heparin compared with animals receiving saline. We also examined polyP inhibitors in a mouse arterial thrombosis model driven by FeCl3 application to the carotid artery. Intravenous administration of generation 1.0 dendrimer, low MW polyethyleneimine, or polymyxin B before FeCl3 application reduced or slowed vessel occlusion (Figure 7C). Log-rank analyses showed that median patency time was significantly prolonged in mice injected with generation 1.0 dendrimer (P < .01; n = 8), polymyxin B sulfate (P < .01; n = 10), or low MW polyethyleneimine (P < .01; n = 8) versus mice injected with vehicle (n = 11). PolyP inhibitors therefore significantly attenuate both venous and arterial thrombosis.
In vivo antithrombotic and anti-inflammatory efficacies of polyP inhibitors. (A-B) Murine model of venous thrombosis. Inhibitors were administered intravenously to mice before initiation of electrolytic injury of the femoral vein (time = 0 in the graphs). Data are mean relative intensities for accumulation of fluorescently labeled fibrin-specific antibodies (A) or labeled platelets (B) in the developing thrombus for mice receiving: red circles, 4 μg/g generation 1.0 dendrimer (n = 10); blue squares, 2 μg/g polymyxin B (n = 8); orange inverted triangles, 100 units/kg unfractionated heparin (n = 5); or open triangles, vehicle only (n = 14). Bars represent 1 SE. (C) Murine model of arterial thrombosis, with Kaplan-Meier curves showing percentage of mice with patent arteries. Inhibitors were injected retro-orbitally 10 minutes before ferric chloride injury to the carotid artery. Blood flow was monitored by Doppler, with occlusion defined as no flow for 1 minute. Log-rank analyses indicated that median patency time was significantly longer for mice injected with 8 μg/g generation 1.0 dendrimer (P < .01, n = 8), 4 μg/g polymyxin B (P < .01, n = 10), or 5 μg/g low MW polyethyleneimine (P < .01, n = 8) versus mice injected with vehicle (n = 11). (D) Murine model of polyP-induced vascular leakage. Mice were given separate retro-orbital injections with Evans blue dye and either a polyP inhibitor (48 μg/g generation 1.0 dendrimer or 20 μg/g polymyxin B) or vehicle. After 40 minutes, saline, bradykinin, and polyP were injected intradermally at 3 respective sites on the back. After an additional 30 minutes, mice were killed and dye was extracted from skin biopsies for quantification. Plots show median (central horizontal lines), mean (triangles), 25th-75th percentile (top and bottom of boxes), and 10th-90th percentile (whiskers) concentrations of extracted dye. Dendrimer administration resulted in significantly less dye leakage at the site of polyP injection compared with control animals (P < .001). Each group (no inhibitor, dendrimer, and polymyxin B) contained 15 mice.
In vivo antithrombotic and anti-inflammatory efficacies of polyP inhibitors. (A-B) Murine model of venous thrombosis. Inhibitors were administered intravenously to mice before initiation of electrolytic injury of the femoral vein (time = 0 in the graphs). Data are mean relative intensities for accumulation of fluorescently labeled fibrin-specific antibodies (A) or labeled platelets (B) in the developing thrombus for mice receiving: red circles, 4 μg/g generation 1.0 dendrimer (n = 10); blue squares, 2 μg/g polymyxin B (n = 8); orange inverted triangles, 100 units/kg unfractionated heparin (n = 5); or open triangles, vehicle only (n = 14). Bars represent 1 SE. (C) Murine model of arterial thrombosis, with Kaplan-Meier curves showing percentage of mice with patent arteries. Inhibitors were injected retro-orbitally 10 minutes before ferric chloride injury to the carotid artery. Blood flow was monitored by Doppler, with occlusion defined as no flow for 1 minute. Log-rank analyses indicated that median patency time was significantly longer for mice injected with 8 μg/g generation 1.0 dendrimer (P < .01, n = 8), 4 μg/g polymyxin B (P < .01, n = 10), or 5 μg/g low MW polyethyleneimine (P < .01, n = 8) versus mice injected with vehicle (n = 11). (D) Murine model of polyP-induced vascular leakage. Mice were given separate retro-orbital injections with Evans blue dye and either a polyP inhibitor (48 μg/g generation 1.0 dendrimer or 20 μg/g polymyxin B) or vehicle. After 40 minutes, saline, bradykinin, and polyP were injected intradermally at 3 respective sites on the back. After an additional 30 minutes, mice were killed and dye was extracted from skin biopsies for quantification. Plots show median (central horizontal lines), mean (triangles), 25th-75th percentile (top and bottom of boxes), and 10th-90th percentile (whiskers) concentrations of extracted dye. Dendrimer administration resulted in significantly less dye leakage at the site of polyP injection compared with control animals (P < .001). Each group (no inhibitor, dendrimer, and polymyxin B) contained 15 mice.
In vivo anti-inflammatory activity of polyP inhibitors
PolyP injected intradermally in mice induces vascular leakage that is dependent on localized activation of the contact pathway and concomitant bradykinin production.6 To evaluate whether cationic molecules could inhibit these proinflammatory effects of polyP in vivo, we used a modified Miles vascular leakage assay. Generation 1.0 dendrimer or polymyxin B was administered intravenously to mice along with Evans blue dye. After 40 minutes, polyP or bradykinin was injected intradermally and local vascular leakage detected by extravasation of Evans blue. Generation 1.0 dendrimer significantly abrogated vascular leakage induced by polyP (Figure 7D; P < .001) but not by bradykinin (Figure 7D; P = .504). Results obtained with polymyxin B were highly variable (Figure 7D) and not statistically significantly different from control animals (P = .549).
Discussion
This study demonstrates proof of principle that inhibitors of polyP, including cationic small molecules, polymers, and proteins, can block the procoagulant and pro-inflammatory effects of polyP, both in vitro and in vivo. Using in vitro clotting assays, the potencies of these inhibitors toward polyP varied considerably, as did their specificities toward polyP compared with heparin, RNA, minerals, or tissue factor. Spermine and PPXbd, in particular, were selective for inhibiting the procoagulant activity of polyP over that of other clotting activators. Spermine was also much more effective at blocking the procoagulant activity of polyP than the anticoagulant activity of heparin/antithrombin. On the other hand, both generation 1.0 dendrimer and polymyxin B, although more potent toward polyP, also significantly reduced the procoagulant activity of RNA and nonphysiologic, mineral-based activators of the contact pathway. Inhibitors such as these may thus have utility as general inhibitors of the contact pathway of blood clotting triggered by anionic polymers and surfaces in vivo. In particular, the generation 1.0 dendrimer was highly effective in vivo at reducing severity of venous thrombosis, arterial thrombosis, and polyP-mediated vascular leakage.
The experiments in Figure 5 on real-time thrombin generation in platelet-rich plasma show that both PPXbd and generation 1.0 dendrimer prolonged the lag time to thrombin generation as well as the time to peak thrombin but had no effect on the peak thrombin concentration itself. This nicely reflects our earlier findings on real-time thrombin generation using platelet-poor plasma to which polyP was added. In that study, we reported that polyP shortened the lag time and the time to peak thrombin generation but not effecting the peak thrombin concentration or the endogenous thrombin potential.5 The fact that we now also report that generation 1.0 dendrimer reduced thrombus size and decreased fibrin accumulation in murine thrombosis models is consistent with the idea that slowing the kinetics of the thrombin burst can significantly attenuate thrombosis in vivo.
Similar to our results in this study with the generation 1.0 dendrimer, Jain et al have very recently reported that a more highly branched cationic PAMAM dendrimer (generation 3.0) inhibited the procoagulant activity of polyP and nucleic acids in vitro, and prevented thrombosis in mice without increasing surgical bleeding.30
Our finding that spermine, norspermine, and spermidine are potent polyP inhibitors is noteworthy because the naturally occurring polyamines, spermine, spermidine, and putrescine inhibit aggregation of human platelets,31-37 and systemic administration of polyamines is reported to prevent carotid artery occlusion in a canine thrombosis model.38 The latter investigators concluded that the antithrombotic activity of polyamines was the result of inhibition of platelet aggregation, although it is tempting to speculate that inhibition of platelet-secreted polyP procoagulant activity may have also contributed to their protective effects. Direct tissue injection of spermine was effective in preventing footpad edema in a rodent model,39 and spermine administration was neuroprotective in an ischemic brain injury model in rats.40 Interestingly, brain contains long-chain polyP,41 and deficiency of contact pathway clotting factors is neuroprotective in mouse models of cerebral artery ischemia-reperfusion.13 It would therefore be interesting to examine the in vivo utility of polyP inhibitors in brain injury models.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Chen Zhang of the University of Illinois HTSF for assistance with screening of compound libraries.
This work was supported by the National Institutes of Health, National Heart, Lung, and Blood Institute (R01 HL047014, J.H.M.; and F30 HL107089, S.H.C.).
National Institutes of Health
Authorship
Contribution: S.A.S., J.N.R.C., R.J.T., B.C.C., and S.H.C. performed experiments and contributed to data analysis and manuscript preparation; and J.H.M. contributed to experimental design, data analysis, and manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: James H. Morrissey, Department of Biochemistry, University of Illinois, 419 Roger Adams Lab, MC-712, 600 S Mathews Ave, Urbana, IL 61801; e-mail: jhmorris@illinois.edu.
References
Author notes
S.A.S. and S.H.C. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal