Abstract
Extracellular ATP (eATP), the most abundant among nucleotides, can act as a mediator during inflammatory responses by binding to plasmamembrane P2 purinergic receptors, which are widely expressed on cells of the immune system. eATP is generally considered as a classical danger signal, which stimulates immune responses in the presence of tissue damage. Converging evidence from several studies using murine models of chronic inflammation have supported this hypothesis; however, the role of eATP in the regulation of human immune function appears to be more complex. Chronic stimulation with micromolar eATP concentrations inhibits the proliferation of T and NK lymphocytes and enhances the capacity of dendritic cells to promote tolerance. The effect of eATP depends on multiple factors, such as the extent of stimulation, eATP concentration, presence/absence of other mediators in the microenvironment, and pattern of P2 receptor engagement. Small but significant differences in the pattern of P2 receptor expression in mice and humans confer the diverse capacities of ATP in regulating the immune response. Such diversity, which is often overlooked, should therefore be carefully considered when evaluating the role of eATP in human inflammatory and autoimmune diseases.
Sources of eATP
Nucleotides are the constituents of nucleic acids, represent the energy store of the cell, and are involved in intracellular signaling as well as in cell-to-cell communication.1 During the past 2 decades, increasing evidence has shown that extracellular ATP (eATP) is an important immune modulator.2 ATP is present at relatively high levels in the cytoplasm of cells, where its concentration ranges from 1 to 10mM. In the extracellular space, its physiologic concentration is considerably lower, ranging between 1 and 10nM. Because of the steep concentration gradient as well as its small size and high mobility, ATP can be rapidly released together with other cellular components after mechanical stress, cell damage, or death. Increased ATP concentration in the extracellular milieu is therefore closely associated with tissue stress or damage.3-6 However, nonlytic nucleotide release may occur in many cell types under a variety of conditions. Living cells in the steady-state release ATP through passive leakage.7,8 Activated platelets represent a relevant source of ATP and release the nucleotide concomitantly with several inflammatory mediators during clot formation.9 T lymphocytes release ATP during the early stages of activation through pannexin 1 (panx1) channels.10 In addition, ATP is released during exercise from skeletal muscle as well as from vascular endothelial cells during conditions of increased blood flow or on mechanical stimulus.11-13 Moreover, nonlytic ATP secretion from endothelial cells and leukocytes may be induced by pathogen-associated molecules, such as lipopolysaccharide.14-17 ATP is also increased in draining lymph nodes during a contact hypersensitivity reaction.18 ATP is released during the early phases of apoptosis19 through pannexin 1 hemichannels20 and attracts monocytes and macrophages to ensure efficient clearance of apoptotic cells. Recently, commensal bacteria in the gut have been shown to secrete discrete amounts of ATP that exert important modulatory effects on immune responses.21
Purinergic receptors
Once in the extracellular compartment, nucleotides are recognized by plasma membrane receptors belonging to the P2 purinergic family, which are expressed by virtually all cell types. Their activation elicits diverse responses depending on the nucleotide concentration, cell type, and receptors expressed. Two P2 receptor subfamilies, called P2X and P2Y, have been described (Figure 1). Members of the 2 groups differ in protein structure, pharmacology, and function.22-24 Table 1 summarizes the expression patterns of P2X and P2Y receptor subtypes25-28 and their affinity for ATP.7,27
Type 2 purinergic receptors and extracellular ATP metabolism. Extracellular ATP exerts its effects on cells by binding to type 2 purinergic receptors. A prototypic P2X receptor is shown. In the active conformation, 3 peptide subunits are assembled to form an ion-permeable channel pore. Each one of the subunits is formed by 2 membrane-spanning domains, a large extracellular loop, and intracellular carboxyl and amino termini. Binding of ATP is crucial for subunit oligomerization, which leads to the opening of pores and the determination of the influx/efflux of cations. ATP can bind P2Y receptors, which are 7 membrane-spanning, G-protein–coupled receptors. Binding of nucleotides induces different cascades of intracellular signaling events, depending on the specific type of G protein associated with the receptor (P2Y1, 6, 14: Gq/11; P2Y2, 4: Gq/11-Gi; P2Y11: Gs, Gq/11; P2Y12, 13: Gi/o). ATP in the extracellular space is metabolized by the plasma membrane-bound ectoenzyme CD39 that catalyzes the hydrolysis of ATP into ADP/AMP and CD73, which subsequently hydrolyzes AMP into adenosine. The activity of these enzymes contributes to the removal of ATP from the extracellular space, thus limiting the duration of signaling. In turn, adenosine engages purinergic P1 receptors (adenosine receptors).
Type 2 purinergic receptors and extracellular ATP metabolism. Extracellular ATP exerts its effects on cells by binding to type 2 purinergic receptors. A prototypic P2X receptor is shown. In the active conformation, 3 peptide subunits are assembled to form an ion-permeable channel pore. Each one of the subunits is formed by 2 membrane-spanning domains, a large extracellular loop, and intracellular carboxyl and amino termini. Binding of ATP is crucial for subunit oligomerization, which leads to the opening of pores and the determination of the influx/efflux of cations. ATP can bind P2Y receptors, which are 7 membrane-spanning, G-protein–coupled receptors. Binding of nucleotides induces different cascades of intracellular signaling events, depending on the specific type of G protein associated with the receptor (P2Y1, 6, 14: Gq/11; P2Y2, 4: Gq/11-Gi; P2Y11: Gs, Gq/11; P2Y12, 13: Gi/o). ATP in the extracellular space is metabolized by the plasma membrane-bound ectoenzyme CD39 that catalyzes the hydrolysis of ATP into ADP/AMP and CD73, which subsequently hydrolyzes AMP into adenosine. The activity of these enzymes contributes to the removal of ATP from the extracellular space, thus limiting the duration of signaling. In turn, adenosine engages purinergic P1 receptors (adenosine receptors).
Expression patterns of P2X and P2Y receptor subtypes and their affinity for ATP
| Receptor . | Agonists . | Agonist binding affinities EC50, μM . | Main downstream signaling events . | Expression . |
|---|---|---|---|---|
| P2X receptors | ||||
| P2X1 | ATP | 0.05-1 | Ca2+ and Na+ influx | Smooth muscle cells, megakaryocytes, platelets, lymphocytes, DCs, epithelial cells, ventricular myocardium, and neurons |
| P2X2 | ATP | 1-30 | Ca2+ influx | Pancreatic cells, and neurons |
| P2X3 | ATP | 0.3-1 | Cation influx | Neurons, keratinocytes, and CD34+ hematopoietic precursors |
| P2X4 | ATP | 1-10 | Ca2+ influx | Neurons, CD34+ stem cells, lymphocytes, macrophages, MoDCs, fibroblasts, keratinocytes, and placenta |
| P2X5 | ATP | 1-10 | Ion influx | Neurons, keratinocytes, and thyrocytes |
| P2X6 | ATP | 1-12 | Ion influx | Neurons, keratinocytes, and thyrocytes |
| P2X7 | ATP | > 100 | Ca2+ and Na+ influx, K+ efflux pore formation | Neurons, lymphocytes, NK cells, neutrophils, monocytes, macrophages, DCs, and placenta |
| P2Y receptors | ||||
| P2Y1 | ADP | 8 | PLC-β activation | Neurons, heart, skeletal muscle, platelets, liver, and digestive tract |
| P2Y2 | ATP, UTP | 0.1 (ATP), 0.2 (UTP) | PLC-β activation cAMP inhibition | Skeletal muscle, heart, lung, spleen, placenta, kidney, and neutrophils |
| P2Y4 | UTP (ATP, UTP) | 2.5 | PLC-β activation cAMP inhibition | Intestine, lung, and placenta |
| P2Y6 | UDP, UTP | 0.3 (UDP), 6 (UTP) | PLC-β activation | Spleen, thymus, placenta, intestine, lung, and brain |
| P2Y11 | ATP | 17 | cAMP production, PLC-β activation | Corneal epithelia, endothelial cells, pancreatic duct cells, DCs, and lymphocytes |
| P2Y12 | ADP | 0.07 | cAMP inhibition | CD34+ stem cells, mast cells, vascular smooth muscle cells, and platelets |
| P2Y13 | ADP, ATP | 0.06 (ADP), 0.26 (ATP) | cAMP inhibition | Bone marrow, spleen, liver, brain, airway epithelial cells, red blood cells, monocytes, DCs, and T lymphocytes |
| P2Y14 | UDP-glucose | 0.1-0.5 | PLC-β activation | Hematopoietic cells, MoDCs, and human airway epithelial cells |
| Receptor . | Agonists . | Agonist binding affinities EC50, μM . | Main downstream signaling events . | Expression . |
|---|---|---|---|---|
| P2X receptors | ||||
| P2X1 | ATP | 0.05-1 | Ca2+ and Na+ influx | Smooth muscle cells, megakaryocytes, platelets, lymphocytes, DCs, epithelial cells, ventricular myocardium, and neurons |
| P2X2 | ATP | 1-30 | Ca2+ influx | Pancreatic cells, and neurons |
| P2X3 | ATP | 0.3-1 | Cation influx | Neurons, keratinocytes, and CD34+ hematopoietic precursors |
| P2X4 | ATP | 1-10 | Ca2+ influx | Neurons, CD34+ stem cells, lymphocytes, macrophages, MoDCs, fibroblasts, keratinocytes, and placenta |
| P2X5 | ATP | 1-10 | Ion influx | Neurons, keratinocytes, and thyrocytes |
| P2X6 | ATP | 1-12 | Ion influx | Neurons, keratinocytes, and thyrocytes |
| P2X7 | ATP | > 100 | Ca2+ and Na+ influx, K+ efflux pore formation | Neurons, lymphocytes, NK cells, neutrophils, monocytes, macrophages, DCs, and placenta |
| P2Y receptors | ||||
| P2Y1 | ADP | 8 | PLC-β activation | Neurons, heart, skeletal muscle, platelets, liver, and digestive tract |
| P2Y2 | ATP, UTP | 0.1 (ATP), 0.2 (UTP) | PLC-β activation cAMP inhibition | Skeletal muscle, heart, lung, spleen, placenta, kidney, and neutrophils |
| P2Y4 | UTP (ATP, UTP) | 2.5 | PLC-β activation cAMP inhibition | Intestine, lung, and placenta |
| P2Y6 | UDP, UTP | 0.3 (UDP), 6 (UTP) | PLC-β activation | Spleen, thymus, placenta, intestine, lung, and brain |
| P2Y11 | ATP | 17 | cAMP production, PLC-β activation | Corneal epithelia, endothelial cells, pancreatic duct cells, DCs, and lymphocytes |
| P2Y12 | ADP | 0.07 | cAMP inhibition | CD34+ stem cells, mast cells, vascular smooth muscle cells, and platelets |
| P2Y13 | ADP, ATP | 0.06 (ADP), 0.26 (ATP) | cAMP inhibition | Bone marrow, spleen, liver, brain, airway epithelial cells, red blood cells, monocytes, DCs, and T lymphocytes |
| P2Y14 | UDP-glucose | 0.1-0.5 | PLC-β activation | Hematopoietic cells, MoDCs, and human airway epithelial cells |
MoDCs indicates monocyte-derived dendritic cells.
P2X receptors are membrane cation channels that are gated by eATP, and their opening determines Ca2+ and Na+ influx as well as K+ efflux. These receptors are oligomers of 3 subunits, which are brought together by binding to the agonist. Each P2X subunit is formed by an extracellular loop, 2 transmembrane domains, and 2 (amino- and carboxyl-terminal) cytoplasmic domains. Agonist binding induces the subunits to assemble and form homomultimeric or, in some cases, heteromultimeric channels that are permeable to monovalent and divalent cations.29-32 Seven P2X subtypes have been cloned to date (P2X1-7) and found to be expressed on lymphocytes, NK cells, macrophages, dendritic cells (DCs), and neutrophils.33 ATP activates all P2X subtypes, among which P2X7 is the best characterized. It is a low affinity, nondesensitizing receptor with the peculiar capacity to undergo a permeability transition from a cationic selective channel to a plasma membrane pore on stimulation with high (1-10mM) or pulsed ATP doses.34 Although eATP is the only known physiologic agonist of P2X7, receptor activation in the mouse may also occur after ADP ribosylation, which is catalyzed by ADP ribosyltransferase (ART) enzymes in the presence of extracellular NAD+.35 In the mouse, the ART2.2 subtype mediates ADP ribosylation of P2X7. Because a murine ART2.2 ortholog is not present in the human genome, NAD+-induced P2X7 activation does not occur in humans.36 P2X7 is expressed in humans as 9 different splice variants (P2X7A-J). Of these, P2X7A represents the canonical P2X7 receptor. In 4 of the variants (P2X7B, P2X7E, P2X7G, and P2X7J), removal of the C-terminus prevents the formation of a large, nonselective plasma membrane pore,37 which leads to cell death.
P2Y receptors are 7 membrane-spanning, G-protein–coupled receptors, whose activation triggers the generation of inositol 1,4,5-trisphosphate and release of Ca2+ from intracellular stores.38 Eight P2Y subtypes have been cloned (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13, and P2Y14).38 The P2Y receptor subfamily is composed of adenine nucleotide-preferring receptors (P2Y1, P2Y11, P2Y12, and P2Y13), uracil nucleotide-preferring receptors (P2Y4 and P2Y6), a receptor of mixed specificity (P2Y2), and a receptor for UDP-glucose (P2Y14; Table 1). P2Y receptors can be divided into 2 functional subgroups. The first group, which includes P2Y1, 2, 4, 6, and 11, is coupled to Gq/G11 proteins and leads to the activation of the PLC-β/IP3 pathway as well as the mobilization of intracellular calcium. The second group, including P2Y12, P2Y13, and P2Y14, is coupled to Gi/o proteins, which inhibit adenylate cyclases.38 Among P2 receptors, P2Y11 has 2 unique characteristics: (1) its activation is associated with an increased intracellular concentration of cyclic AMP39-41 ; and (2) there is no ortholog gene of human P2Y11 in rodents.38 ATP is the preferred physiologic ligand of P2Y11,42-44 but receptor activation can also be triggered by extracellular NAD+.45
Extracellular metabolism of nucleotides
The interplay between eATP and purinergic receptors is influenced by the activity of ectoenzymes that hydrolyze extracellular nucleotides (Figure 1), and maintain their concentration in the low nanomolar range. These enzymes share the ability to hydrolyze triphosphate and/or diphosphate nucleosides, but not monophosphate nucleosides.46 They are named ecto-nucleoside triphosphate diphosphohydrolases (E-NTDPase) because they are capable of hydrolyzing purines and pyrimidines. Eight subtypes of E-NTDPase have been cloned; of these, 4 (NTPDase1, 2, 3, and 8) are expressed on the cell surface. E-NTPD1, which is also known as CD39, is found on monocytes, DCs, neutrophils, and B lymphocytes as well as on some subsets of NK and T cells.47,48 Because of its distribution and activity, CD39 has a crucial role in modulating the effect of eATP in inflammation. AMP generated by CD39 activity is further hydrolyzed into adenosine by the membrane-bound ecto-5′-nucleotidase/CD73 (Figure 1), which is expressed at different levels on circulating leukocytes.46 Of note, CD39 and CD73 are coexpressed on the surface of murine regulatory T lymphocytes (Tregs) and on a subset of human Treg cells.49
Other ectoenzymes, such as those that metabolize extracellular NAD+, can indirectly influence P2 receptors activity. The extracellular NAD+ concentration is regulated by the activity of ecto-NAD glycohydrolases, such as CD38 and CD157, which are both expressed on DCs.36 CD38 is also expressed on T and B lymphocytes and CD157 is expressed on neutrophils.36 These membrane bound glycoproteins catalyze the hydrolysis of NAD+ into adenosine 5′-diphosphate-ribose and the subsequent conversion of adenosine 5′-diphosphate-ribose into cyclic adenosine 5′-diphosphate-ribose.
Extracellular ATP as danger-associated trigger for immune responses
The inflammatory response is crucial for host defense against invasive pathogens as well as for tissue and wound repair. Stimuli that trigger the inflammatory response are composed of molecules expressed by pathogenic microorganisms as well as endogenous molecules associated with tissue stress or damage, which are called danger-associated molecular patterns. These host-derived molecules share the ability to initiate or amplify inflammation by triggering pattern recognition receptors on innate immune cells.
ATP belongs to the group of constitutive danger-associated molecular patterns released as a consequence of cellular damage during the course of infection or in sterile inflammation. In several animal models of inflammatory and autoimmune diseases, eATP has been shown to promote inflammation through the following mechanisms: (1) P2X7-mediated activation of the inflammasome on innate immune cells with consequent release of IL-1β and IL-1850 ; (2) stimulation of effector T-cell functions through the activation of P2X2, P2X4, and P2X751,52 ; (3) selective depletion of the immunomodulatory Treg cell subset, which is highly sensitive to P2X7-dependent cell death53 ; and (4) enhancement of neutrophil microbicidal activity, which is induced by the P2X7 receptor.54
Activation of caspase 1 and the consequent processing and release of mature IL-1β and IL-18 is the best characterized function of P2X7. The opening of the P2X7 channel induces K+ efflux and the subsequent decrease in intracellular K+ concentration, which is a crucial event that causes Nacht Domain–, Leucine-Rich Repeat–, and PYD-Containing Protein 3 (NLRP3) activation.55 The process that drives the release of IL-1β and IL-18 starts with the assembly of the NLRP3 inflammasome. Activated NLRP3 induces the recruitment of the adaptor protein apoptosis-associated speck-like protein, which in turn activates caspase 1 to catalyze the proteolytic maturation of pro-IL-1β and pro-IL-18.50 As P2X7 activation leads to NLRP3 inflammasome assembly, P2X7-deficient mice display impaired inflammasome activation, reduced IL-1β release, and decreased severity of disease, in the murine models of allergic contact dermatitis56 and collagen-induced arthritis.57 Moreover, P2X7 deficiency or the administration of receptor antagonists protect mice from the development of experimental autoimmune encephalomyelitis.58,59 In addition, in ovalbumin-sensitized and ovalbumin-challenged asthmatic mice, the engagement of P2 receptors by eATP modulates the function of DCs and plays a key pathogenic role. Although increased levels of eATP were found in the bronchoalveolar fluid of allergen-challenged asthmatic patients, the exact pathogenic role of eATP and the receptors involved in this disease remains to be elucidated.60
ATP can act as a proinflammatory molecule not only by stimulating innate immune responses, but also by favoring effector T-cell activation. ATP released from activated T lymphocytes acts in a paracrine and autocrine manner, and mainly activates P2X receptors. The influx of extracellular Ca2+ through opened channels contributes to the activation of nuclear factor of activated T cells (NFAT) and promotes IL-2 gene transcription and T-cell proliferation. P2X1, P2X4, and panx1 translocate to the immune synapse after T-cell stimulation. Their silencing leads to a dramatic reduction in NFAT activation and IL-2 expression.51 Similarly, T lymphocytes secrete ATP, which stimulates P2X7 in an autocrine manner and induces IL-2 gene transcription and proliferation.52 Several groups have described antiapoptotic and proliferation-inducing effects of P2X7 in lymphoid cell lines,61 embryonic stem cell cultures,62 and microglia63 in vitro as well as P2X7 tumor growth-promoting activity in vivo.64 Such effects are associated with an increased cytoplasmic Ca2+ concentration, activation of NFATc1,65 and may be related to the expression of C-terminal truncated splice variants that lack the domain involved in pore formation.
Interestingly, ATP can also indirectly support effector T-cell expansion and innate immunity activation by inhibiting the immunosuppressive activity of CD4+CD25+Foxp3+ Tregs. These cells, compared with effector T lymphocytes, display higher sensitivity to P2X7-mediated cytotoxicity on exposure to eATP or, in the murine system, in the presence of extracellular NAD+.53,66 Indeed, selective depletion of Treg cells can be induced in mice by systemic administration of NAD+ in an ART2.2- and P2X7-dependent fashion.67 Moreover, ATP neutralization, early P2X7 blockade, or genetic deficiency of P2X7 improved animal survival in a murine model of GVHD by dampening inflammation and reducing the effector/regulatory T-cell ratio.68 Similarly, P2X7-deficient mice showed a delayed onset of type 1 diabetes, which was probably the result of prolonged survival of Treg cells in β-pancreatic islets.69
Neutrophils actively secrete ATP and express P2Y1, 2, 4, 6, 11, and 14, P2X1, 4, 5, and 7 receptor subtypes, CD39, and CD73.27 During chemotaxis, ATP secretion occurs through panx hemichannels and connexin 4370,71 at the leading edge of the cell, where it acts in an autocrine fashion by binding to the P2Y2 receptor.71 Concomitantly, ATP is rapidly hydrolyzed to adenosine, which engages the Gi-coupled adenosine A3 receptor. The activation of P2Y2 and A3 amplifies the chemotactic signal, which controls neutrophil migration. Moreover, ATP synergizes with IL-8 to activate neutrophil chemotaxis.72 eATP also promotes the adhesion of neutrophils to endothelial cells by increasing the expression of E-selectin and β2-integrin macrophage antigen-1 (CD11b/CD18).27 Furthermore, by activating P2X7, eATP induces augmented production of reactive oxygen species, enhances microbicidal activity,54 and triggers degranulation of neutrophils in the presence of a costimulus, such as N-formyl-methionine-leucine-phenylalanine.73 In addition, the binding of eATP to P2Y11 on neutrophils has been shown to deliver a prosurvival signal through type I cAMP-dependent protein kinases.74
Dampening immune function by extracellular nucleotides
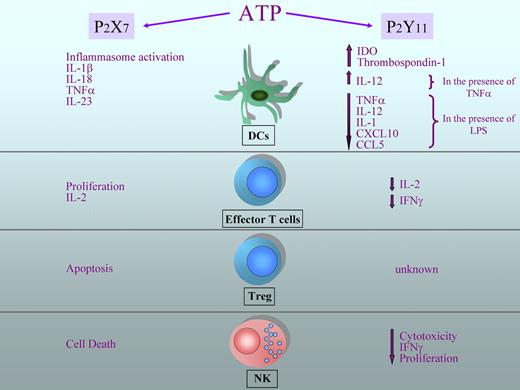
As a danger signal, eATP triggers immune responses when tissue injury occurs. However, several studies in human cells suggest a more complex role for eATP in the regulation of immune responses.75,76 Indeed eATP can potently inhibit several immune functions of different leukocyte subsets, including DCs, NK cells, and T lymphocytes. Increasing evidence has identified the P2Y11 receptor as the main mediator of the immunosuppressive effects of eATP. As outlined in Figure 2, P2Y11 mediates several eATP-induced inhibitory effects on different immune cell subsets, whereas P2X7 plays a pivotal role in stimulating proinflammatory and effector functions.
Diverse regulation of leukocyte function exerted by ATP. ATP can exert different effects on immune cells depending on which receptor is engaged. On DCs, ATP induces inflammasome activation and secretion of proinflammatory cytokines on binding to P2X7, whereas the activation of P2Y11 leads to a reduced production of proinflammatory cytokines and chemokines (in the presence of lipopolysaccharide) and to the production of immunosuppressive molecules, such as indoleamine 2,3-dioxygenase (IDO) and thrombospondin-1. On the other hand, in the presence of TNF-α, the secretion of IL-12 from DCs is increased. Activation of P2X7 on effector T lymphocytes increases IL-2 secretion and proliferation; in contrast, binding of P2Y11 determines a reduction in INF-γ and IL-2 production and limits proliferation. ATP has a crucial role in maintaining the homeostasis of regulatory T cells, as these lymphocytes are particularly sensitive to apoptosis induced by P2X7 activation. The effects of P2Y11 activation on Tregs are currently not known. ATP reduces IFN-γ production, proliferation, and cytotoxic activity in NK cells through the activation of P2Y11, whereas the engagement of P2X7 leads to cell death.
Diverse regulation of leukocyte function exerted by ATP. ATP can exert different effects on immune cells depending on which receptor is engaged. On DCs, ATP induces inflammasome activation and secretion of proinflammatory cytokines on binding to P2X7, whereas the activation of P2Y11 leads to a reduced production of proinflammatory cytokines and chemokines (in the presence of lipopolysaccharide) and to the production of immunosuppressive molecules, such as indoleamine 2,3-dioxygenase (IDO) and thrombospondin-1. On the other hand, in the presence of TNF-α, the secretion of IL-12 from DCs is increased. Activation of P2X7 on effector T lymphocytes increases IL-2 secretion and proliferation; in contrast, binding of P2Y11 determines a reduction in INF-γ and IL-2 production and limits proliferation. ATP has a crucial role in maintaining the homeostasis of regulatory T cells, as these lymphocytes are particularly sensitive to apoptosis induced by P2X7 activation. The effects of P2Y11 activation on Tregs are currently not known. ATP reduces IFN-γ production, proliferation, and cytotoxic activity in NK cells through the activation of P2Y11, whereas the engagement of P2X7 leads to cell death.
Many of the eATP inhibitory effects are mimicked by other cAMP-inducing agents or by cell-permeable cAMP analogs, which is in agreement with the capacity of activated P2Y11 to increase intracellular cAMP concentration and with the well-established role of intracellular cAMP as a potent immunosuppressant. Therefore, eATP may directly exert cAMP-mediated immune suppression through engagement of the P2Y11 receptor. For example, human DCs do not undergo “classical” maturation when exposed to micromolar concentrations of eATP. Although eATP stimulates DCs to up-regulate costimulatory membrane molecules, eATP also blocks inflammatory cytokine and chemokine production induced by lipopolysaccharide, including TNF-α, IL-1, IL-12, CCL2, CCL3, CCL5, and CXCL10, whereas the anti-inflammatory cytokine IL-10 and the IL-1 receptor antagonist are either unaffected or up-regulated.77-79 The production of TNF-α and IL-12 was restored by specifically blocking the P2Y11 receptor (F. Molinari and A.l.S., unpublished data, June 2009). Moreover, eATP induces DCs to produce large amounts of thrombospondin-1 and synergizes with IFN-γ to up-regulate indoleamine 2,3-dioxygenase, which inhibits T-cell proliferation.80 These effects are mimicked by the nonhydrolyzable ATP analog ATP-γ-S (an agonist of P2Y11) and by several cAMP-elevating agents, as well as by the administration of cell-permeable cAMP analogs.80-82 Schnurr et al reported that P2Y11 activity inhibits IL-27 production.83 In the same article, the authors reported the capacity of eATP to increase mRNA levels of IL-23, which potentially fosters the expansion of the proinflammatory Th17 lymphocyte subset.83 However, IL-23 stimulation was not mediated by the P2Y11 receptor. In addition, eATP synergizes with TNF-α to induce IL-12p70 production by human DCs.41,84 Therefore, depending on the microenvironment, eATP can induce DCs to acquire a tolerogenic phenotype or promote IL-12 expression.
Micromolar concentrations of eATP have been shown to inhibit human NK cell proliferation.85 In addition, Gorini et al showed that eATP-induced P2Y11 receptor activation increased intracellular cAMP levels and markedly inhibited CX3CL1-induced chemotaxis and cytotoxicity, in NK cells.86 Moreover, eATP inhibits IFN-γ and IL-2 expression as well as the proliferation of human T lymphocytes. Although the mechanism and the receptors involved have not yet been identified, such effects are hypothesized to be the result of an increase in intracellular cAMP concentration elicited by eATP.87,88
P2Y11 activation by extracellular NAD+ also enhances the capacity of human bone marrow–derived mesenchymal stem cells to suppress effector T-cell proliferation.89 Thus, P2Y11 seems to be involved in the immunomodulatory activity of mesenchymal stem cells in the bone marrow as well. Because no rodent ortholog of P2Y11 exists,23,38 eATP may increase the intracellular cAMP concentration and exert immune suppression in humans, but not in naturally P2Y11-deficient murine cells (Figure 3). Moreover, because of the absence of a human ortholog of ART2.2, extracellular NAD+, which is a P2Y11 agonist in humans, does not support P2X7 activation. Interestingly, through membrane bound CD39 and CD73, murine Treg cells can exert immune suppression using a dual mechanism: removing the proinflammatory eATP danger signal and concomitantly generating adenosine, which exerts cAMP-dependent immunosuppressive action through the engagement of the adenosine A2a Gs protein–coupled receptor.49,90 However, because eATP can act as a direct cAMP-elevating agent in humans, the removal of eATP in humans may result in a less pronounced immunosuppressive or even stimulatory effect compared with that observed in mice. Future research focusing on the interaction between human Treg cells, DCs, and effector T lymphocytes is needed to address the role of eATP in such crosstalk to validate the observations made in animal models.
eATP differentially modulates inflammation in mice and humans. In mice (left), eATP activates the ionotropic receptor P2X7, which can also be activated by NAD+ through ART2.2-mediated ADP ribosylation. P2X7 activation leads to K+ efflux that triggers NLRP3 inflammasome activation, thereby promoting inflammation (red lines). P2X7 can also induce apoptosis of Tregs, thus amplifying inflammation. On the other hand, Tregs contribute to a reduction in eATP concentration by expressing the CD39 ectoenzyme, which hydrolyzes ATP into ADP and AMP (blue lines). In humans (right), the high-affinity ATP receptor P2Y11 mediates immunosuppressive effects by increasing the intracellular cAMP concentration. Moreover, because of the absence of a human ortholog of the murine ART2.2 gene, NAD+ does not activate P2X7.
eATP differentially modulates inflammation in mice and humans. In mice (left), eATP activates the ionotropic receptor P2X7, which can also be activated by NAD+ through ART2.2-mediated ADP ribosylation. P2X7 activation leads to K+ efflux that triggers NLRP3 inflammasome activation, thereby promoting inflammation (red lines). P2X7 can also induce apoptosis of Tregs, thus amplifying inflammation. On the other hand, Tregs contribute to a reduction in eATP concentration by expressing the CD39 ectoenzyme, which hydrolyzes ATP into ADP and AMP (blue lines). In humans (right), the high-affinity ATP receptor P2Y11 mediates immunosuppressive effects by increasing the intracellular cAMP concentration. Moreover, because of the absence of a human ortholog of the murine ART2.2 gene, NAD+ does not activate P2X7.
To date, 2 studies have identified 2 different single nucleotide polymorphisms of the P2Y11 gene that are associated with acute myocardial infarction and narcolepsy, respectively. In one study, a broad population of subjects carrying the common Ala-87-Thr P2Y11 polymorphism showed increased risk of acute myocardial infarction associated with augmented inflammation.91 Although the consequences of this polymorphism on receptor function have not yet been characterized, the nonpolar to polar amino acid shift is likely to affect ligand binding affinity or receptor function. In addition, a single nucleotide polymorphism in the 3′-untranslated region of the P2Y11 gene was shown to be associated with narcolepsy and causes reduced receptor expression in CD8+ T lymphocytes and NK cells.92 This disease is caused by the loss of hypocretin-producing neurons in the hypothalamus and is associated with the expression of a particular HLA haplotype as well as a specific T-cell receptor-α gene variant. Moreover, autoantibodies against the protein Trib2 expressed by hypocretin-producing neurons have been detected in the sera of patients with recent-onset narcolepsy. These findings suggest the possible autoimmune nature of this disease and the requirement of intact P2Y11-mediated signaling for control of the self-damaging cytotoxic activity of CD8+ T and NK cells. In this context, the development of transgenic animals expressing the human P2Y11 would be a significant step toward a more accurate definition of the pathogenic role of eATP in inflammatory and autoimmune human diseases and may help address some unanswered questions, such as: (1) how the inflammatory power of eATP is changed by the translation from mouse to humans, (2) how P2Y11 regulates immune function in vivo, and (3) whether P2Y11 is a suitable target for therapeutic approaches to treat inflammatory disorders.
In conclusion, compelling evidence from murine models of inflammatory diseases has outlined a well-defined role for eATP as a classical danger signal that activates immunity in an adjuvant-like fashion. However, a different and more complex picture has occurred from observations made in human cells, where eATP can also exert inhibitory effects. This discrepancy might result from the presence in humans, but not in rodents, of the P2Y11 receptor, which has the unique ability (among P2 receptors) to increase intracellular cAMP concentration and thus exert immune suppression. Whether eATP promotes protective immunity or immune tolerance is determined by the integration of different signals evoked by eATP itself, as well as by other mediators in the microenvironment. In humans, the ability of eATP to also act as a negative regulator of immune functions suggests that it might play a different role in human inflammatory diseases compared with what animal models have shown. In humans, concurrent activation of P2Y11 and P2X7 occurs in the presence of relatively high concentrations of eATP, such as in areas surrounding acute cell death (Figure 4). Lower ATP concentrations that activate P2Y11, but not P2X7, may be present in the extracellular milieu after regulated release of active ATP by healthy cells, or during the resolving phase of inflammation, when eATP levels may be decreased by ectonucleotidases. The different capacities of eATP to regulate immunity in human and murine systems should be taken into consideration when planning to exploit the purinergic network for the design of therapies to treat human chronic inflammatory and autoimmune diseases.
Different outcomes of immune cells exposed to eATP. Depending on the P2 receptor subtype expressed, the concentration in the extracellular milieu, and the duration of stimulation, eATP may induce pro- or anti-inflammatory responses. Millimolar concentrations of eATP open the P2X7 channel. Whereas the opening of the P2X7 channel for a short period of time leads to the activation of a number of mechanisms that sustain inflammation, prolonged stimulation of the P2X7 channel induces apoptosis of cells and consequent immune suppression. Relatively low (micromolar) concentrations of eATP activate the high-affinity receptor P2Y11, which is coupled to a Gs protein and induces an increase in intracellular concentration of cAMP, a well-known negative regulator of immune cells. Engagement of P2Y11 inhibits several effector mechanisms, including NK- and T-cell proliferation, cytokine production, and cytotoxic activity, thus contributing to the resolving phase of inflammation. In this model, ATP would act both as an initiator and terminator of immune response.
Different outcomes of immune cells exposed to eATP. Depending on the P2 receptor subtype expressed, the concentration in the extracellular milieu, and the duration of stimulation, eATP may induce pro- or anti-inflammatory responses. Millimolar concentrations of eATP open the P2X7 channel. Whereas the opening of the P2X7 channel for a short period of time leads to the activation of a number of mechanisms that sustain inflammation, prolonged stimulation of the P2X7 channel induces apoptosis of cells and consequent immune suppression. Relatively low (micromolar) concentrations of eATP activate the high-affinity receptor P2Y11, which is coupled to a Gs protein and induces an increase in intracellular concentration of cAMP, a well-known negative regulator of immune cells. Engagement of P2Y11 inhibits several effector mechanisms, including NK- and T-cell proliferation, cytokine production, and cytotoxic activity, thus contributing to the resolving phase of inflammation. In this model, ATP would act both as an initiator and terminator of immune response.
Acknowledgments
The authors thank Prof Luigi Racioppi, Prof Giuseppina Ruggiero, and Prof Giuseppe Terrazzano for critically reviewing the manuscript and providing helpful suggestions.
This work has been supported in part by the Italian Ministry of Health (Ricerca Corrente 2010-2011); Fondazione Roma, Type 2 Diabetes Mellitus: Role of Inflammation and Innate Immunity in the Pathogenesis of Endothelial Dysfunction and Atherosclerosis; and the Commission of European Union (2012-2014, ERA-NET NEURON program, Role of Danger Signals in Stroke and Therapeutic Targeting by Nanobodies).
Authorship
Contribution: L.V., S.G., G.R., and A.l.S. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrea la Sala, Laboratory of Molecular and Cellular Immunology, IRCCS San Raffaele Pisana, Via di Val Cannuta, 247 00166 Rome, Italy; e-mail: andrea.lasala@sanraffaele.it.