Age-group analyses were conducted of patients in the prophylactic platelet dose trial (PLADO), which evaluated the relation between platelet dose per transfusion and bleeding. Hospitalized patients with treatment-induced hypoproliferative thrombocytopenia were randomly assigned to 1 of 3 platelet doses: 1.1 × 1011, 2.2 × 1011, or 4.4 × 1011 platelets/m2 per transfusion, given for morning counts of ≤ 10 000 platelets/μL. Daily hemostatic assessments were performed. The primary end point (percentage of patients who developed grade 2 or higher World Health Organization bleeding) was evaluated in 198 children (0-18 years) and 1044 adults. Although platelet dose did not predict bleeding for any age group, children overall had a significantly higher risk of grade 2 or higher bleeding than adults (86%, 88%, 77% vs 67% of patients aged 0-5 years, 6-12 years, 13-18 years, vs adults, respectively) and more days with grade 2 or higher bleeding (median, 3 days in each pediatric group vs 1 day in adults; P < .001). The effect of age on bleeding differed by disease treatment category and was most pronounced among autologous transplant recipients. Pediatric subjects were at higher risk of bleeding over a wide range of platelet counts, indicating that their excess bleeding risk may be because of factors other than platelet counts. This trial was registered at www.clinicaltrials.gov as #NCT00128713.
Medscape EDUCATION Continuing Medical Education online
This activity has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education through the joint sponsorship of Medscape, LLC and the American Society of Hematology. Medscape, LLC is accredited by the ACCME to provide continuing medical education for physicians.
Medscape, LLC designates this Journal-based CME activity for a maximum of 1.0 AMA PRA Category 1 Credit(s)™. Physicians should claim only the credit commensurate with the extent of their participation in the activity.
All other clinicians completing this activity will be issued a certificate of participation. To participate in this journal CME activity: (1) review the learning objectives and author disclosures; (2) study the education content; (3) take the post-test with a 70% minimum passing score and complete the evaluation at http://www.medscape.org/journal/blood; and (4) view/print certificate. For CME questions, see page 925.
Disclosures
The authors, the Associate Editor Mortimer Poncz, and CME questions author Laurie Barclay, freelance writer and reviewer, Medscape, LLC, declare no competing financial interests.
Learning objectives
Upon completion of this activity, participants will be able to:
Describe the association of age with risk of grade 2 or higher bleeding among patients with treatment-induced hypoproliferative thrombocytopenia enrolled in prophylactic platelet dose trial (PLADO).
Describe factors affecting the association of age with risk of grade 2 or higher bleeding among patients with treatment-induced hypoproliferative thrombocytopenia enrolled in PLADO.
Describe the association of age with risk of grade 3 or higher bleeding among patients with treatment-induced hypoproliferative thrombocytopenia enrolled in PLADO.
Release date: July 26, 2012; Expiration date: July 26, 2013
Introduction
Both pediatric and adult patients undergoing hematopoietic stem cell transplants (HSCTs) and/or chemotherapy for leukemia and other malignant conditions experience hypoproliferative thrombocytopenia, placing them at risk for bleeding. However, the incidence of bleeding in children compared with adults has not been well characterized. Likewise, the precise relations between bleeding and patient platelet count or prophylactic platelet transfusion dose in children are unknown.
One of the few studies that analyzed the relation between bleeding and platelet counts in children with leukemia was a retrospective review by Roy et al conducted between 1950 and 1955,1 when platelets were transfused to treat, rather than to prevent, overt bleeding. When patient platelet counts were either < 11 000/μL or between 11 000 and 20 000/μL, minor bleeding occurred on 53% and 47% of days, respectively, and clinically significant bleeding (gross gastrointestinal bleeding or hematuria) occurred on 26% and 10% of days, respectively. However, current relevance of these data is limited because children in that study undoubtedly received aspirin; none received modern chemotherapy, radiation treatment, or a HSCT; retrospective review may have failed to detect some bleeds.
Subsequent studies suggested that prophylactic platelet transfusions could reduce bleeding in thrombocytopenic patients with leukemia, both in adults2 and children.3 In the 1970s, Murphy et al found that children with leukemia experienced an average of 7.9 significant bleeds (nasal/oral bleeding requiring packing, gross gastrointestinal or genitourinary bleeding, CNS bleeding, or bleeding that required red cell transfusion) per 100 months of observation without prophylactic platelet transfusions versus 1.9 for children given prophylactic platelet transfusions.3 However, relevance of these data is still questionable for the reasons discussed earlier, and a prophylactic platelet transfusion trigger of 20 000 platelets/μL was used versus the 10 000 platelets/μL trigger that is currently recommended.4 Furthermore, the source of platelet concentrates (apheresis vs whole blood–derived) and the quality and quantity of platelets transfused were probably quite different and perhaps inferior, compared with current practice.
Although there is evidence that prophylactic platelet transfusions reduce the incidence of bleeding,3 the optimal dose of platelets to transfuse is controversial.5 Roy et al randomly assigned 62 patients to receive 1 of 2 doses, based on units per body weight, when the platelet count was ≤ 25 000/μL.1 Both doses were equally efficacious in reducing bleeding incidence, but the small sample size precluded definitive dosing conclusions.
On the basis of the paucity of data and the difficulty of applying these historic studies to current practice, a secondary analysis of the recently reported Determination of the Optimal Platelet Dose Strategy to Prevent Bleeding in Thrombocytopenic Patients (PLADO) clinical trial6 was conducted to determine whether bleeding outcomes differed among the 3 pediatric age groups or between any pediatric age group versus adults.
Methods
The method and overall results of the PLADO study have previously been reported.6 PLADO was registered on www.clinicaltrials.gov with identifier NCT00128713.
Study population
Briefly, the PLADO study was a prospective, multicenter, randomized controlled trial of patients who were hospitalized and expected to become thrombocytopenic (platelet counts of ≤ 10 000 platelets/μL for ≥ 5 days) as a result of HSCT or chemotherapy for hematologic malignancy or solid tumor. The institutional review boards at participating hospitals approved the PLADO study. Adult patients were required to provide written informed consent in accordance with the Declaration of Helsinki, and, for children, a parent or legal guardian provided consent. Assent from children was obtained in accordance with local policy.
Other eligibility criteria included a body weight of 10-135 kg, prothrombin time (PT) and partial thromboplastin times (PTT) ≤ 1.3 times the upper limit of normal for the laboratory, a fibrinogen level of ≥ 100 mg/dL, and no previous platelet transfusions during the current hospitalization. Patients were ineligible if they currently had World Health Organization (WHO) grade 2 or higher bleeding7 (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), platelet refractoriness within the past 30 days, known panel reactive HLA Abs ≥ 20%, acute promyelocytic leukemia, idiopathic or thrombotic thrombocytopenic purpura, hemolytic-uremic syndrome, planned prophylactic platelet transfusions at platelet counts of > 10 000 platelets/μL, planned use of bedside-leukoreduced platelets, major surgery within 2 weeks, use of prothrombotic or antithrombotic drugs, pregnancy, or previous enrollment in the PLADO trial.
Stratification, randomization, and blinding
Randomization was stratified with 4 disease treatment categories (autologous or syngeneic HSCT, allogeneic HSCT, chemotherapy without HSCT for hematologic malignancy, or chemotherapy without HSCT for solid tumor) and was balanced by hospital.8 Randomization did not take patient age into account. Enrolled patients were randomly assigned in a 1:1:1 ratio, by computer-generated permuted blocks within disease treatment categories, to receive prophylactic platelet transfusions with the use of 1 of 3 doses, based on the patient's body surface area (BSA): a medium dose (MD), 2.2 × 1011 platelets/m2; a lower dose, 1.1 × 1011 platelets/m2 (half the MD); or a higher dose, 4.4 × 1011 platelets/m2 (2 times the MD). The MD was selected to correspond to the usual adult platelet dose of either 1 pool of whole blood–derived platelets from 4 to 6 donors or 1 apheresis collection per transfusion. Blood bank technical staff members knew both the patient's target dose and the acceptable dose range (0.75-1.25 times the target dose) but were not told the patient's randomization assignment. Staff members who performed the daily hemostatic assessments were not given any information about the patient's assigned dose, but they may not have been completely blinded because of differences in transfusion volumes between the dose groups.
Transfusions
The prophylactic platelet transfusion trigger was a morning platelet count of ≤ 10 000 platelets/μL. Platelets could also be administered at any time for acute bleeding or in association with an invasive procedure. If clinically indicated, the dose or trigger for prophylactic platelet transfusions could be altered at the discretion of the attending physician. However, once the patient stabilized, the study dose and trigger guidelines were to be reinstituted. HLA-selected platelet units were transfused completely, regardless of the patient's assigned dose, to avoid wasting the product. Red blood cells (RBCs) were transfused according to local guidelines. Both platelets and RBCs were leukoreduced.
Hemostatic assessments
Daily assessments were performed by trained research staff members and included physical assessments, patient/family interviews, and medical record reviews. Data were collected on all bleeding manifestations, excluding urine dipstick and stool guaiac tests. A computer algorithm then calculated each patient's daily bleeding grade, based on both the bleeding signs and symptoms and red cell transfusion data. Daily platelet counts and hematocrits were collected.
Study completion
Patients completed study participation the first time any of the following occurred: (1) 30 days after their first platelet transfusion, (2) 10 days without a platelet transfusion, (3) hospital discharge, (4) death, or (5) study withdrawal.
Statistical analysis
Analyses are restricted to the 1272 PLADO patients who received ≥ 1 platelet transfusion while on study. Patients were analyzed in their assigned dose group, even if some or all of their platelet transfusions were outside their dose range. For these current posthoc analyses of patient age, patients were divided into 4 age groups according to the likelihood of biologic and physiologic similarities within each group: 0-5 years, 6-12 years, 13-18 years, and ≥ 19 years. The age groups were compared for baseline characteristics, bleeding outcomes, morning platelet counts, number of transfusion episodes, total quantity of blood products received, and compliance with both the study dose and the prophylactic platelet transfusion trigger of 10 000 platelets/μL. The data were analyzed with SAS Version 9.2 software.9
For unadjusted age-group comparisons, Fisher exact test was used for binary and categorical variables; ANOVA was used for variables with approximately normal distributions in each age group, and the Kruskal-Wallis test was used for continuous variables that were not normally distributed. The time from stem cell transplantation to onset of grade 2 or higher bleeding was compared with Cox proportional hazards regression.10 Because many outcome variables were skewed, medians and quartiles are presented rather than means and SDs.
For multiple predictor models, logistic regression was used for binary outcomes, and generalized linear models were used for continuous outcomes. For analyses with patient-day as the unit of analysis, within-person correlation was taken into account. Interaction tests were used to assess whether the age-group effect differed by disease treatment category or by randomized dose group. For variables that were not normally distributed, ANCOVA on the ranks was used for interaction tests. The group receiving chemotherapy without HSCT for solid tumor was omitted from interaction analyses by disease treatment category because of the small number of patients (n = 7).
For each age-group comparison, a P value is reported for the 3-degrees-of-freedom test in which the null hypothesis is that there are no differences between the 4 age groups and the alternative hypothesis is that ≥ 1 pair of age groups differs. When this overall comparison was significant at the .05 level, P values for all 6 pairwise comparisons of age groups were calculated, and pairwise comparisons that were significant at the .05 level are reported.
No adjustment was made for analyzing multiple outcomes.
Results
The PLADO trial enrolled 1351 patients during 2004-2007 at 26 hospitals across the United States. Overall, 1272 patients, including 200 pediatric patients, had ≥ 1 study platelet transfusion. At 18 hospitals, > 90% of patients were adults, including 11 hospitals that solely enrolled adults. At 6 hospitals, > 90% of patients were children, including 3 hospitals that exclusively enrolled children. Similar numbers of patients were enrolled in each of the pediatric groups: 0-5 years (n = 66), 6-12 years (n = 69), and 13-18 years (n = 65), whereas most patients were adults aged ≥ 19 years (n = 1072). The minimum subject's age was 9 months and the maximum age was 83 years.
Baseline characteristics
Comparisons of baseline characteristics among the 4 age groups are presented in Table 1. No significant differences were observed in platelet dose assignments, sex, spleen status, or baseline hemoglobin value. Children in all 3 age groups were more likely than adults to have received prior platelet and RBC transfusions. Of particular note are the differences in disease treatment categories among the age groups: the percentage of patients undergoing autologous or syngeneic HSCT varied widely between age groups (44% for ages 0-5 years, 17% for ages 6-12 years, 18% for ages 13-18 years, and 35% for adults) as did the percentage of subjects undergoing allogeneic HSCT (41%, 65%, 60%, and 38%, respectively). Chemotherapy without HSCT for hematologic malignancy was less common in the pediatric age groups than in adults (11%, 16%, 17%, and 26%, respectively). No adults and few patients in each pediatric age group received chemotherapy without HSCT for solid tumors (n = 7).
Comparison of baseline characteristics among the 4 age groups
| Characteristic . | 0-5 y . | 6-12 y . | 13-18 y . | ≥ 19 y . | Total . | P for overall comparison of the 4 age groups . | Significant pairwise comparisons* . |
|---|---|---|---|---|---|---|---|
| No. of patients | 66 | 69 | 65 | 1072 | 1272 | ||
| Treatment arm | .65 | ||||||
| Lower dose | 23 (35) | 19 (28) | 26 (40) | 349 (33) | 417 (33) | ||
| Medium dose | 25 (38) | 26 (38) | 18 (28) | 354 (33) | 423 (33) | ||
| Higher dose | 18 (27) | 24 (35) | 21 (32) | 369 (34) | 432 (34) | ||
| Sex | .34 | ||||||
| Male | 41 (62) | 40 (58) | 46 (71) | 641 (60) | 768 (60) | ||
| Female | 25 (38) | 29 (42) | 19 (29) | 431 (40) | 504 (40) | ||
| Age, y | 3.2 (2.2, 4.6) | 8.9 (7.4, 11.7) | 15.6 (14.8, 16.7) | 52.3 (41.6, 60.4) | 48.8 (31.9, 59.0) | NA | |
| Weight, kg | 14.2 (11.9, 17.6) | 30.9 (24.6, 38.5) | 63.9 (55.2, 82.5) | 81.1 (69.2, 94.2) | 78.1 (63.0, 92.2) | < .001 | |
| Height, cm | 96.1 (85.0, 102.6) | 131 (122.5, 145.7) | 168.2 (162.0, 176.0) | 171.6 (164.0, 178.0) | 170 (161.0, 177.0) | < .001 | |
| BSA, m2 | 0.6 (0.5, 0.7) | 1.0 (0.9, 1.3) | 1.8 (1.6, 2.0) | 2.0 (1.8, 2.1) | 1.9 (1.7, 2.1) | < .001 | |
| Disease treatment category | < .001 | A, B, C, E, F | |||||
| Autologous or syngeneic stem cell transplantation | 29 (44) | 12 (17) | 12 (18) | 376 (35) | 429 (34) | ||
| Allogeneic stem cell transplantation | 27 (41) | 45 (65) | 39 (60) | 412 (38) | 523 (41) | ||
| Chemotherapy without HSCT for hematologic malignancy | 7 (11) | 11 (16) | 11 (17) | 284 (26) | 313 (25) | ||
| Chemotherapy without HSCT for solid tumor† | 3 (5) | 1 (1) | 3 (5) | 0 (0) | 7 (1) | ||
| Primary diagnosis | < .001 | A, B, C, E, F | |||||
| Acute lymphocytic leukemia | 9 (14) | 19 (28) | 14 (22) | 74 (7) | 116 (9) | ||
| Acute myelogenous leukemia | 11 (17) | 22 (32) | 25 (38) | 411 (38) | 469 (37) | ||
| Chronic myelogenous leukemia | 1 (2) | 2 (3) | 6 (9) | 45 (4) | 54 (4) | ||
| Chronic lymphocytic leukemia | 0 (0) | 0 (0) | 0 (0) | 21 (2) | 21 (2) | ||
| Chronic myelomonocytic leukemia | 0 (0) | 0 (0) | 0 (0) | 6 (1) | 6 (< 1) | ||
| Non-Hodgkin lymphoma | 1 (2) | 8 (12) | 5 (8) | 190 (18) | 204 (16) | ||
| Hodgkin lymphoma | 0 (0) | 2 (3) | 2 (3) | 75 (7) | 79 (6) | ||
| Myelodysplastic syndromes | 0 (0) | 0 (0) | 0 (0) | 74 (7) | 74 (6) | ||
| Plasma cell disorders | 0 (0) | 0 (0) | 0 (0) | 157 (15) | 157 (12) | ||
| Non–hematopoietic solid tumor carcinoma | 9 (14) | 0 (0) | 1 (2) | 4 (< 1) | 14 (1) | ||
| Non–hematopoietic solid tumor sarcoma | 0 (0) | 1 (1) | 3 (5) | 1 (< 1) | 5 (< 1) | ||
| Non–hematologic solid tumor (nonsarcoma, noncarcinoma) | 24 (36) | 5 (7) | 5 (8) | 3 (< 1) | 37 (3) | ||
| Aplastic anemia congenital and acquired | 5 (8) | 7 (10) | 3 (5) | 9 (1) | 24 (2) | ||
| Other | 6 (9) | 3 (4) | 1 (2) | 2 (< 1) | 12 (1) | ||
| Desired type of platelets | < .001 | B, C, E | |||||
| Apheresis | 62 (94) | 57 (83) | 51 (78) | 711 (66) | 881 (69) | ||
| Pooled whole blood–derived platelet concentrates | 4 (6) | 12 (17) | 14 (22) | 361 (34) | 391 (31) | ||
| Previous platelet transfusion | < .001 | C, E, F | |||||
| Yes | 60 (91) | 59 (86) | 55 (85) | 550 (52) | 724 (57) | ||
| No | 6 (9) | 10 (14) | 10 (15) | 517 (48) | 543 (43) | ||
| Unknown | 0 | 0 | 0 | 5 | 5 | ||
| Previous RBC transfusion | < .001 | C, E, F | |||||
| Yes | 63 (95) | 65 (94) | 60 (92) | 768 (72) | 956 (75) | ||
| No | 3 (5) | 4 (6) | 5 (8) | 300 (28) | 312 (25) | ||
| Unknown | 0 | 0 | 0 | 4 | 4 | ||
| Spleen | .72 | ||||||
| Splenectomy | 3 (5) | 1 (1) | 1 (2) | 28 (3) | 33 (3) | ||
| Enlarged | 2 (3) | 1 (1) | 1 (2) | 49 (5) | 53 (4) | ||
| Not enlarged | 61 (92) | 67 (97) | 63 (97) | 995 (93) | 1186 (93) | ||
| Laboratory values | |||||||
| Hemoglobin level, g/dL | 9.4 (8.7, 10.6) | 10 (8.9, 10.7) | 9.8 (8.9, 11.5) | 9.8 (9.1, 10.7) | 9.8 (9.0, 10.7) | .23 | |
| Unknown | 1 | 0 | 0 | 3 | 4 | ||
| Hematocrit, % | 27.3 (24.2, 30.0) | 28 (26.0, 30.8) | 27.6 (25.9, 32.6) | 28.3 (26.0, 31.0) | 28 (26.0, 31.0) | .05 | |
| Unknown | 1 | 0 | 0 | 8 | 9 | ||
| Platelet count, 103/μL | 91 (44.0, 155.0) | 50 (25.0, 147.0) | 44 (25.0, 105.0) | 36 (25.0, 54.0) | 38 (25.0, 61.0) | < .001 | B, C, E, F |
| Unknown | 1 | 0 | 0 | 3 | 4 | ||
| PT, × upper limit of normal | 0.96 (0.89, 1.02) | 0.96 (0.90, 1.04) | 1.01 (0.93, 1.07) | 0.91 (0.83, 0.97) | 0.92 (0.84, 0.99) | < .001 | C, E, F |
| Unknown | 11 | 9 | 12 | 170 | 202 | ||
| INR | 1 (0.9, 1.1) | 1 (1.0, 1.1) | 1 (1.0, 1.2) | 1.1 (1.0, 1.1) | 1 (1.0, 1.1) | .02 | C |
| Unknown | 1 | 0 | 0 | 13 | 14 | ||
| No. with neither PT nor INR | 1 | 0 | 0 | 11 | 12 | .77 | |
| PTT, sec | 32.2 (29.0, 34.8) | 31.3 (28.9, 35.0) | 32.7 (29.9, 36.7) | 28.7 (26.0, 32.0) | 29 (26.2, 32.7) | < .001 | C, E, F |
| Unknown | 1 | 0 | 0 | 11 | 12 | ||
| Fibrinogen level, mg/dL | 306 (29.0, 357.0) | 328 (266.0, 422.0) | 344 (276.0, 410.0) | 369 (283.0, 480.0) | 360 (278.5, 466.5) | < .001 | B, C, E, F |
| Unknown | 1 | 0 | 0 | 15 | 16 | ||
| Lymphocytotoxic antibody screen‡ | |||||||
| 0% | 50 (79) | 55 (83) | 53 (85) | 736 (74) | 894 (75) | .04 | A |
| 1%-19% | 4 (6) | 9 (14) | 6 (10) | 156 (16) | 175 (15) | ||
| ≥ 20% | 9 (14) | 2 (3) | 3 (5) | 107 (11) | 121 (10) | ||
| Unknown | 3 | 3 | 3 | 73 | 82 |
| Characteristic . | 0-5 y . | 6-12 y . | 13-18 y . | ≥ 19 y . | Total . | P for overall comparison of the 4 age groups . | Significant pairwise comparisons* . |
|---|---|---|---|---|---|---|---|
| No. of patients | 66 | 69 | 65 | 1072 | 1272 | ||
| Treatment arm | .65 | ||||||
| Lower dose | 23 (35) | 19 (28) | 26 (40) | 349 (33) | 417 (33) | ||
| Medium dose | 25 (38) | 26 (38) | 18 (28) | 354 (33) | 423 (33) | ||
| Higher dose | 18 (27) | 24 (35) | 21 (32) | 369 (34) | 432 (34) | ||
| Sex | .34 | ||||||
| Male | 41 (62) | 40 (58) | 46 (71) | 641 (60) | 768 (60) | ||
| Female | 25 (38) | 29 (42) | 19 (29) | 431 (40) | 504 (40) | ||
| Age, y | 3.2 (2.2, 4.6) | 8.9 (7.4, 11.7) | 15.6 (14.8, 16.7) | 52.3 (41.6, 60.4) | 48.8 (31.9, 59.0) | NA | |
| Weight, kg | 14.2 (11.9, 17.6) | 30.9 (24.6, 38.5) | 63.9 (55.2, 82.5) | 81.1 (69.2, 94.2) | 78.1 (63.0, 92.2) | < .001 | |
| Height, cm | 96.1 (85.0, 102.6) | 131 (122.5, 145.7) | 168.2 (162.0, 176.0) | 171.6 (164.0, 178.0) | 170 (161.0, 177.0) | < .001 | |
| BSA, m2 | 0.6 (0.5, 0.7) | 1.0 (0.9, 1.3) | 1.8 (1.6, 2.0) | 2.0 (1.8, 2.1) | 1.9 (1.7, 2.1) | < .001 | |
| Disease treatment category | < .001 | A, B, C, E, F | |||||
| Autologous or syngeneic stem cell transplantation | 29 (44) | 12 (17) | 12 (18) | 376 (35) | 429 (34) | ||
| Allogeneic stem cell transplantation | 27 (41) | 45 (65) | 39 (60) | 412 (38) | 523 (41) | ||
| Chemotherapy without HSCT for hematologic malignancy | 7 (11) | 11 (16) | 11 (17) | 284 (26) | 313 (25) | ||
| Chemotherapy without HSCT for solid tumor† | 3 (5) | 1 (1) | 3 (5) | 0 (0) | 7 (1) | ||
| Primary diagnosis | < .001 | A, B, C, E, F | |||||
| Acute lymphocytic leukemia | 9 (14) | 19 (28) | 14 (22) | 74 (7) | 116 (9) | ||
| Acute myelogenous leukemia | 11 (17) | 22 (32) | 25 (38) | 411 (38) | 469 (37) | ||
| Chronic myelogenous leukemia | 1 (2) | 2 (3) | 6 (9) | 45 (4) | 54 (4) | ||
| Chronic lymphocytic leukemia | 0 (0) | 0 (0) | 0 (0) | 21 (2) | 21 (2) | ||
| Chronic myelomonocytic leukemia | 0 (0) | 0 (0) | 0 (0) | 6 (1) | 6 (< 1) | ||
| Non-Hodgkin lymphoma | 1 (2) | 8 (12) | 5 (8) | 190 (18) | 204 (16) | ||
| Hodgkin lymphoma | 0 (0) | 2 (3) | 2 (3) | 75 (7) | 79 (6) | ||
| Myelodysplastic syndromes | 0 (0) | 0 (0) | 0 (0) | 74 (7) | 74 (6) | ||
| Plasma cell disorders | 0 (0) | 0 (0) | 0 (0) | 157 (15) | 157 (12) | ||
| Non–hematopoietic solid tumor carcinoma | 9 (14) | 0 (0) | 1 (2) | 4 (< 1) | 14 (1) | ||
| Non–hematopoietic solid tumor sarcoma | 0 (0) | 1 (1) | 3 (5) | 1 (< 1) | 5 (< 1) | ||
| Non–hematologic solid tumor (nonsarcoma, noncarcinoma) | 24 (36) | 5 (7) | 5 (8) | 3 (< 1) | 37 (3) | ||
| Aplastic anemia congenital and acquired | 5 (8) | 7 (10) | 3 (5) | 9 (1) | 24 (2) | ||
| Other | 6 (9) | 3 (4) | 1 (2) | 2 (< 1) | 12 (1) | ||
| Desired type of platelets | < .001 | B, C, E | |||||
| Apheresis | 62 (94) | 57 (83) | 51 (78) | 711 (66) | 881 (69) | ||
| Pooled whole blood–derived platelet concentrates | 4 (6) | 12 (17) | 14 (22) | 361 (34) | 391 (31) | ||
| Previous platelet transfusion | < .001 | C, E, F | |||||
| Yes | 60 (91) | 59 (86) | 55 (85) | 550 (52) | 724 (57) | ||
| No | 6 (9) | 10 (14) | 10 (15) | 517 (48) | 543 (43) | ||
| Unknown | 0 | 0 | 0 | 5 | 5 | ||
| Previous RBC transfusion | < .001 | C, E, F | |||||
| Yes | 63 (95) | 65 (94) | 60 (92) | 768 (72) | 956 (75) | ||
| No | 3 (5) | 4 (6) | 5 (8) | 300 (28) | 312 (25) | ||
| Unknown | 0 | 0 | 0 | 4 | 4 | ||
| Spleen | .72 | ||||||
| Splenectomy | 3 (5) | 1 (1) | 1 (2) | 28 (3) | 33 (3) | ||
| Enlarged | 2 (3) | 1 (1) | 1 (2) | 49 (5) | 53 (4) | ||
| Not enlarged | 61 (92) | 67 (97) | 63 (97) | 995 (93) | 1186 (93) | ||
| Laboratory values | |||||||
| Hemoglobin level, g/dL | 9.4 (8.7, 10.6) | 10 (8.9, 10.7) | 9.8 (8.9, 11.5) | 9.8 (9.1, 10.7) | 9.8 (9.0, 10.7) | .23 | |
| Unknown | 1 | 0 | 0 | 3 | 4 | ||
| Hematocrit, % | 27.3 (24.2, 30.0) | 28 (26.0, 30.8) | 27.6 (25.9, 32.6) | 28.3 (26.0, 31.0) | 28 (26.0, 31.0) | .05 | |
| Unknown | 1 | 0 | 0 | 8 | 9 | ||
| Platelet count, 103/μL | 91 (44.0, 155.0) | 50 (25.0, 147.0) | 44 (25.0, 105.0) | 36 (25.0, 54.0) | 38 (25.0, 61.0) | < .001 | B, C, E, F |
| Unknown | 1 | 0 | 0 | 3 | 4 | ||
| PT, × upper limit of normal | 0.96 (0.89, 1.02) | 0.96 (0.90, 1.04) | 1.01 (0.93, 1.07) | 0.91 (0.83, 0.97) | 0.92 (0.84, 0.99) | < .001 | C, E, F |
| Unknown | 11 | 9 | 12 | 170 | 202 | ||
| INR | 1 (0.9, 1.1) | 1 (1.0, 1.1) | 1 (1.0, 1.2) | 1.1 (1.0, 1.1) | 1 (1.0, 1.1) | .02 | C |
| Unknown | 1 | 0 | 0 | 13 | 14 | ||
| No. with neither PT nor INR | 1 | 0 | 0 | 11 | 12 | .77 | |
| PTT, sec | 32.2 (29.0, 34.8) | 31.3 (28.9, 35.0) | 32.7 (29.9, 36.7) | 28.7 (26.0, 32.0) | 29 (26.2, 32.7) | < .001 | C, E, F |
| Unknown | 1 | 0 | 0 | 11 | 12 | ||
| Fibrinogen level, mg/dL | 306 (29.0, 357.0) | 328 (266.0, 422.0) | 344 (276.0, 410.0) | 369 (283.0, 480.0) | 360 (278.5, 466.5) | < .001 | B, C, E, F |
| Unknown | 1 | 0 | 0 | 15 | 16 | ||
| Lymphocytotoxic antibody screen‡ | |||||||
| 0% | 50 (79) | 55 (83) | 53 (85) | 736 (74) | 894 (75) | .04 | A |
| 1%-19% | 4 (6) | 9 (14) | 6 (10) | 156 (16) | 175 (15) | ||
| ≥ 20% | 9 (14) | 2 (3) | 3 (5) | 107 (11) | 121 (10) | ||
| Unknown | 3 | 3 | 3 | 73 | 82 |
Values for categorical variables are presented as n (%); values for continuous variables are presented as median (quartile 1, quartile 3).
INR indicates international normalized ratio; and NA, not applicable.
A: 0-5 years vs 6-12 years; B: 0-5 years vs 13-18 years; C: 0-5 years vs ≥ 19 years; D: 6-12 years vs 13-18 years; E: 6-12 years vs ≥ 19 years; F: 13-18 years vs ≥ 19 years.
Excluded from analyses of interaction between age group and disease treatment category because of small sample sizes.
Patients already known to have panel reactive antibody ≥ 20% were excluded from PLADO. However, some patients were found to have values ≥ 20% on the study's baseline test.
Furthermore, within each HSCT disease treatment category, the primary diagnoses differed significantly by age group (supplemental Table 2). Pediatric patients undergoing autologous or syngeneic HSCT usually had nonhematologic solid tumors (eg, neuroblastoma, Wilms tumor, brain tumors), whereas adults (> 90%) had multiple myeloma and lymphoma. Among subjects undergoing allogeneic transplantations, acute myelogenous leukemia (AML) was common in all age groups, whereas acute lymphocytic leukemia (ALL) and aplastic anemia were more common in pediatric patients, and leukemias and myelodysplastic syndromes were more common in adults. In both pediatric and adult patients who received chemotherapy without HSCT for hematologic malignancy, ALL and AML were the most common diagnoses.
Protocol compliance
Compliance with randomized prophylactic platelet transfusion doses.
A total of 6030 prophylactic platelet transfusions were given. After excluding 588 transfusions that were HLA-selected or volume-reduced, and 88 transfusions with missing data, 5384 transfusions were analyzed for compliance with the assigned target dose. Of these, 4925 transfusions (91%) were compliant. The predominant noncompliance in all age groups was issuing more platelets than the patient was randomly assigned to receive. For age groups 0-5, 6-12, 13-18, and ≥ 19 years, doses were compliant for 85%, 93%, 77%, and 93% of transfusions, respectively (P = .06, adjusting for within-person correlation). In models that also adjusted for randomized dose assignment or for disease treatment category, age group was still not a significant predictor of dose compliance.
Compliance with prophylactic platelet transfusion trigger of 10 000 platelets/μL.
On the basis of clinical events, the protocol allowed either a temporary or permanent increase in the transfusion trigger at the discretion of the patient's physician. Of 24 690 patient-days on study, the 10 000 trigger was adhered to on 22 704 days (92%). In all age groups, nearly all noncompliance was because of providing prophylactic platelets to a patient with a morning platelet count > 10 000 platelets/μL. For age groups 0-5, 6-12, 13-18, and ≥ 19 years, the trigger was adhered to on 93%, 92%, 89%, and 92% of study days, respectively (P = .22, adjusting for within-person correlation). In models that also adjusted for randomized dose group or for disease treatment category, age group was still not a significant predictor of trigger compliance.
Bleeding outcomes
Age group was a significant predictor of all but one of the bleeding outcomes analyzed. In addition, interaction tests showed that for many of the outcomes related to bleeding of grade 2 and higher, the effect of age differed by disease treatment category and was more pronounced in the group of patients receiving autologous/syngeneic HSCT.
WHO bleeding grades
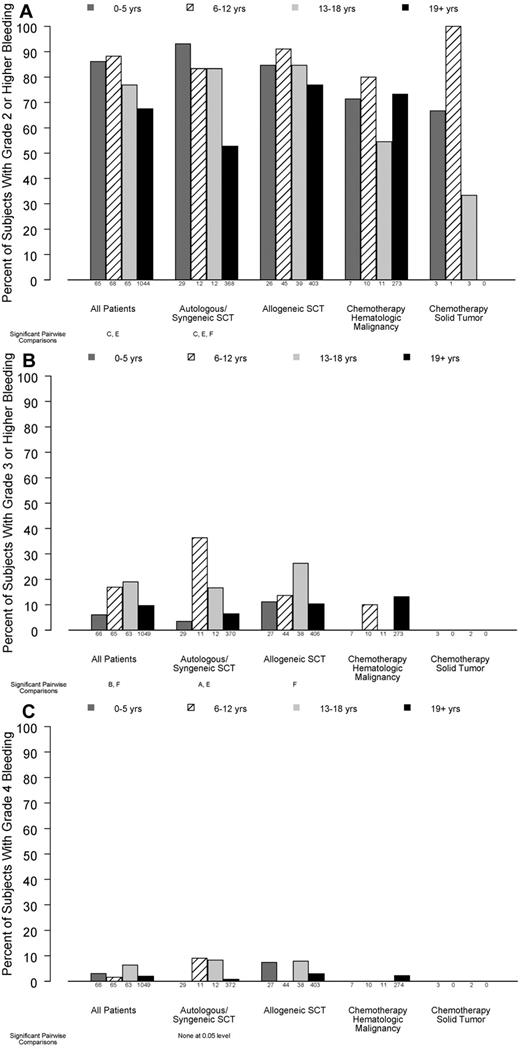
As shown in Figure 1A, younger children were significantly more likely than adults to have ≥ 1 day of grade 2 or higher bleeding while on study (86%, 88%, 77%, and 67%, for ages 0-5, 6-12, 13-18, and ≥ 19 years, respectively; P < .001). This effect differed by disease treatment category (P for interaction = .04). In patients receiving autologous/syngeneic HSC transplant, bleeding of grade 2 or higher occurred in 93%, 83%, 83%, and 53% of patients in the 4 age groups, respectively (P < .001). In patients who received allogeneic HSCT, bleeding of grade 2 or higher occurred in 85%, 91%, 85%, and 77% of the patients in the 4 age groups, respectively (P = .10). In the small number of patients receiving chemotherapy without HSCT for hematologic malignancy, age group was not a significant predictor of grade 2 or higher bleeding (71%, 80%, 55%, and 73% respectively; P = .53).
Relation between age group and percentage of patients experiencing bleeding of various grades, in all patients and stratified by disease treatment category. Age group comparisons with pairwise P values < .05 are noted (A: 0-5 years vs 6-12 years; B: 0-5 years vs 13-18 years; C: 0-5 years vs ≥ 19 years; D: 6-12 years vs 13-18 years; E: 6-12 years vs ≥ 19 years; F: 13-18 years vs ≥ 19 years). (A) At least 1 day with grade 2 or higher bleeding (overall test for age group, P < .001; interaction of age group with disease treatment category, P = .04), (B) ≥ 1 day with grade 3 or higher bleeding (overall test for age group, P = .02; P for interaction = .13), (C) ≥ 1 day with grade 4 bleeding (P = .12, P for interaction not estimable).
Relation between age group and percentage of patients experiencing bleeding of various grades, in all patients and stratified by disease treatment category. Age group comparisons with pairwise P values < .05 are noted (A: 0-5 years vs 6-12 years; B: 0-5 years vs 13-18 years; C: 0-5 years vs ≥ 19 years; D: 6-12 years vs 13-18 years; E: 6-12 years vs ≥ 19 years; F: 13-18 years vs ≥ 19 years). (A) At least 1 day with grade 2 or higher bleeding (overall test for age group, P < .001; interaction of age group with disease treatment category, P = .04), (B) ≥ 1 day with grade 3 or higher bleeding (overall test for age group, P = .02; P for interaction = .13), (C) ≥ 1 day with grade 4 bleeding (P = .12, P for interaction not estimable).
The percentage of subjects who experienced grade 3 or higher bleeding also differed by age group (P = .02; Figure 1B). Only 28 patients experienced grade 4 bleeding, and this did not differ by age group (P = .12; Figure 1C).
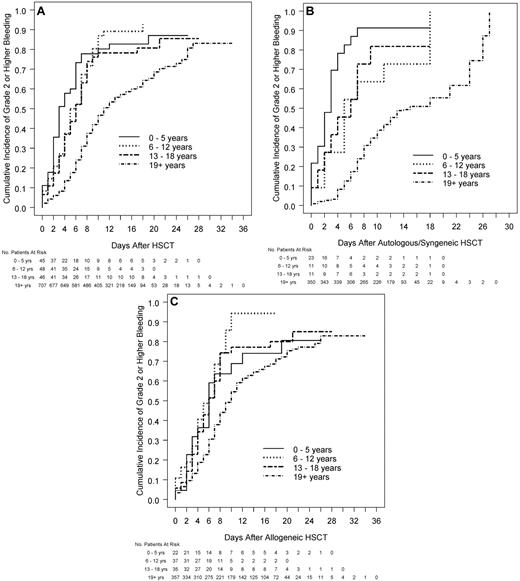
The median number of days with grade 2 or higher bleeding was 3 in each pediatric age group versus 1 in adults (Table 2; P < .001). Among patients who received HSCT, children had significantly shorter times from transplantation to grade 2 or higher bleeding than adults (median days, 3.0, 5.5, 6.0, and 11.0 for the 4 age groups, respectively; P < .001; Figure 2A). This was true both for patients receiving autologous/syngeneic HSCT (Figure 2B) and for patients receiving allogeneic HSCT (Figure 2C). Further analyses showed that the effect of age on the bleeding outcomes was not affected by the assigned platelet dose. In models that included age group and platelet dose assignment, age group remained a significant predictor of the bleeding outcomes, but dose was not a significant predictor of any of the bleeding outcomes.
Number of days with grade 2 or higher bleeding by age group, overall, and within disease treatment category
| Outcome . | P for interaction between age group and disease treatment category . | 0-5 y . | 6-12 y . | 13-18 y . | ≥ 19 y . | Total . | P for overall comparison of the 4 age groups . | Significant pairwise comparisons* . |
|---|---|---|---|---|---|---|---|---|
| No. of patients | 66 | 69 | 65 | 1072 | 1272 | |||
| No. of days with bleeding of grade 2 or higher, all patients, median (quartile 1, quartile 3) | < .001 | 3 (1, 6.5) | 3 (1, 6) | 3 (0, 9.5) | 1 (0, 4) | 1 (0, 4) | < .001 | C, E, F |
| Unknown, n | 6 | 13 | 13 | 115 | 147 | |||
| No. of days with bleeding of grade 2 or higher, within disease treatment category | ||||||||
| Autologous or syngeneic stem cell transplantation, median (quartile 1, quartile 3) | 4 (2, 7) | 2 (1, 8) | 2 (1, 8) | 0 (0, 1) | 1 (0, 2) | < .001 | C, E, F | |
| Unknown, n | 3 | 1 | 2 | 31 | 37 | |||
| Allogeneic stem cell transplantation, median (quartile 1, quartile 3) | 3 (1, 6.5) | 3.5 (1.5, 6) | 5 (2, 17) | 2 (0, 5) | 2 (1, 6) | .002 | E, F | |
| Unknown, n | 3 | 5 | 8 | 47 | 63 | |||
| Chemotherapy without HSCT for hematologic malignancy, median (quartile 1, quartile 3) | 1 (0, 3) | 1 (0, 1) | 0 (0, 3) | 2 (0, 4) | 2 (0, 4) | .30 | ||
| Unknown, n | 0 | 6 | 2 | 37 | 45 | |||
| Chemotherapy without HSCT for solid tumor, median (quartile 1, quartile 3) | 2 (0, 3) | 0 (0, 0) | 0 (0, 2) | .20 | ||||
| Unknown, n | 0 | 1 | 1 | 0 | 2 |
| Outcome . | P for interaction between age group and disease treatment category . | 0-5 y . | 6-12 y . | 13-18 y . | ≥ 19 y . | Total . | P for overall comparison of the 4 age groups . | Significant pairwise comparisons* . |
|---|---|---|---|---|---|---|---|---|
| No. of patients | 66 | 69 | 65 | 1072 | 1272 | |||
| No. of days with bleeding of grade 2 or higher, all patients, median (quartile 1, quartile 3) | < .001 | 3 (1, 6.5) | 3 (1, 6) | 3 (0, 9.5) | 1 (0, 4) | 1 (0, 4) | < .001 | C, E, F |
| Unknown, n | 6 | 13 | 13 | 115 | 147 | |||
| No. of days with bleeding of grade 2 or higher, within disease treatment category | ||||||||
| Autologous or syngeneic stem cell transplantation, median (quartile 1, quartile 3) | 4 (2, 7) | 2 (1, 8) | 2 (1, 8) | 0 (0, 1) | 1 (0, 2) | < .001 | C, E, F | |
| Unknown, n | 3 | 1 | 2 | 31 | 37 | |||
| Allogeneic stem cell transplantation, median (quartile 1, quartile 3) | 3 (1, 6.5) | 3.5 (1.5, 6) | 5 (2, 17) | 2 (0, 5) | 2 (1, 6) | .002 | E, F | |
| Unknown, n | 3 | 5 | 8 | 47 | 63 | |||
| Chemotherapy without HSCT for hematologic malignancy, median (quartile 1, quartile 3) | 1 (0, 3) | 1 (0, 1) | 0 (0, 3) | 2 (0, 4) | 2 (0, 4) | .30 | ||
| Unknown, n | 0 | 6 | 2 | 37 | 45 | |||
| Chemotherapy without HSCT for solid tumor, median (quartile 1, quartile 3) | 2 (0, 3) | 0 (0, 0) | 0 (0, 2) | .20 | ||||
| Unknown, n | 0 | 1 | 1 | 0 | 2 |
A: 0-5 years vs 6-12 years; B: 0-5 years vs 13-18 years; C: 0-5 years vs ≥ 19 years; D: 6-12 years vs 13-18 years; E: 6-12 years vs ≥ 19 years; F: 13-18 years vs ≥ 19 years.
Relation between age group and time from HSCT to grade 2 or higher bleeding in all patients who received HSCT and by type of HSCT. (A) All patients who received HSCT (overall test for age group, P < .001, each pediatric age group significantly different from adults; interaction of age group with type of transplantation, P < .001). (B) Patients who receive autologous/syngeneic HSCT (overall test for age group, P < .001, each pediatric age group significantly different from adults). (C) Patients who received allogeneic HSCT (overall test for age group, P < .001, 6-12 and 13-18 years significantly different from adults).
Relation between age group and time from HSCT to grade 2 or higher bleeding in all patients who received HSCT and by type of HSCT. (A) All patients who received HSCT (overall test for age group, P < .001, each pediatric age group significantly different from adults; interaction of age group with type of transplantation, P < .001). (B) Patients who receive autologous/syngeneic HSCT (overall test for age group, P < .001, each pediatric age group significantly different from adults). (C) Patients who received allogeneic HSCT (overall test for age group, P < .001, 6-12 and 13-18 years significantly different from adults).
Relation between morning platelet count and the occurrence of grade 2 or higher bleeding
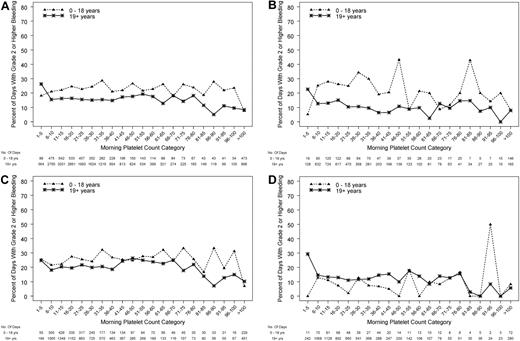
On average, morning platelet counts in pediatric subjects (0-18 years) were ∼ 12 000 platelets/μL higher than in adults (P < .001). Figure 3A depicts the relation between the morning platelet count and whether grade 2 or higher bleeding occurred during that day, treating each patient-day as the unit of analysis. Platelet counts were divided into categories each spanning 5000 platelets/μL. Some combinations of age group and morning platelet count categories were rare; therefore, the 3 pediatric age groups were combined for these analyses. In both pediatric and adult patients, the percentage of days with bleeding for both groups was fairly constant for morning platelet counts over the wide range of 6000-80 000 platelets/μL. However, over this wide range, children were significantly more likely to experience bleeding than were adults (P < .001 for age group, adjusting for morning count category and within-person correlation).
Relation between morning platelet count category and the occurrence of grade 2 or higher bleeding on that day, in pediatric and adult age groups. (A) All patients (P ≤ .001 for age group and P < .001 for platelet count category), (B) autologous/syngeneic HSCT (P ≤ .001 for age group and P < 0.001 for platelet count category), (C) allogeneic HSCT (P = .053 for age group, and P = .004 for platelet count category), (D) chemotherapy without HSCT for hematologic malignancy (logistic model did not converge because of some categories having no days with bleeding).
Relation between morning platelet count category and the occurrence of grade 2 or higher bleeding on that day, in pediatric and adult age groups. (A) All patients (P ≤ .001 for age group and P < .001 for platelet count category), (B) autologous/syngeneic HSCT (P ≤ .001 for age group and P < 0.001 for platelet count category), (C) allogeneic HSCT (P = .053 for age group, and P = .004 for platelet count category), (D) chemotherapy without HSCT for hematologic malignancy (logistic model did not converge because of some categories having no days with bleeding).
In further analyses of morning platelet count as a predictor of grade 2 or higher bleeding, the age group effect differed by disease treatment category (P for interaction < .001). Among patients who received either type of HSCT, children had higher bleeding risk than adults (P < .001, Figure 3A). This reached statistical significance for patients who received autologous/syngeneic HSCT (P < .001; Figure 3B) and approached significance for patients who received allogeneic HSCT (P = .053; Figure 3C). The logistic model did not converge for patients who received chemotherapy without HSCT for hematologic malignancies because some combinations of age group and morning platelet count had no days with bleeding, but it appears that, in this disease treatment category, children have similar or somewhat lower risk of bleeding than adults over a wide range of platelet counts (Figure 3D).
Organ system bleeding
The WHO bleeding score is based on bleeding manifestations in 8 organ systems and on hemodynamic instability (supplemental Table 1). Figure 4 depicts the percentage of patients in each age group that experienced grade 2 or higher bleeding in each organ system or bleeding that resulted in hemodynamic instability. Supplemental Table 3 provides further detail on specific bleeding manifestations in each age group.
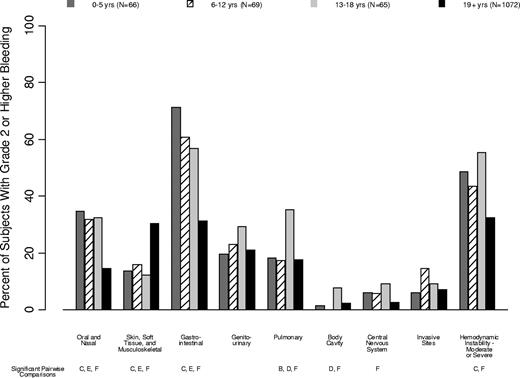
Percentage of patients in each age group that experienced grade 2 or higher bleeding in each organ system. Age group comparisons with pairwise P values < .05 are noted (A: 0-5 years vs 6-12 years; B: 0-5 years vs 13-18 years; C: 0-5 years vs ≥ 19 years; D: 6-12 years vs 13-18 years; E: 6-12 years vs ≥ 19 years; F: 13-18 years vs ≥ 19 years).
Percentage of patients in each age group that experienced grade 2 or higher bleeding in each organ system. Age group comparisons with pairwise P values < .05 are noted (A: 0-5 years vs 6-12 years; B: 0-5 years vs 13-18 years; C: 0-5 years vs ≥ 19 years; D: 6-12 years vs 13-18 years; E: 6-12 years vs ≥ 19 years; F: 13-18 years vs ≥ 19 years).
Oral/nasal bleeding was more common in children than in adults (35%, 32%, 32%, and 14% in the 4 age groups, respectively; P < .001). Gastrointestinal bleeding, independent of swallowed nasal blood, was also more common in children than in adults (71%, 61%, 57%, and 31%, respectively; P < .001). Skin/soft tissue/musculoskeletal bleeding was less common in children than in adults (14%, 16%, 12%, and 30% of patients, respectively; P < .001).
Pulmonary bleeding was most common in patients aged 13-18 years (18%, 17%, 35%, 18%; P = .01), as was visible blood in a body cavity (2%, 0%, 8%, 2%; P = .05), CNS bleeding (6%, 6%, 9%, 3%; P = .009), and moderate-to-severe hemodynamic instability (48%, 43%, 55%, 33%; P < .001).
Resource utilization outcomes
Data on platelet and RBC utilization outcomes by age group are shown in Figure 5A through D. For many of the resource utilization outcomes, interaction tests showed that the effect of age group differed by disease treatment category.
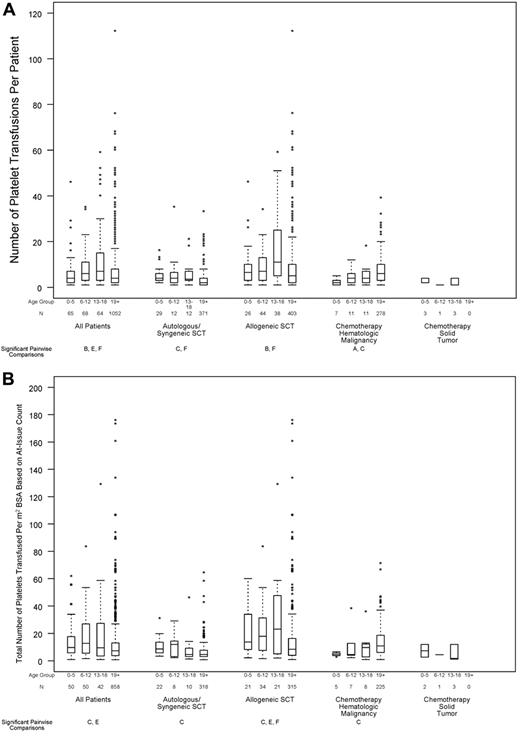
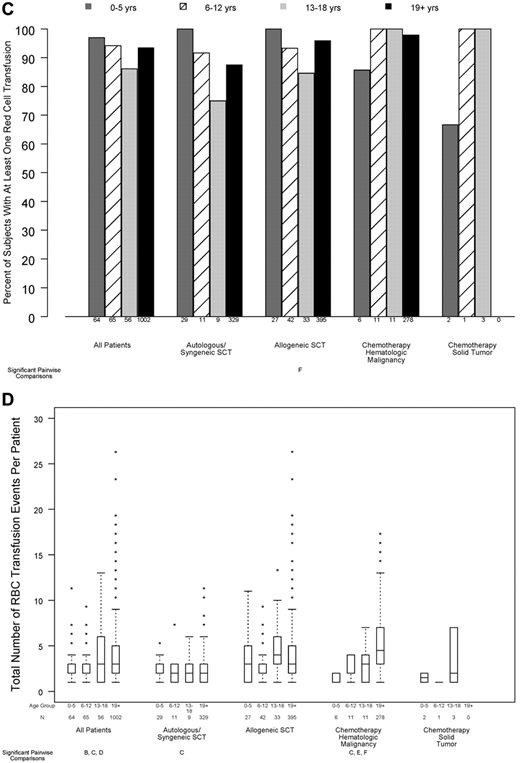
Relation between age group and transfusion resource requirements, in all patients and within disease treatment category. Age group comparisons with pairwise P values < .05 are noted (A: 0-5 years vs 6-12 years; B: 0-5 years vs 13-18 years; C: 0-5 years vs ≥ 19 years; D: 6-12 years vs 13-18 years; E: 6-12 years vs ≥ 19 years; F: 13-18 years vs ≥ 19 years). (A) Number of platelet transfusions per patient (overall test for age group, P < .001; interaction between age group and disease treatment category, P < .001), (B) total number of platelets transfused per BSA, based on at-issue count (overall test for age group, P < .001; interaction between age group and disease treatment category, P < .001). (C) Percentage of patients who received ≥ 1 red cell transfusion (overall test for age group, P = .11; P for interaction not estimable), (D) total number of RBC transfusion events (overall test for age group, P = .045; interaction between age group and disease treatment category, P < .001).
Relation between age group and transfusion resource requirements, in all patients and within disease treatment category. Age group comparisons with pairwise P values < .05 are noted (A: 0-5 years vs 6-12 years; B: 0-5 years vs 13-18 years; C: 0-5 years vs ≥ 19 years; D: 6-12 years vs 13-18 years; E: 6-12 years vs ≥ 19 years; F: 13-18 years vs ≥ 19 years). (A) Number of platelet transfusions per patient (overall test for age group, P < .001; interaction between age group and disease treatment category, P < .001), (B) total number of platelets transfused per BSA, based on at-issue count (overall test for age group, P < .001; interaction between age group and disease treatment category, P < .001). (C) Percentage of patients who received ≥ 1 red cell transfusion (overall test for age group, P = .11; P for interaction not estimable), (D) total number of RBC transfusion events (overall test for age group, P = .045; interaction between age group and disease treatment category, P < .001).
In the 1249 patients with complete data on number of platelet transfusion episodes, age was a significant predictor of this outcome (Figure 5A; P < .001). Of particular note, children ages 0-5 years received significantly fewer platelet transfusions than subjects ages 13-18 years, whereas children ages 6-12 years and ages 13-18 years had significantly more platelet transfusions than patients aged ≥ 19 years. After adjusting for the assigned platelet dose arm, the same age comparisons remained statistically significant. The assigned dose was also a significant predictor for the number of platelet transfusions given for all ages. For all ages, there was a trend toward more platelet transfusions in the lower dose arm. Further analyses found that the effect of age on the number of platelet transfusions did not differ by assigned dose arm (P for interaction = .80).
The 1000 patients with no missing data on product platelet counts were evaluated for the total number of platelets transfused per square meter of BSA. In an unadjusted analysis, age was a significant predictor of the total number of platelets transfused per square meter of BSA (Figure 5B; P < .001). Children ages 0-5 and 6-12 years had significantly more platelets transfused per BSA than patients aged ≥ 19 years. When adjusted for the assigned platelet dose, the same age comparisons remained statistically significant (P = .004 and P = .001, respectively).
More than 90% of patients required ≥ 1 RBC transfusion while on study, and this percentage did not significantly differ by age group (Figure 5C; P = .11). However, there was a small, but statistically significant, difference in the number of RBC transfusions per patient, which was lower in the 2 youngest age groups (Figure 5D; P = .045).
Discussion
The current secondary analysis of the PLADO study was conducted to determine whether either bleeding outcomes or the relation between platelet dose per transfusion and bleeding might vary with age, despite the primary finding of the original report that, when all age groups were combined, there was no such platelet dose effect overall or within any disease treatment category.6 For this secondary analysis, patients were divided into 3 pediatric age groups according to the likelihood of biologic and physiologic similarities of subjects within each group (0-5 years, 6-12 years, and 13-18 years), as well as an adult group (≥ 19 years) to determine whether there were age-related differences in study outcomes. The 4 age groups differed significantly in many baseline characteristics, including disease treatment categories and primary diagnosis (Table 1).
Importantly, large and statistically significant differences were found between age groups in the primary end point of the PLADO study, that is, the percentage of patients having ≥ 1 days of grade 2 or higher bleeding. This outcome occurred in 86% of patients ages 0-5 years, 88% of patients ages 6-12 years, 77% of patients ages 13-18 years, and 67% of adults. Similar to the overall results of the PLADO trial,6 no effect of prophylactic platelet transfusion dose was observed in any age group. The PLADO data do not indicate that pediatric patients would benefit from a higher prophylactic transfusion trigger than the 10 000 platelets/μL trigger used in this study, because children, particularly those undergoing HSCT, were at higher risk of bleeding than adults over a wide range of morning platelet counts (Figure 3A-C), suggesting that factors other than platelet count are responsible for the higher incidence of bleeding in children.
Patient age was also found to be a significant predictor of whether ≥ grade 3 bleeding occurred (P = .02), and this outcome was most common in children ages 6-12 and 13-18 years. Nearly all patient-days with bleeding grade 3 or higher were assigned that grade because the patient received a RBC transfusion for which the stated indication was to treat active bleeding; other types of grade 3 and 4 bleeding were rare. It is possible that there were differences among age groups in RBC transfusion guidelines or in how it was determined whether RBC transfusions were given for active bleeding versus anemia for patients with similar clinical situations. However, the PLADO study did not collect data to address these possible explanations. In contrast to grade 3 or higher bleeding, patient age was not found to be predictive of grade 4 bleeding. This is not surprising, given the overall low percentage of patients experiencing this level of bleeding. In addition, age group was a significant predictor of the number of platelet transfusion episodes and the total number of platelets transfused per square meter of BSA. Platelet utilization was also generally higher in children than in adults.
Further analyses found that the increased risk of bleeding in pediatric patients was most pronounced in the patients who received autologous/syngeneic HSCT. Among adult patients, the autologous/syngeneic HSCT group was at markedly lower risk of bleeding than the allogeneic or chemotherapy groups (53%, 77%, and 73%, respectively). However, in the pediatric age groups, the patients who received autologous/syngeneic HSCT had similar or higher bleeding risk than patients in the other disease treatment categories (Figure 1A).
This secondary analysis provided insight into the potential role of factors that might contribute to the findings of increased bleeding risk in children versus adults. The number of platelets transfused during each transfusion episode and the concentration of circulating platelets are probably not responsible for increased bleeding in pediatric versus adult patients, because the increased risk of bleeding in pediatric patients was similar in all 3 randomized dose groups and was present across a wide range of morning platelet counts. Furthermore, the types of bleeding that occurred differed between children and adults. Compared with adults, a higher percentage of children experienced oral, nasal, and gastrointestinal bleeding, and a lower percentage experienced skin, soft tissue, and musculoskeletal bleeding. This may reflect their degree of mucositis because of intense chemotherapeutic treatment regimens for their underlying disease.
One hypothesis to explain the increased bleeding risk in pediatric patients within specific organs is that there may be functional differences between age groups in the interaction between platelets and vascular endothelium in certain tissues. Endothelial structure and function probably vary with age and also may be affected pathophysiologically by age group differences in the expression of the underlying diseases present, in the differing intensity of chemotherapeutic treatments and HSCT conditioning regimens, and in the interplay between these factors, all present within the context of hypoproliferative thrombocytopenia. These hypothetical age-dependent effects on endothelial function and structure probably vary among different organ systems.
First, endothelial cell injury and multiple factors involved in endothelial regeneration in pediatric patients who receive allogeneic HSCT have recently been found by McPherson et al to be associated with a poor response to platelet transfusions.11 In preliminary studies, these investigators found that angiopoietin-2 (ang-2) and vascular endothelial growth factor (VEGF) increased, whereas platelet-derived growth factor-BB (PDGF-BB) and soluble PECAM decreased after conditioning regimens that used high-dose chemotherapy, often including total body irradiation. These investigators observed both an increased number of bleeding events and accelerated platelet consumption during a period of “vascular regeneration.”
Second, normal (not elevated) levels of VEGF are thought to be required for maintenance of endothelial cell stability and function,12 as well as for regulation of vascular filterability (absence of leakiness to plasma water).13 Indeed, platelets are rich sources of VEGF, sphinogosine-1 phosphate, and angiopoietins,14,,–17 which are released at low levels in the microvasculature to mediate endothelial cell survival and permeability. Consistent with this, Kitchens18 found by electron microscopy that, compared with normal rabbits, tongue capillaries in rabbits made severely thrombocytopenic by busulfan had thinner endothelial cell walls with focal gaps or fenestrations.
Another hypothesis is that the greater risk of bleeding in children may be because of one or more important functional or structural differences among the endothelium of younger children, older children, and adults, variations that may render younger children more susceptible than adults to bleeding in the setting of chemotherapy- and radiation therapy–induced thrombocytopenia. There may be an age difference in endothelial structure, as suggested by measurements of the pulmonary capillary filtration and reflection coefficients in newborn versus adult rabbits.19 These measurements show that the permeability to water (filtration coefficient) of newborn rabbits is twice that of adults and that the number of vascular endothelial pores increases and the pore radius decreases, continuously even beyond 4 weeks of age.20 These animal studies support the possibility of age-dependent differences in structure and function of the human endothelium.
Compounding this vulnerability, some chemotherapeutic regimens used to manage pediatric patients, especially for neuroblastoma and brain tumors during autologous HSCT, may be significantly more toxic to naive endothelium than the regimens administered in adult autologous HSCT.21 In addition, children are frequently prescribed relatively higher doses per m2 BSA of chemotherapeutic agents compared with adults. These high intensity chemotherapeutic regimens have increased cure rates in some pediatric malignancies to as high as 95%, and > 30% in more recalcitrant malignancies such as stage IV Neuroblastoma.22,23 However, relatively high doses of chemotherapy may contribute to increased bleeding in children by disrupting and more severely damaging endothelial cells that line vessel walls of various organs in comparison to the relatively lower doses recommended for adults.
These differences in treatment regimen and intensity of treatment support the speculation that the increased risk of bleeding in pediatric patients undergoing HSCT or chemotherapy may be related to difficulty in maintaining a sufficient level of integrity of the vascular endothelium, as an organ system, to provide optimal hemostasis.11,22,24
Although the PLADO trial included more children undergoing prophylactic platelet transfusions than prior studies, these post hoc analyses of the PLADO trial are, nonetheless, limited by the relatively small sample size in each of the pediatric age groups, particularly within individual disease treatment categories. Although the P values indicate that the higher bleeding risk in children is unlikely to be because of chance, especially when the P values are < .01, chance is still a possible explanation. Another important limitation is that data on specific treatment regimens and doses of chemotherapeutic agents and radiation were not collected in the PLADO trial. Therefore, no analyses could be performed to assess whether specific treatment regimens may have contributed to bleeding risk, making it difficult to extrapolate our findings to predict bleeding risks for individual patients. Although GVHD may affect bleeding, this study did not collect data on GVHD diagnosed before study entry, and only 3 subjects developed GVHD while on study. Therefore, no analyses could be performed to assess whether the degree of GVHD explained differences in bleeding between age groups. Information about allergic rhinitis history was not collected and therefore could not be included or excluded from contributing to epistaxis. Future studies are needed to determine which treatment regimens and other factors are most probable to increase the risk of bleeding in patients of different ages and to better characterize the physiology of normal pediatric endothelial cells and their response to injury.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Transfusion Medicine/Hemostasis Clinical Trials Network investigators, study coordinators, research staff, and patients who participated in this study.
This work was supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health to the Data Coordinating Center at New England Research Institutes (grant HL072268), Case Western Reserve University (grant HL072033), Children's Hospital Boston (grant HL072291), Cornell University (grant HL072196), Duke University (grant HL072291), Emory University (grant HL072248), Johns Hopkins University (grant HL072191), Massachusetts General Hospital (grant HL072299), Puget Sound Blood Center (grant HL072305), Tulane (grant HL072274), University of Iowa (grant HL072028), University of Maryland (grant HL072359), University of Minnesota (grant HL072027), University of North Carolina (grant HL072355), University of Oklahoma (grant HL072283), University of Pennsylvania (grant HL072346), University of Pittsburgh (grant HL072331), and the Blood Center of Wisconsin (grant HL072290).
National Institutes of Health
Authorship
Contribution: All authors contributed to the writing of the manuscript; C.D.J., S.F.A., M.-I.C., R.G.S., S.J.S., J.M.J., J.B., E.F.G., E.J.N., and S.R.S. designed the research; C.D.J., M.I.C., R.G.S., S.J.S., M.E.S., J.M.J., C.D.T., J.B., E.F.G., E.J.N., and S.R.S. performed the research and collected the data; C.D.J., S.G., S.F.A., R.G.S., S.J.S., C.D.T., E.F.G., E.J.N., W.S., and S.R.S. interpreted the data; and S.G. and S.F.A. performed statistical analyses.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Cassandra D. Josephson, Children's Healthcare of Atlanta, Department of Pathology, 1405 Clifton Rd NE, Atlanta, GA 30322; e-mail: cjoseph@emory.edu.