The balance between actions of procoagulant and anticoagulant factors protects organisms from bleeding and thrombosis. Thus, antithrombin deficiency increases the risk of thrombosis, and complete quantitative deficiency results in intrauterine lethality. However, patients homozygous for L99F or R47C antithrombin mutations are viable. These mutations do not modify the folding or secretion of the protein, but abolish the glycosaminoglycan-induced activation of antithrombin by affecting the heparin-binding domain. We speculated that the natural β-glycoform of antithrombin might compensate for the effect of heparin-binding mutations. We purified α- and β-antithrombin glycoforms from plasma of 2 homozygous L99F patients. Heparin affinity chromatography and intrinsic fluorescence kinetic analyses demonstrated that the reduced heparin affinity of the α-L99F glycoform (KD, 107.9 ± 3nM) was restored in the β-L99F glycoform (KD, 53.9 ± 5nM) to values close to the activity of α-wild type (KD, 43.9 ± 0.4nM). Accordingly, the β-L99F glycoform was fully activated by heparin. Similar results were observed for recombinant R47C and P41L, other heparin-binding antithrombin mutants. In conclusion, we identified a new type of mosaicism associated with mutations causing heparin-binding defects in antithrombin. The presence of a fully functional β-glycoform together with the activity retained by these variants helps to explain the viability of homozygous and the milder thrombotic risk of heterozygous patients with these specific antithrombin mutations.
Introduction
Since 1965, when E. Egeberg described the first family with an association between antithrombin deficiency and venous thrombosis,1 many data have sustained the key hemostatic role of this anticoagulant. Actually, heterozygous deficiency significantly increases the risk of thrombosis, making its diagnosis essential for possible prophylactic or therapeutic treatment of carriers.2 Two types of antithrombin deficiencies have been distinguished on the basis of functional and immunologic assays. Type I antithrombin deficiency is classically detected by a low level of functional and immunologic antithrombin assays, whereas type II antithrombin deficiency occurs when a dysfunctional variant is present in the circulation. Within type II deficiencies, 3 subtypes have been established depending on the location in the antithrombin molecule: reactive site, heparin-binding site, or pleiotropic.3 However, although this classification seeks to distinguish better between the type II deficiencies, it sometimes ignores the inherent complexity of the disease.
The key hemostatic role of antithrombin is also recognized by the embryonic lethality of homozygotes.4 Two relevant exceptions have been identified: antithrombin Toyama (R47C) and, especially, antithrombin Budapest-III (L99F), which are associated with a moderate risk of thrombosis in heterozygosis and embryonic viability in homozygous state.5,–7 Neither of the mutations impair the folding or secretion of the protein, but they do cause a type II deficiency by affecting the heparin-binding domain,5,–7 and thereby abolish the activation of antithrombin by glycosaminoglycans—a crucial process for effective anticoagulant activity.8 Antithrombin Toyama has the most pronounced effect because the amino acid modification displaces one of the primary contact sites with heparin.5,8,9 In contrast, the leucine to phenylalanine change of antithrombin Budapest-III seems to alter the interaction between helix C and the N-terminal end of helix D, thereby perturbing the geometry of the domain, which also leads to a reduced heparin affinity.10 Another mutation not involved in the direct contact with heparin, but also impairing heparin affinity, is antithrombin Basel (P41L), in which a profound structural change inferred by the replacement of proline to leucine (with increased helical structure and hydrophobicity) has been speculated.11 This mutation is also associated with an only moderate risk of thrombosis, although no homozygous patients have been described.
Although the heparin cofactor activity of these mutations with a heparin-binding defect is severely impaired, none of these mutations significantly interferes with the progressive activity, which has been postulated to explain the mild clinical phenotype of heterozygotes and the viability in homozygous state.3
In this study, we propose that a posttranslational mosaicism affecting this molecule may constitute a new mechanism that contributes to a lower risk of thrombosis and the viability of patients carrying these mutations in homozygous state. For this purpose, we have characterized the antithrombin glycoforms present in the plasma of 2 homozygous brothers for the L99F mutation, and the recombinant glycoforms of R47C and P41L antithrombin variants. Our data support the idea that the β-glycoform may compensate for the effect of these mutations by supplying a limited source of heparin-activated molecules, which improve the anticoagulant capacity of heterozygous and especially homozygous subjects, thus reducing the risk of thrombosis and contributing to their viability.
Methods
Functional and genetic analysis of patients and family members
Anti-FXa activity and Ag levels of antithrombin from plasma of patients and family members, as well as PCR amplification and sequencing of the SERPINC1 gene were carried out essentially as reported previously.12 Tests for thrombophilia included quantification of free protein S and protein C activity, detection of antiphospholipid Abs, and genotyping of FV Leiden and prothrombin G20210A polymorphisms. Crossed-immunoelectrophoresis of antithrombin with unfractionated sodium heparin Rovi was performed as previously reported.12
Off-gel protein fractionation
Semiliquid phase isoelectrofocusing fractionation was performed on an Off-Gel 3100 apparatus (Agilent Technologies) using a 24-cm strip with immobilized pH gradient gels in the 4-7 range. Plasma samples, 10 μL, were mixed with isoelectric focusing buffer (Agilent Technologies) and loaded onto the strips. Protein fractionation was performed for 26 hours with maximum 8000 V for a total of 60 000 V/h, with a last electroelution step according to the manufacturer's protocol. The resulting liquid fractions (150 μL) with a 0.25 pH range were collected, and aliquots of proteins further resolved on 10% acrylamide SDS-PAGE for Western blot probing.
Recombinant expression of WT and mutant antithrombins
Site-directed mutagenesis was performed using the Stratagene QuikChange Site-Directed Mutagenesis kit and the appropriate primers to obtain R47C and P41L mutants on the pCEP4/AT plasmid containing the WT cDNA sequence of the human antithrombin molecule. This plasmid was generated by site-directed mutagenesis of the pCEP4/AT-S137A, generously provided by Dr J. Huntington (Cambridge Institute for Medical Research [CIMR], Cambridge, United Kingdom), to return to the WT sequence that produces similar amounts of α- and β-glycoforms. Human embryonic kidney cells expressing the Epstein-Barr nuclear Ag 1 (HEK-EBNA) were grown to 60% confluence at 37°C, 5% CO2, in DMEM with GlutaMAX-I medium (Invitrogen), supplemented with 5% FBS (Sigma-Aldrich). We transfected 200 μg/mL WT and mutant plasmids for 30 minutes in OptiMEM with LTX (Invitrogen), as suggested by the manufacturer. After 24 hours, the cells were washed with PBS and changed to a CD-CHO medium (Invitrogen) supplemented with 4mM l-glutamine and 0.25 mg/mL Geneticin (Invitrogen). Cells were grown at 37°C for 10 days. The media was harvested and replaced by a fresh medium every 2 days.
Protein purification
Antithrombin from plasma of healthy blood donors or from patients was purified by heparin affinity chromatography on HiTrap Heparin columns (GE Healthcare), using an ÄKTA Purifier (GE Healthcare) in 100mM Tris-HCl and 10mM citric acid, in a gradient from 0.15 to 2M NaCl. Fractions with antithrombin were applied to a HiTrap Q column (GE Healthcare). Finally, proteins were eluted in 3 different peaks and desalted over 5 mL of HiTrap Desalting columns (GE Healthcare) and stored at −70°C. The α- and β-glycoforms of WT and mutant recombinant proteins were purified from media harvests using the same columns as described for the plasma protein. The use of samples from human donors and patients was approved by the internal ethical committee of the University of Murcia.
Electrophoretic evaluation
Purity and separation of proteins were checked by SDS-PAGE, performed in 10% (w/v) polyacrylamide and nondenaturing PAGE in presence and absence of 6M urea, essentially performed as indicated elsewhere.13
MALDI-TOF-MS analysis
A solution of 3,5-dimethoxy-4-hydroxycinnamic acid (10 g/L) in acetonitrile/water/trifluoroacetic acid (50:50:0.1 by volume) was chosen for protein analysis. Experiments were carried out on a Voyager-DE STR Biospectrometry workstation (Applied Biosystems), equipped with an N2 laser (337 nm). Recorded data were processed with Data Explorer Software (Applied Biosystems). Antithrombin samples were also digested in 100mM NH4HCO3 (pH 7.8) containing trypsin (ratio enzyme/substrate 1:50) at 37°C for 16 hours. Peptide mixtures from in situ digestion of proteins were desalted in a GELoader tip packed with 0.5 μL of POROS-10 R2 (PerSeptive Biosystem) slurry and subsequently analyzed by matrix-assisted laser desorption/ionization–time of flight–mass spectrometry (MALDI-TOF–MS) using α-cyano-4-hydroxycinnamic acid (20 g/L) in acetonitrile/water/trifluoroacetic acid (80:20:0.1 by volume).
Intrinsic fluorescence studies
Equilibrium dissociation constants (KD) for the antithrombin-heparin interaction were determined essentially as described previously.14 Briefly, the change in intrinsic fluorescence of antithrombin (25-50nM) on titration of the unfractionated heparin was monitored at 340nM on a PerkinElmer Life Sciences 50B spectrofluorometer, with excitation at 280nM and using bandwidths of 3.5nM for both excitation and emission. All titrations were carried out at room temperature under physiologic ionic strength (I = 0.15) in 20mM Na2HPO4, 100mM NaCl, 0.1mM EDTA, 0.1% polyethylene glycol 8000, pH 7.4. Fluorescence emission intensity was taken as the average of 100 measurements recorded at 1-second intervals for each addition of heparin. Data were fitted as previously described.14
Results
Identification and clinical characteristics of patients with heparin-binding defects
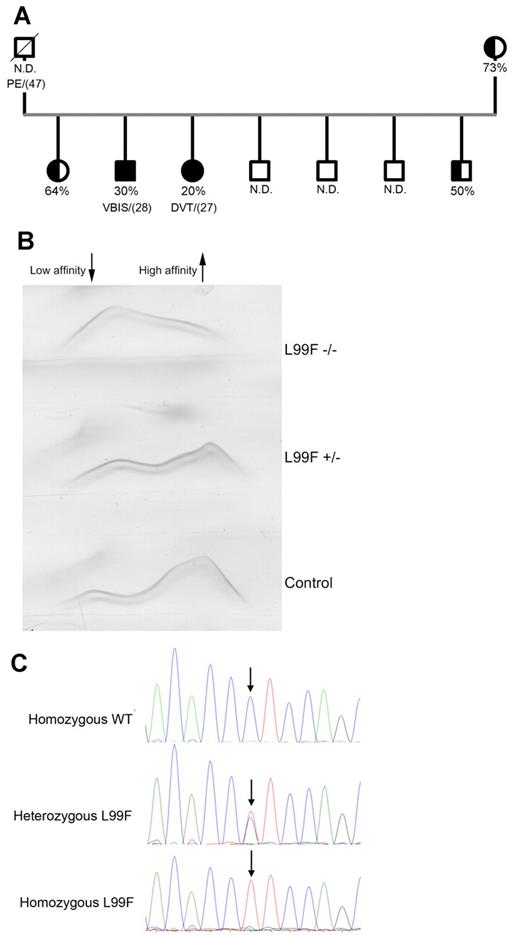
We identified a thrombophilic family where 2 members developed thrombotic episodes—deep venous thrombosis and a cerebrovascular ischemic attack—at young ages (Figure 1A). Testing for thrombophilia only revealed an antithrombin deficiency, with plasma heparin cofactor anti-FXa values significantly < 50% (30% and 20%). The other siblings and the mother had reduced anti-FXa activity with no reported thrombotic events. The father died from pulmonary embolism at the age of 47 years (Figure 1A). Plasma antithrombin Ag levels (70%-75%), together with an increased fraction of antithrombin with low heparin affinity (Figure 1B) suggested a type II deficiency with a heparin-binding defect. Sequencing of the SERPINC1 gene revealed a single base pair mutation responsible for the L99F change, which was homozygous in both symptomatic patients (Figure 1C). Interestingly, crossed immunoelectrophoresis of both homozygous patients revealed the existence of antithrombin forms that bound to heparin (Figure 1B).
Characterization of the homozygous Budapest-III patients. (A) Pedigree of the thrombophilic family. Filled symbols indicate homozygous carriers, and half-filled symbols indicate heterozygous carriers. Anti-FXa activity values and the age of the first thrombotic episode (in brackets) are also indicated. N.D. indicates those cases where anti-FXa activity was unknown. N.D. indicates nondetermined; PE, pulmonary embolism; VBIS, vertebral-basilar ischemic stroke; and DVT, deep venous thrombosis. (B) Crossed immunoelectrophoresis in the presence of unfractionated heparin of the plasma of homozygous and heterozygous Budapest-III patients and control plasma. The antithrombin forms of high and low heparin affinity are indicated. (C) Electropherogram of the PCR-amplified product of exon 2 in a control, a heterozygous patient, and a homozygous patient for the L99F mutation. ↓ indicates the position of the mutation.
Characterization of the homozygous Budapest-III patients. (A) Pedigree of the thrombophilic family. Filled symbols indicate homozygous carriers, and half-filled symbols indicate heterozygous carriers. Anti-FXa activity values and the age of the first thrombotic episode (in brackets) are also indicated. N.D. indicates those cases where anti-FXa activity was unknown. N.D. indicates nondetermined; PE, pulmonary embolism; VBIS, vertebral-basilar ischemic stroke; and DVT, deep venous thrombosis. (B) Crossed immunoelectrophoresis in the presence of unfractionated heparin of the plasma of homozygous and heterozygous Budapest-III patients and control plasma. The antithrombin forms of high and low heparin affinity are indicated. (C) Electropherogram of the PCR-amplified product of exon 2 in a control, a heterozygous patient, and a homozygous patient for the L99F mutation. ↓ indicates the position of the mutation.
Purification and biochemical characterization of antithrombin variants from plasma of patients
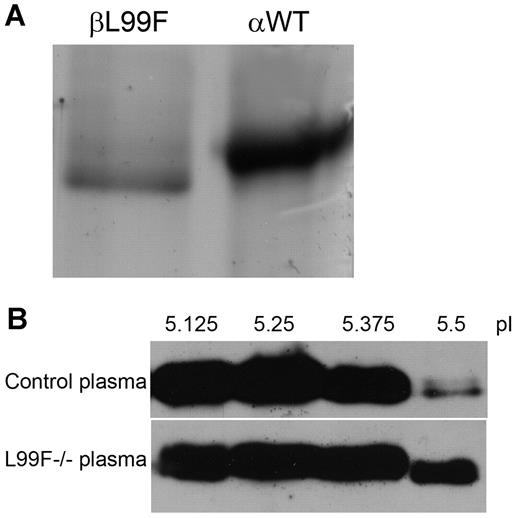
Thanks to the great generosity of the 2 L99F homozygous patients, we were able to purify antithrombin from 300 mL of plasma by heparin affinity chromatography. As expected, most protein eluted at a low salt concentration (∼ 150mM NaCl) supporting the low heparin affinity associated with this mutation. However, in accordance with the crossed-immunoelectrophoretic result, we detected antithrombin in fractions where normally the α-WT isoform elutes (1M NaCl). Electrophoretic analysis of these fractions revealed a molecule slightly lower than the α-WT isoform (Figure 2A). After further purification of this molecule by ion exchange chromatography, mass spectrometry confirmed the change to phenylalanine at residue 99 and an exact mass of 55 kDa. Although we were not able to detect the peptide containing N135, all available data, together with its higher isoelectric point (pI; 5.7) determined by isoelectrofocusing (Figure 2B), strongly suggest that this component was the mutant β-glycoform.
Antithrombin L99F (antithrombin Budapest-III); characterization of purified plasma glycoforms of homozygous patients. (A) SDS-PAGE and silver staining of β-L99F and α-wild type (αWT) glycoforms present in the heparin affinity chromatography fractions eluting at 1M NaCl from plasma of L99F and wild-type homozygous subjects. (B) Isoelectrofocusing of plasma samples. Isoelectrofocusing fractions from control plasma and one of the homozygous Budapest-III (L99F−/−) collected with an off-gel system were run under reduced SDS conditions and detected by Western blot. The fractions obtained between pI 5.5 and 5.63 mainly contained the β-glycoform of antithrombin.
Antithrombin L99F (antithrombin Budapest-III); characterization of purified plasma glycoforms of homozygous patients. (A) SDS-PAGE and silver staining of β-L99F and α-wild type (αWT) glycoforms present in the heparin affinity chromatography fractions eluting at 1M NaCl from plasma of L99F and wild-type homozygous subjects. (B) Isoelectrofocusing of plasma samples. Isoelectrofocusing fractions from control plasma and one of the homozygous Budapest-III (L99F−/−) collected with an off-gel system were run under reduced SDS conditions and detected by Western blot. The fractions obtained between pI 5.5 and 5.63 mainly contained the β-glycoform of antithrombin.
We further characterized the heparin affinity of the α- and β-glycoforms of both the WT and the L99F variant, purified from plasma of healthy subjects and the 2 homozygous patients, by fluorescence studies. Equilibrium dissociation constants confirmed a ∼ 2-fold increased heparin affinity for the β-L99F (KD, 53.9 ± 5nM) than for the α-L99F (KD, 107.9 ± 3nM). Indeed, the heparin affinity of the β-L99F was similar to that of the α-WT form under physiologic ionic strength conditions (KD, 43.9 ± 0.4nM). According to this result and its full progressive activity, the β-L99F variant was activated by heparin, inhibiting thrombin at similar rates than the α-WT antithrombin. These results suggest that the higher heparin affinity of the β-antithrombin may also compensate for the effect of mutations causing heparin-binding defects.
Characterization of R47C and P41L recombinant variants
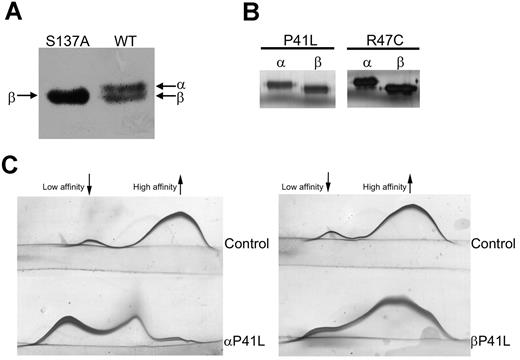
We next sought to confirm whether these results obtained from the 2 homozygous patients with a mutation indirectly affecting the heparin-binding domain are a general phenomenon that can be extrapolated to other mutations causing a heparin-binding defect, including those affecting residues directly involved in primary contacts with heparin. Because of the lack of homozygous subjects with other heparin-binding defects, a recombinant model was used. We used pCEP4/AT transiently expressed in HEK-EBNA cells.15 The original β-background of the plasmid (with a S137A mutation to generate only β-glycoform, which facilitates the purification of recombinant antithrombins),15 was first returned to the WT background, by site-directed mutagenesis. This plasmid generated similar amounts of both α- and β-glycoforms in the conditioned medium (Figure 3A). On this background, we generated the 2 mutations affecting the heparin-binding domain identified in patients with antithrombin deficiency, P41L and R47C, by site-directed mutagenesis. α- and β-glycoforms from WT and mutants were purified by heparin affinity and anion exchange chromatography (Figure 3B). We ensured that no transformation to latent or polymer occurred during the purification of recombinant proteins by detecting progressive anti-FXa activity in all cases (data not shown). In the case of P41L, we were able to purify enough of both glycoforms to evaluate the capacity of heparin binding by crossed immunoelectrophoresis. As expected, the β-P41L glycoform retained a component with high heparin affinity (Figure 3C). In addition, fluorescence analysis also revealed a higher heparin affinity for the β-glycoform that was comparable with the value obtained for the β-WT glycoform (Table 1). In the case of R47C, we only obtained protein for fluorescence analysis, but the results verified that the β-glycoform compensated for the effect of the mutation (Table 1).
Electrophoretic properties and biochemical characterization of recombinant antithrombin glycoforms. (A) Antithrombin glycoforms expressed in HEK-EBNA when transfected with pCEP4/AT-S137A (left) or with pCEP4/AT WT (right) plasmid. The medium containing the secreted recombinant protein was run under reduced SDS conditions and detected by Western blot. (B) SDS-PAGE and silver staining of recombinant glycoforms of WT, R47C, and P41L antithrombin purified by heparin affinity chromatography and anion exchange chromatography. (C) Crossed immunoelectrophoresis in the presence of unfractionated heparin of purified recombinant α- and β-P41L glycoforms. The antithrombin forms of high and low heparin affinity are indicated.
Electrophoretic properties and biochemical characterization of recombinant antithrombin glycoforms. (A) Antithrombin glycoforms expressed in HEK-EBNA when transfected with pCEP4/AT-S137A (left) or with pCEP4/AT WT (right) plasmid. The medium containing the secreted recombinant protein was run under reduced SDS conditions and detected by Western blot. (B) SDS-PAGE and silver staining of recombinant glycoforms of WT, R47C, and P41L antithrombin purified by heparin affinity chromatography and anion exchange chromatography. (C) Crossed immunoelectrophoresis in the presence of unfractionated heparin of purified recombinant α- and β-P41L glycoforms. The antithrombin forms of high and low heparin affinity are indicated.
Equilibrium dissociation constants for the binding of unfractionated heparin to antithrombin
| . | KD, nM . |
|---|---|
| Plasma | |
| α-WT | 43.9 ± 0.4 |
| β-WT | 33.2 ± 1.6 |
| α-L99F | 107.9 ± 0.3 |
| β-L99F | 53.9 ± 0.2 |
| Recombinant | |
| α-WT | 48.9 ± 2.5 |
| β-WT | 37.6 ± 2.2 |
| α-P41L | 87.1 ± 1.9 |
| β-P41L | 39.7 ± 2.1 |
| α-R47C | 91.0 ± 1.3 |
| β-R47C | 57.8 ± 1.7 |
| . | KD, nM . |
|---|---|
| Plasma | |
| α-WT | 43.9 ± 0.4 |
| β-WT | 33.2 ± 1.6 |
| α-L99F | 107.9 ± 0.3 |
| β-L99F | 53.9 ± 0.2 |
| Recombinant | |
| α-WT | 48.9 ± 2.5 |
| β-WT | 37.6 ± 2.2 |
| α-P41L | 87.1 ± 1.9 |
| β-P41L | 39.7 ± 2.1 |
| α-R47C | 91.0 ± 1.3 |
| β-R47C | 57.8 ± 1.7 |
KD were measured by global fitting of fluorescence titrations with unfractionated heparin as described.14
KD indicates dissociation constant; and WT, wild type.
Discussion
The potential deleterious consequences of a mutation impairing the function of a protein can be eluded or at least attenuated by different mechanisms. Redundancy, the existence of several genes in the genome of an organism that to some extent perform the same role, is one of these protective mechanisms.16 Pharmacologic rescue has also been described, and particularly interesting in this setting is the rescue of conformationally defective proteins by pharmacologic chaperones.17 On the other hand, somatic mosaicism caused by in vivo reversion of inherited mutations has been described in human genetic disorders, such as Fanconi anemia, hemophilia, or Duchenne muscular dystrophy among others.18,–20 Finally, there are some examples of how the functional and pathologic consequences of a mutation depend on the isoform of a protein. The most evident examples concern those isoforms produced by alternative splicing.21 We show here a new example of how a mutation might have functional consequences in different isoforms. In this case, the isoforms are caused by a posttranslational modification, the N-glycosylation, which compensates for the effect of certain mutations affecting the heparin affinity of antithrombin.
The occurrence of antithrombin glycoforms is derived from the existence of a less efficient consensus sequence (N-X-S) at N135 that affects the functional rate of the oligosaccharyl transferase which incorporates the N-glycan during protein translation and folding.22 Two glycoforms are present in plasma: (1) α-isoform, the main circulating antithrombin (90%), with 4 N-glycans, and (2) β-isoform, the minority glycoform (10%), lacking the N-glycan at N135. The different site occupancy affects the size, pI, and, more relevantly from a functional point of view, the heparin affinity of the molecule. Thus, the absence of the N-glycan at N135 increases 2- to 4-fold the affinity for heparin23 by affecting the heparin-induced conformational change in the second binding step.24 Hence, the β-glycoform could be mainly responsible for the control of thrombin at the endothelial surface,25,26 but it also explains its increased clearance rate, which contributes to the smaller amount of β-antithrombin in plasma.27 The results of this study suggest that the higher heparin affinity of the β-antithrombin may also compensate for the effect of mutations causing heparin-binding defects (Table 1). Thus, the data obtained with antithrombin purified from the plasma of 2 homozygous patients for the L99F mutation support the idea that the β-glycoform compensates the effect of the mutation (Table 1, Figures 1B, 2A). This mutation reduces the heparin affinity of the α-glycoform, but does not avoid the binding to heparin and the final activation of the β-glycoform. Similar results were obtained for P41L, another mutation that indirectly reduces the heparin affinity of the α-glycoform (Table 1, Figure 3C). In these patients, the presence of even small amounts of molecules that are fully activated by heparin, together with the progressive activity of the variant, may explain the milder thrombotic risk and the higher prevalence in the general population. This rule may even be extrapolated to the R47C mutation, affecting a residue involved in the primary contact with the heparin, as the recombinant β-isoform could compensate for the strong effects of this mutation in the interaction with heparin (Table 1).
In conclusion, the data obtained from the purification and characterization of the antithrombin glycoforms from 2 homozygous patients with L99F mutation and the recombinant glycoforms of P41L and R47C sustain a natural posttranslational mosaicism. These patients have a main α-glycoform in plasma that retains progressive activity but has reduced heparin affinity, and a less abundant β-glycoform that maintains the heparin affinity and that is fully activated by this cofactor. These results, together with the significant progressive inhibitory function of these variants explain the milder thrombotic risk of mutations with heparin-binding defect and will also contribute to the viability of affected homozygotes compared with the lethality of other mutations that directly affect expression or inhibitory activity. It is difficult to quantify the proportional contributions of the 2 factors to the survival of affected homozygotes but the findings presented here will draw attention to the hitherto overlooked role of β-antithrombin as a backup resource.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by SAF2009-08993 (Ministerio de Ciencia y Tecnologia and Fondo Europeo de Desarrollo Regional [FEDER]), Redes Temáticas de Investigación Cooperativa Red Temática de Investigación Cooperativa en Enfermedades Cardiovasculares RD06/0014/0039 (Instituto de Salud Carlos III and FEDER), Fundación Séneca (04515/GERM/06), and Fundación Mutua Madrileña. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Authorship
Contribution: I.M.-M., J.N.-F., A.Ø., R.G.-G., J.P., N.B., A.M., M.E.d.l.M.-B., and S.Á. performed the laboratory work for this study; I.M.-M., V.V., and J.C. wrote the manuscript; C.P. and S.R.K. recruited the patient samples; C.M., S.P., V.V., and J.C. coordinated the research; C.M., S.P., and S.R.K. critically reviewed the manuscript; and J.C. was the principal investigator and takes primary responsibility for the manuscript.
Conflict-of-interest disclosure: I.M.-M. and C.M. are researchers from Fundación para la Formación e Investigación Sanitarias. J.N.-F. holds postdoctoral contract Sara Borrell from ISCIII. M.E.d.l.M.-B. holds a predoctoral research grant from ISCIII, and S.Á. holds a Formación de Personal Investigador grant from Ministerio de Ciencia y Tecnología. The remaining authors declare no competing financial interests.
Correspondence: Dr Javier Corral, University of Murcia, Centro Regional de Hemodonación, Ronda de Garay S/N, Murcia 30003, Spain; e-mail: javier.corral@carm.es.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal