Abstract
Although the overproduction of immunoglobulins by short-lived plasma cells accompanying an immune response links with their apoptosis, how long-lived plasma cells adapt to ensure their longevity in this context is obscure. Here, we show that apoptosis signal–regulating kinase 1 (ASK1) contributes to apoptosis of plasma cells because ASK1 activity was induced during differentiation of short-lived plasma cells, and, when produced by ASK1-deficient mice, these cells survived better than those of control mice. Moreover, antigen-specific long-lived plasma cells generated by immunization accumulated in ASK1-deficient mice, suggesting ASK1 also plays a negative role in survival of long-lived plasma cells. In malignant plasma cells, ASK1 transcription was directly suppressed by B lymphocyte–induced maturation protein-1 (Blimp-1). The expression of ASK1 and Blimp-1 showed an inverse correlation between normal human mature B cells and bone marrow plasma cells from patients with multiple myeloma (MM). Suppression of ASK1 is crucial for cell survival because its enforced expression in MM cells caused apoptosis in vitro and lowered MM load in a xenograft animal model; furthermore, alteration of ASK1 activity affected MM cell survival. Our findings indicate a novel mechanism underlying the regulation of survival in normal and malignant plasma cells by ASK1.
Introduction
Plasma cells, the terminally differentiated antibody-secreting cells, are generated from mature B cells once they encounter antigens.1 The generation of plasma cells can be aided by follicular helper T cells or independent of T cells.2 Plasma cells that arise in lymphoid organs either receive survival signals from the local microenvironment or home to bone marrow under the guidance of the bone marrow–derived chemoattractant, CXCL12, wherein they become competent for long-term survival; otherwise, they serve as short-lived plasma cells that eventually undergo apoptosis.1 Plasma cell survival in bone marrow requires several external factors, such as IL-6, B cell–activating factor, and a proliferation-inducing ligand secreted by bone marrow stromal cells or osteoclasts.3-5 In the lymphoid organs/tissues, plasma cells survive in the presence of B cell–activating factor or IL-10, each of which is secreted by epithelial cells6 ; in the presence of IL-6 secreted by dendritic cells; or in the presence of a proliferation-inducing ligand secreted by macrophages.7 Many of these factors are also important for the survival of the malignant counterparts of long-lived plasma cells,8 such as multiple myeloma (MM) cells.1 However, the intracellular factors that regulate the survival of either short-lived or long-lived plasma cells need to be further investigated.
During an immune response, the mass production of immunoglobulins in plasma cells can invoke a cellular adaptive response, termed the endoplasmic reticulum (ER) stress response, to cope with the consequent accumulation of unfolded immunoglobulins in the ER lumen.9 Apoptosis can be triggered on prolonged ER stress.10 Apoptosis signal–regulating kinase 1 (ASK1), also known as mitogen-activated protein 3K5, plays a role in inducing apoptosis in response to cellular stress.11 Mitogen-activated protein 3K5 is a member of MAP kinase kinase kinase family, and it selectively activates the JNK and p38 MAPK pathways on receiving various intracellular and extracellular stress signals, including ER stress and treatment with lipopolysaccharide (LPS).12-14 Activation of ASK1 is tightly regulated by phosphorylation of a threonine (T) residue in ASK1 (T838 in human; T845 in mouse) that resides in the loop of the kinase domain.15 ASK1 can be activated by autophosphorylation.15 By contrast, protein phosphatase 5 (PP5) negatively regulates ASK1 by dephosphorylating T838.16 ASK1 is activated during stress responses, and the subsequent activation of JNK is required for stress-induced apoptosis. In fact, activation of ASK1 plays a role in a variety of diseases, such as those involving neuronal degeneration.13
B lymphocyte–induced maturation protein-1 (Blimp-1) is a crucial regulator of plasma cell development. B cell–specific deletion of Prdm1, the gene encoding Blimp-1, in mice showed defective production of antibody-secreting plasma cells generated after exposure to either thymus-dependent (TD) or thymus-independent (TI) antigens.17 Blimp-1 suppresses the transcription of genes responsible for germinal center B-cell or mature B-cell identity and for proliferation.18 In addition to its role in orchestrating plasma cell differentiation, Blimp-1 is also required for maintenance of long-lived plasma cells.19,20 In our previous study, we reported that survival of MM cells or mouse plasmacytoma cells requires continuous Blimp-1 expression because depletion of Blimp-1 in these cells causes apoptosis.20 In addition, MM cells treated with bortezomib, a therapeutic agent for MM relapse,21 had a reduced level of Blimp-1.20 However, the detailed mechanism of how Blimp-1 ensures the survival of plasma cells remains elusive.
Because immunoglobulin overproduction in plasma cells can cause ER stress and ASK1 is involved in ER stress–induced apoptosis, we hypothesized that long-lived plasma cells possess a mechanism by which ER stress–induced apoptosis is prevented; thus, we investigated whether the regulation of ASK1 is relevant to plasma cell survival.
Methods
Cell lines and reagents
NCI-H929 (H929), U266, IM9 human MM cells, SKW6.4 lymphoblastoid cells, Raji mature B cells, and stable transfectants of WI-L2 cells were maintained as described.18,20 Arachidonic acid (AA) and okadaic acid (OA) were purchased from Calbiochem. The small interfering RNA (siRNA) targeting human ASK1 (Stealth RNAi siRNA duplex) or negative-control siRNA duplexes were purchased from Invitrogen.
Animals and primary B-cell culture
Splenic B cells (purified with B220 microbeads; Miltenyi Biotec) from 8- to 12-week-old C57BL/6 mice (BioLASCO), Ask1 knockout (KO) mice were generated as described,22 and their littermate control wild-type (WT) mice were stimulated with LPS (2 μg/mL; Sigma-Aldrich) or IL-21 (100 ng/mL; eBioscience) plus αCD40 (5 μg/mL; BD PharMingen) and αIgM (10 μg/mL; Jackson ImmunoResearch Laboratories). The procedures for immunization of mice are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Primary human CD138+ plasma cells (isolated by CD138 MicroBeads; Miltenyi Biotech) were obtained from patients with MM at the National Taiwan University Hospital. Primary CD19+ human B cells were isolated as described23 and cultured in RPMI-1640 that contained 10% fetal bovine serum (Gibco), penicillin/streptomycin, and 50μM β-mercaptoethanol at an initial cell density of 1 × 106/mL. All the human samples were obtained with the approval of both the Human Subject Research Ethics Committee of Academia Sinica and the National Taiwan University Hospital.
Plasmids
cDNAs encoding full-length human ASK1 and constitutively active (CA) ASK1 lacking the N-terminal residues 1-649 were amplified by RT-PCR from H929 cells. The lentiviral vector that produces the shRNA against Blimp-1 and the negative control were described previously.20 Detailed information on the generation of the dominant-negative ASK1, various expression vectors encoding different forms of ASK1, ASK1 promoter–driven luciferase reporter construct, and the constructs for establishment of Tet-on-inducible transfectants are described in supplemental Methods.
Generation and maintenance of luciferase-expressing inducible transfectants
H929 and IM9 cells stably expressing luciferase were generated by selecting cells that carry the integrated luciferase expression vector with puromycin (2 μg/mL; Sigma-Aldrich). See supplemental Methods for details.
Retroviral and lentiviral vector preparation and transduction
Flow cytometry
Cells were harvested and washed with phosphate-buffered saline once and then suspended in annexin V binding buffer (BD PharMingen) at a density of 106/mL. The antibodies used in this study were purchased from BD PharMingen and are described in supplemental Methods. Cellular fluorescence intensity was analyzed by FACSCanto (Becton Dickinson) and FCS Express 3.0 software (De Novo Software).
Immunoblotting
Total cell lysates (30-50 μg) or nuclear extracts (10 μg), prepared as described,25 were subjected to SDS-PAGE and immunoblotting with the use of primary antibodies against Blimp-1, actin, α-tubulin, FLAG, Bim, Bcl-2, Bcl-xL, and Mcl-1 as described.20 Otherwise, the other antibodies are described in supplemental Methods. The immunoreactive proteins were detected as described.20
RT-QPCR
Isolation of total RNA, cDNA synthesis, and subsequent quantitative PCR (QPCR) analysis in an ABI Prism 7300 sequence detection system (Applied Biosystems) were performed as described.23 RT-QPCR analysis of cDNA was performed with the use of primers for the SYBR green method, and the primer sequences used are described in supplemental Methods.
Chromatin immunoprecipitation
Chromatin immunoprecipitation was performed essentially as described.23 See supplemental Methods for details.
Transfection and luciferase reporter assay
siRNA (1μM) against human ASK1 or negative-control siRNA was delivered into 2 × 106 H929 cells by Nucleofector II (Lonza) according to the supplier's suggested protocol. The procedure for the luciferase reporter assay is described in supplemental Methods.
Xenograft animal model
Luciferase-expressing ASK1-inducible and control Tet response element (TRE) H929 or IM9 transfectants (1 × 107 cells) were intravenously injected into sublethally irradiated female SCID mice (BioLASCO) via the tail vein. At 6 days after engraftment, normal drinking water was replaced by 5% sucrose lacking doxycycline (Dox) or supplemented with Dox (0.2 mg/mL). Meanwhile, mice that had received a xenograft were orally administrated with or without Dox by gavage (1 mg in 1 mL per mice) twice a week. Bioluminescence was measured after intraperitoneal injection with D-luciferin (150 μg/g of body weight; Caliper), followed by acquiring bioluminescent images with the use of the Xenogen IVIS system.
TUNEL assay
Mice that had received a xenograft were killed 20 days after engraftment, and the lungs were fixed in 10% formaldehyde (Mallinckrodt Chemicals) at 4°C. Samples were dehydrated, followed by slicing paraffin-embedded sections at a thickness of 3 μm. Subsequent TUNEL staining was performed with an in situ cell death detection kit and TMR red (Roche) according to the manufacturer's suggestions Images, acquired with Leica DMG000B microscope equipped with a 20×/0.7 HC-Plan APO objective and Leica DFC490 camera, were analyzed with Leica FW4000 software (Leica Microsystems).
Statistics
Statistical analysis was performed with a paired, 2-tailed Student t test. Log-rank tests were used to calculate the statistical significance in Kaplan-Meier survival curves. P < .05 was considered statistically significant.
Results
ASK1 is involved in regulating apoptosis of short-lived plasma cells
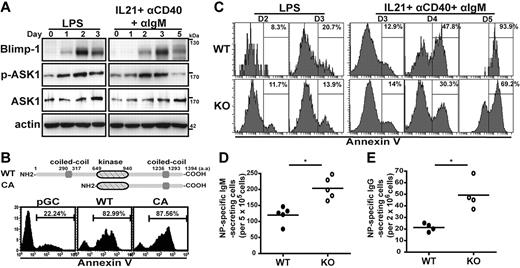
We first examined if the expression level or activity of ASK1 is involved in generating short-lived plasma cells. Toward this goal, we generated short-lived plasma cells in vitro from murine splenic B cells that had been stimulated with LPS (which mimics a TI stimulus) or the combination of IL-21, αIgM, and αCD40 (which mimics a TD stimulus)26 ; plasma cells typically begin to die at day 3 (TI-like stimulus) or day 5 (TD-like stimulus) after stimulation. As expected, Blimp-1 was induced after stimulation in both cultured cells as analyzed by immunoblotting, showing that these cells were committed to plasma cell fate (Figure 1A). The temporal expression and activation of ASK1 in these culture systems was next examined. The phosphorylation of ASK1 at T845 was increased in response to both types of stimulation, indicating that ASK1 was activated under both culture conditions; notably, the overall levels of ASK1 protein did not change substantially (Figure 1A). We next examined whether ASK1 contributed to stimulus-induced apoptosis during differentiation of short-lived plasma cells. WT or CA ASK127 was expressed from a bi-cistronic retroviral vector that coexpressed yellow fluorescent protein (yfp) in TD stimulant–treated splenic B cells, and apoptosis of plasma cells (B220lowCD138+) was assessed by annexin V staining in the yfp+ gate. Ectopic expression of WT or CA ASK1 increased apoptosis compared with plasma cells transduced with the control vector (pGC; Figure 1B). We also examined apoptosis of short-lived plasma cells produced by TI or TD stimulant–treated Ask1 KO and WT splenic B cells; the B220lowCD138+ plasma cells generated by KO B cells showed less apoptosis than WT plasma cells on day 3 (TI stimulation) and days 4-5 (TD stimulation; Figure 1C). Therefore, our data indicated that ASK1 was activated and played a role in enhancing apoptosis during the generation of short-lived plasma cells in vitro.
ASK1 enhances apoptosis during the generation of short-lived plasma cells. (A) ASK1 is activated during the generation of short-lived plasma cells. Splenic B cells treated with LPS (2 μg/mL) or the combination of IL-21 (100 ng/mL), αCD40 (5 μg/mL), and αIgM (10 μg/mL) for various periods were subjected to immunoblotting with the indicated antibodies. Actin was used as the loading control. Phosphorylated ASK1 is denoted as p-ASK1. (B) Ectopic expression of ASK1 promotes apoptosis of short-lived plasma cells. Murine splenic B220+ B cells stimulated with IL-21, αCD40, and αIgM were transduced with retroviral vectors producing either WT or CA ASK1 or with a mock virus (pGC) followed by annexin V staining and FACS to measure apoptosis in the yfp+B220lowCD138+ gate at 2 days after transduction. The diagram above the histograms shows the linear domain map of ASK1. (C) Plasma cells gated from the stimulated Ask1 KO splenic B-cell culture show decreased apoptosis. B220+ splenic B cells isolated from WT or Ask1 KO mice were cultured with LPS or the combination of IL-21, αCD40, and αIgM for various periods. The extent of apoptosis of B220lowCD138+ plasma cells was analyzed by annexin V+ staining. (D-E) Increased NP-specific antibody-secreting cells were detected in the spleen (D) or bone marrow (E) of Ask1 KO mice. Splenocytes or bone marrow cells from WT and Ask1 KO mice harvested at 1 week (D) and 6 weeks (E) after NP-Ficoll (D) or NP–keyhole limpet hemocyanin (E) immunization were used to quantify NP-specific IgM-secreting (D) or IgG-secreting (E) cells by ELISPOT analysis. Each closed or open circle represents an individual WT or Ask1 KO mouse (D: n = 5; E: n = 4). *P < .05.
ASK1 enhances apoptosis during the generation of short-lived plasma cells. (A) ASK1 is activated during the generation of short-lived plasma cells. Splenic B cells treated with LPS (2 μg/mL) or the combination of IL-21 (100 ng/mL), αCD40 (5 μg/mL), and αIgM (10 μg/mL) for various periods were subjected to immunoblotting with the indicated antibodies. Actin was used as the loading control. Phosphorylated ASK1 is denoted as p-ASK1. (B) Ectopic expression of ASK1 promotes apoptosis of short-lived plasma cells. Murine splenic B220+ B cells stimulated with IL-21, αCD40, and αIgM were transduced with retroviral vectors producing either WT or CA ASK1 or with a mock virus (pGC) followed by annexin V staining and FACS to measure apoptosis in the yfp+B220lowCD138+ gate at 2 days after transduction. The diagram above the histograms shows the linear domain map of ASK1. (C) Plasma cells gated from the stimulated Ask1 KO splenic B-cell culture show decreased apoptosis. B220+ splenic B cells isolated from WT or Ask1 KO mice were cultured with LPS or the combination of IL-21, αCD40, and αIgM for various periods. The extent of apoptosis of B220lowCD138+ plasma cells was analyzed by annexin V+ staining. (D-E) Increased NP-specific antibody-secreting cells were detected in the spleen (D) or bone marrow (E) of Ask1 KO mice. Splenocytes or bone marrow cells from WT and Ask1 KO mice harvested at 1 week (D) and 6 weeks (E) after NP-Ficoll (D) or NP–keyhole limpet hemocyanin (E) immunization were used to quantify NP-specific IgM-secreting (D) or IgG-secreting (E) cells by ELISPOT analysis. Each closed or open circle represents an individual WT or Ask1 KO mouse (D: n = 5; E: n = 4). *P < .05.
To further understand the physiologic relevance of ASK1 is regulating plasma cell survival in vivo, we examine the production of plasma cells after immunization. Seven days after immunization with TI antigen, (4-hydroxy-3-nitrophenyl) acetyl (NP)–Ficoll, KO mice produced significantly higher numbers of NP-specific IgM-secreting cells, which reflect short-lived plasma cells,17 in spleen compared with those produced by WT mice (Figure 1D), consistent with the notion that ASK1 promotes apoptosis of short-lived plasma cells. Along this line, we wondered if the numbers of long-lived plasma cells, generated by the TD antigen, NP–keyhole limpet hemocyanin, in bone marrow of mice was also affected by a lack of ASK1. Interestingly, at 6 weeks after immunization, the KO mice had more NP-specific IgG-secreting cells in bone marrow than did WT mice (Figure 1E), which coincided with the increased NP-specific IgG titers in the sera of KO mice 3 and 6 weeks after immunization (supplemental Figure 1). These results suggested that loss of ASK1 may prolong plasma cell survival, leading to increased numbers of long-lived and short-lived plasma cells.
ASK1 is directly repressed by Blimp-1
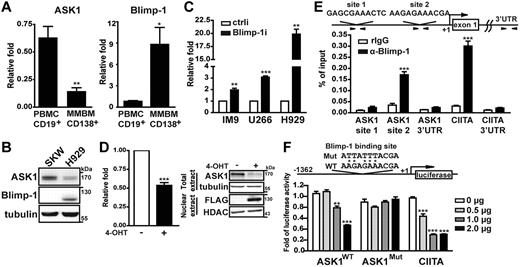
Given that lack of ASK1 increased long-lived plasma cell numbers and that Blimp-1 is important for the maintenance of long-lived plasma cells,19,20 we speculated that Blimp-1 may repress ASK1 in these cells. We examined the association between the expression of Blimp-1 and of ASK1 in CD19+ human mature B cells isolated from peripheral blood of healthy persons and in CD138+ human plasma cells isolated from bone marrow aspirates of patients with MM. As expected, the level of Blimp-1 mRNA was ∼ 8-fold higher in plasma cells than in mature B cells (Figure 2A). Of note, the level of ASK1 mRNA was reduced ∼ 4-fold in plasma cells than in mature B cells (Figure 2A). Consistently, the expression of ASK1 and Blimp-1 protein in a human lymphoblastoid cell line, SKW6.4, and in a MM cell line, H929, was inverse (Figure 2B). The reduced expression of ASK1 appeared to be sensitive to the level of Blimp-1 because Blimp-1 depletion via a lentiviral vector expressing shRNA for Blimp-1, Blimp-1i,20 in MM cell lines (including IM9, U266, and H929) increased ASK1 mRNA levels compared with cells transduced with a control vector expressing a scrambled shRNA sequence (ctrli; Figure 2C). In a reverse trend, the expression of ASK1 mRNA and protein was reduced on induction of Blimp-1 in an established WI–IL-2 B-cell line that expressed inducible FLAG-tagged Blimp-1 fused with estrogen ligand binding domain18 (Figure 2D left panel and right panel, respectively). Together, these opposite expression profiles suggested that Blimp-1 suppresses ASK1 expression.
ASK1 expression is directly repressed by Blimp-1. (A) RT-QPCR analysis of mRNA levels of Blimp-1 and ASK1 in mature B cells isolated from peripheral human blood mononuclear cells (PBMC-CD19+) and plasma cells isolated from bone marrow aspirates from patients with MM (MMBM-CD138+). Results were compared with the internal control GAPDH mRNA and further normalized to PBMC-CD19+. Data are mean ± SD (n = 5). (B) Immunoblotting shows the expression of Blimp-1 and ASK1 in SKW6.4 and H929 cells. Tubulin was the loading control. (C) Knockdown of Blimp-1 increases the expression of ASK1 mRNA. Human MM cell lines IM9, U266, and H929 transduced with a lentiviral vector (Blimp-1i or ctrli) for 4 days were subjected to the analysis of ASK1 mRNA expression by RT-QPCR. GAPDH mRNA was used as the internal control, and the results were normalized to the ctrli sample. Data are mean ± SD (n = 3). (D) Induction of Blimp-1 represses ASK1. Blimp-1 was induced by treatment with CdCl2 (5μM) and 4-OHT (3μM) in WI-IL2Blimp1 fused with estrogen ligand binding domain stable transfectants for 24 hours. Induced or uninduced cells were used to prepare RNA (RT-QPCR analysis) and nuclear extract and total-cell lysates (immunoblot analysis). RT-QPCR results were compared with the uninduced samples. Data are mean ± SD (n = 3). (E) Chromatin from H929 cells was immunoprecipitated with anti–Blimp-1 or rabbit IgG (control), and the precipitated chromatin samples were then amplified by QPCR with the use of primer sets spanning the 2 putative Blimp-1 binding sites (ASK1 site 1 and site 2) in the ASK1 promoter, the known Blimp-1 site in the CIITA promoter, and other control loci. Results were normalized to input DNA. (F) Blimp-1 suppresses ASK1 transcription. Raji cells were transfected by electroporation with the indicated doses of Blimp-1 expression plasmid, RL-tk, for normalization control, and the firefly luciferase reporter constructs driven by −1362 to 100 bp relative to the transcriptional start site of ASK1 containing either a WT or mutated (Mut) Blimp-1 binding site. Cells were harvested after 24 hours and subjected to a luciferase assay. Luciferase was normalized to each luciferase reporter control group that had not been transfected with the Blimp-1 expression vector. Results are mean ± SD from 3 independent experiments. *P < .05, **P < .01, ***P < .001. UTR indicates untranslated region.
ASK1 expression is directly repressed by Blimp-1. (A) RT-QPCR analysis of mRNA levels of Blimp-1 and ASK1 in mature B cells isolated from peripheral human blood mononuclear cells (PBMC-CD19+) and plasma cells isolated from bone marrow aspirates from patients with MM (MMBM-CD138+). Results were compared with the internal control GAPDH mRNA and further normalized to PBMC-CD19+. Data are mean ± SD (n = 5). (B) Immunoblotting shows the expression of Blimp-1 and ASK1 in SKW6.4 and H929 cells. Tubulin was the loading control. (C) Knockdown of Blimp-1 increases the expression of ASK1 mRNA. Human MM cell lines IM9, U266, and H929 transduced with a lentiviral vector (Blimp-1i or ctrli) for 4 days were subjected to the analysis of ASK1 mRNA expression by RT-QPCR. GAPDH mRNA was used as the internal control, and the results were normalized to the ctrli sample. Data are mean ± SD (n = 3). (D) Induction of Blimp-1 represses ASK1. Blimp-1 was induced by treatment with CdCl2 (5μM) and 4-OHT (3μM) in WI-IL2Blimp1 fused with estrogen ligand binding domain stable transfectants for 24 hours. Induced or uninduced cells were used to prepare RNA (RT-QPCR analysis) and nuclear extract and total-cell lysates (immunoblot analysis). RT-QPCR results were compared with the uninduced samples. Data are mean ± SD (n = 3). (E) Chromatin from H929 cells was immunoprecipitated with anti–Blimp-1 or rabbit IgG (control), and the precipitated chromatin samples were then amplified by QPCR with the use of primer sets spanning the 2 putative Blimp-1 binding sites (ASK1 site 1 and site 2) in the ASK1 promoter, the known Blimp-1 site in the CIITA promoter, and other control loci. Results were normalized to input DNA. (F) Blimp-1 suppresses ASK1 transcription. Raji cells were transfected by electroporation with the indicated doses of Blimp-1 expression plasmid, RL-tk, for normalization control, and the firefly luciferase reporter constructs driven by −1362 to 100 bp relative to the transcriptional start site of ASK1 containing either a WT or mutated (Mut) Blimp-1 binding site. Cells were harvested after 24 hours and subjected to a luciferase assay. Luciferase was normalized to each luciferase reporter control group that had not been transfected with the Blimp-1 expression vector. Results are mean ± SD from 3 independent experiments. *P < .05, **P < .01, ***P < .001. UTR indicates untranslated region.
We next verified whether ASK1 is directly repressed by Blimp-1. A genomic region that spanned 1.5 kb upstream of the transcriptional start site of human ASK1 was reported to be necessary for basal transcription from ASK128 and was thus used to analyze the potential Blimp-1 binding sites according to putative Blimp-1 binding sequences.29 We identified 2 putative Blimp-1 binding sites, which are located at approximately −1309 to −1299 and −262 to −253 relative to the transcription start site of ASK1. Chromatin immunoprecipitation was used to examine whether Blimp-1 binds these 2 putative sites in the ASK1 promoter; compared with samples immunoprecipitated with the rabbit IgG control antibody, Blimp-1 bound to CIITA, a known direct target of Blimp-1 as a positive control,30 and to the −262 to −253 region of ASK1 but not to the −1309 to −1299 region or the sequence encoding the 3′ untranslated region (Figure 2E). Likewise, in the mouse Ask1 5′ regulatory region, 2 potential Blimp-1 binding sites were found, and both appeared to be occupied by Blimp-1 in B220lowCD138+ plasma cells sorted from LPS-stimulated splenic B-cell cultures (supplemental Figure 2). To determine whether the putative Blimp-1 binding site in the −262 to −253 region of human ASK1 is critical for Blimp-1 repression, we generated a reporter construct containing the −1.3-kb region of ASK1 fused to luciferase cDNA. A previously generated luciferase reporter driven by CIITA promoter III was used as a positive control for Blimp-1 repression.30 We found that, indeed, Blimp-1 suppressed the WT ASK1 promoter as well as the CIITA promoter activity in a dose-dependent manner (Figure 2F), whereas the Blimp-1–mediated repression was significantly diminished when the Blimp-1 binding site was mutated (Figure 2F). Taken together, these data indicated that Blimp-1 directly repressed ASK1 transcription.
Ectopic expression of ASK1 causes apoptosis in MM cells
Because Blimp-1 suppresses ASK1, we examined whether ectopic expression of ASK1 is sufficient to induce the death of malignant plasma cells, even in the presence of endogenous Blimp-1. A retroviral vector that expressed FLAG-tagged WT or CA ASK1, or yfp alone (pGC), was transduced into the human MM cell lines, H929 and U266 cells, and the retrovirally transduced (yfp+) cells were sorted 1 day later and maintained in culture for various periods to determine the extent of cell death. The expression of the various FLAG-tagged ASK1 proteins was confirmed by immunoblotting with anti-FLAG (Figure 3A). Compared with pGC-transduced cells, expression of WT or CA ASK1 resulted in more pronounced cell death in both the H929 and U266 lines at most time points (Figure 3A). In addition, when H929 cells were transduced with a dominant-negative ASK1, which is a loss-of-kinase-function mutant that carries a lysine-to-arginine point mutation at 709 site (K709R),12 cell death was comparable with that of pGC-transduced cells (Figure 3A left panel), indicating that the kinase activity of ASK1 controls the death of plasma cells. In line with this, Tet-on–based, Dox-dependent, inducible H929 and IM9 stable transfectants that express FLAG-tagged ASK1 were generated to examine cell death after induction of ASK1. Dox induced ASK1 on various days, as confirmed by immunoblotting with anti-FLAG (Figure 3B). As expected, induction of ASK1 expression in these 2 inducible transfectants triggered increased cell death in a time-dependent manner compared with control transfectants that carried only the TRE (Figure 3B). The increased cell death by enforced expression of ASK1 resulted from apoptosis because H929 cells retrovirally transduced with CA or WT ASK1 had a greater proportion of annexin V–positive cells compared with pGC-transduced cells (Figure 3C). Together, these data indicated that ectopic expression of ASK1 triggered apoptotic cell death in malignant plasma cells.
ASK1 triggers apoptosis in human MM cell lines. (A) Ectopic expression of ASK1 increases death of H929 and U266 cells. Retroviral vector producing FLAG-tagged WT, CA, or dominant-negative (DN) ASK1 or the control vector, pGC, was transduced into H929 (left) and U266 (right) cells. The transduced cells were sorted according to the expression of yfp at 1 day after transduction and cultured for the days indicated. The percentage of cell death was determined by trypan blue exclusion. Immunoblotting with anti-FLAG shows the expression of transduced proteins. Tubulin was used as an internal control. (B) Tet-on-inducible stable H929 and IM9 transfectants were treated with Dox (1 μg/mL) for 3 days to induce ASK1 expression. Percentage of cell death was determined by trypan blue exclusion with the use of cells harvested on various days after Dox treatment, and the expression of inducible ASK1 was evaluated by immunoblotting using anti-FLAG. Results represent mean ± SD (n = 3). (C) Ectopic expression of ASK1 causes apoptosis of H929 cells. H929 cells were transduced with retroviral vector to produce WT ASK1 (black line), CA ASK1 (gray line), or pGC control vector (gray fill) for 3 days. The apoptotic cells in the yfp+ gate were determined by annexin V staining. Results are from 1 experiment and are representative of 3 independent experiments. *P < .05, **P < .01.
ASK1 triggers apoptosis in human MM cell lines. (A) Ectopic expression of ASK1 increases death of H929 and U266 cells. Retroviral vector producing FLAG-tagged WT, CA, or dominant-negative (DN) ASK1 or the control vector, pGC, was transduced into H929 (left) and U266 (right) cells. The transduced cells were sorted according to the expression of yfp at 1 day after transduction and cultured for the days indicated. The percentage of cell death was determined by trypan blue exclusion. Immunoblotting with anti-FLAG shows the expression of transduced proteins. Tubulin was used as an internal control. (B) Tet-on-inducible stable H929 and IM9 transfectants were treated with Dox (1 μg/mL) for 3 days to induce ASK1 expression. Percentage of cell death was determined by trypan blue exclusion with the use of cells harvested on various days after Dox treatment, and the expression of inducible ASK1 was evaluated by immunoblotting using anti-FLAG. Results represent mean ± SD (n = 3). (C) Ectopic expression of ASK1 causes apoptosis of H929 cells. H929 cells were transduced with retroviral vector to produce WT ASK1 (black line), CA ASK1 (gray line), or pGC control vector (gray fill) for 3 days. The apoptotic cells in the yfp+ gate were determined by annexin V staining. Results are from 1 experiment and are representative of 3 independent experiments. *P < .05, **P < .01.
ASK1 activity is required for MM cell apoptosis
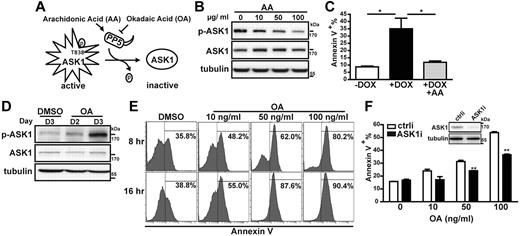
Because ASK1 overexpression could overcome the survival-promoting effect of Blimp-1 and could increase plasma cell death, we next examined whether ASK1 activity is crucial for this effect. ASK1 activity can be negatively regulated by PP5.16 The phosphatase activity of PP5 can be inhibited or activated in vitro by OA31 and AA,32 respectively (Figure 4A). Indeed, the phosphorylation of ASK1 at T838 in the ASK1-inducible H929 cells treated with Dox was blocked by cotreatment with AA in a dose-dependent manner (Figure 4B). Furthermore, the ASK1-induced apoptosis could be prevented by cotreating ASK1-inducible H929 cells with Dox and AA (Figure 4C). Reciprocally, ASK1 in H929 cells could be phosphorylated at T838 3 days after OA treatment (Figure 4D), which dose and time dependently led to enhanced apoptosis of CD138+ human plasma cells isolated from bone marrow of patients with MM (Figure 4E). This effect largely relied on the induction of ASK1 activity by OA treatment because cells depleted of ASK1 showed reduced OA-mediated apoptosis compared with cells treated with ctrli (Figure 4F). Taken together, our data showed that regulation of ASK1 activity can modulate MM cell survival.
Modulation of ASK1 activity affects MM cell survival. (A) Schematic representation of the mechanism by which PP5 regulates ASK1 activity. (B) ASK1-inducible H929 cells simultaneously treated with the indicated doses of AA and Dox (1 μg/mL) for 48 hours were harvested for lysate preparation, followed by immunoblotting with the indicated antibodies. Tubulin was used as an internal control. Phosphorylated ASK1 is denoted as p-ASK1. (C) ASK1-inducible H929 cells treated without Dox (−Dox), with Dox (+Dox; 1 μg/mL), or with Dox plus AA (+Dox at 1 μg/mL +AA at 10 μg/mL) for 72 hours were subjected to annexin V staining. Results show the percentage of annexin V+ cells and are the mean ± SD (n = 3). *P < .05. (D) H929 cells treated with OA (10 ng/mL) for 2 or 3 days or treated with the solvent (DMSO, control) for 3 days were subjected to immunoblotting with the indicated antibodies. (E) Human primary plasma cells from patients with MM isolated with CD138+ beads were treated with OA at the indicated doses or with DMSO (control) and were harvested 8 or 16 hours later for annexin V staining. Data are for 1 patient and are representative of data from 3 patient samples. (F) H929 cells were transfected by electroporation with an siRNA against ASK1 mRNA (ASK1i) or a control siRNA (ctrli). After 24 hours, a portion of cells were harvested for immunoblotting to ensure the knockdown of ASK1. Cells were then treated with 10, 50, or 100 ng/mL OA or with DMSO (0 ng/mL of OA) for an additional 24 hours, followed by annexin V staining. Results show the percentage of annexin V+ cells and are the mean ± SD (n = 2). **P < .01.
Modulation of ASK1 activity affects MM cell survival. (A) Schematic representation of the mechanism by which PP5 regulates ASK1 activity. (B) ASK1-inducible H929 cells simultaneously treated with the indicated doses of AA and Dox (1 μg/mL) for 48 hours were harvested for lysate preparation, followed by immunoblotting with the indicated antibodies. Tubulin was used as an internal control. Phosphorylated ASK1 is denoted as p-ASK1. (C) ASK1-inducible H929 cells treated without Dox (−Dox), with Dox (+Dox; 1 μg/mL), or with Dox plus AA (+Dox at 1 μg/mL +AA at 10 μg/mL) for 72 hours were subjected to annexin V staining. Results show the percentage of annexin V+ cells and are the mean ± SD (n = 3). *P < .05. (D) H929 cells treated with OA (10 ng/mL) for 2 or 3 days or treated with the solvent (DMSO, control) for 3 days were subjected to immunoblotting with the indicated antibodies. (E) Human primary plasma cells from patients with MM isolated with CD138+ beads were treated with OA at the indicated doses or with DMSO (control) and were harvested 8 or 16 hours later for annexin V staining. Data are for 1 patient and are representative of data from 3 patient samples. (F) H929 cells were transfected by electroporation with an siRNA against ASK1 mRNA (ASK1i) or a control siRNA (ctrli). After 24 hours, a portion of cells were harvested for immunoblotting to ensure the knockdown of ASK1. Cells were then treated with 10, 50, or 100 ng/mL OA or with DMSO (0 ng/mL of OA) for an additional 24 hours, followed by annexin V staining. Results show the percentage of annexin V+ cells and are the mean ± SD (n = 2). **P < .01.
Induction of ASK1 decreases MM cell growth in vivo and prolongs animal survival in a xenograft model of human MM
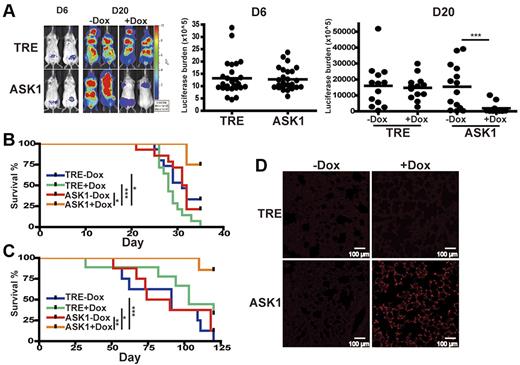
We next investigated whether induction of ASK1 decreases MM cell growth in vivo with the use of a xenograft model of human MM. We adopted a luciferase-based, noninvasive bioluminescent imaging system in a SCID mouse–human MM xenograft model in which the bioluminescence in each individual mouse could be quantified by real-time photon emission that correlates with MM cell number. ASK1-inducible H929 or IM9 transfectants stably expressing luciferase were intravenously xenografted into SCID mice that had received a sublethal dose of radiation. At 6 days after transplantation, mice that had received ASK1-inducible IM9 transfectants were administrated Dox, and in vivo bioluminescent imaging analysis showed that mice carrying ASK1-inducible transfectants or control TRE transfectants exhibited undistinguishable MM cell load before Dox treatment on day 6. The induction of ASK1 after Dox administration in vivo was validated by immunoblotting with the use of lysates from extramedullar solid tumors of mice that received a transplant (supplemental Figure 3A). Remarkably, mice bearing ASK1-inducible IM9 transfectants showed significantly reduced bioluminescence after Dox administration than did mice without Dox treatment on day 20. By contrast, mice engrafted with control TRE IM9 transfectants in the presence or absence of Dox administration had similar bioluminescence (Figure 5A). In addition to the diminished MM cell growth in vivo on ASK1 induction, overall survival was significantly prolonged in mice that received a transplant with ASK1-inducible IM9 transfectants and administrated Dox (Figure 5B) compared with mice that received control TRE IM9 transfectants treated with or without Dox or ASK1-inducible IM9 transfectants without Dox. Similar results, in terms of reduced bioluminescence in vivo (supplemental Figure 3B) and prolonged survival (Figure 5C) after Dox administration, were evident for mice that received a transplant with a stable ASK1-inducible H929 transfectant compared with control TRE H929 transfectants.
Induction of ASK1 reduces MM cell proliferation and prolongs survival in an MM xenograft model. (A) Induction of ASK1 inhibits MM cell proliferation. Luciferase-expressing ASK1-inducible IM9 transfectant (ASK1) or control TRE transfectant (TRE) were intravenously injected into sublethally irradiated SCID mice. Xenografted mice were orally administrated doxycycline (+Dox) by gavage (1 mg in 1 mL per mouse) and in drinking water (0.2 mg/mL Dox) at 6 days after engraftment or left untreated (−Dox). Bioluminescence was measured after injection with D-luciferin intraperitoneally on the days indicated, followed by image acquisition. Thirteen mice were used for each group. Data were acquired for individual mice that received a xenograft, and the horizontal bars indicate the median of each group. (B-C) Induction of ASK1 prolongs animal survival. Kaplan-Meier survival curve of mice engrafted with luciferase-expressing ASK1-inducible IM9 transfectants (B) or luciferase-expressing ASK1-inducible H929 transfectants (C). (D) Induction of ASK1 increases apoptosis of MM cells in vivo. Paraffin-embedded lung sections from luciferase-expressing ASK1-inducible–engrafted mice or control TRE H929 transfectant–engrafted mice treated with or without Dox at 20 days after engraftment were subjected to TUNEL assay. *P < .05, **P < .01, ***P < .001.
Induction of ASK1 reduces MM cell proliferation and prolongs survival in an MM xenograft model. (A) Induction of ASK1 inhibits MM cell proliferation. Luciferase-expressing ASK1-inducible IM9 transfectant (ASK1) or control TRE transfectant (TRE) were intravenously injected into sublethally irradiated SCID mice. Xenografted mice were orally administrated doxycycline (+Dox) by gavage (1 mg in 1 mL per mouse) and in drinking water (0.2 mg/mL Dox) at 6 days after engraftment or left untreated (−Dox). Bioluminescence was measured after injection with D-luciferin intraperitoneally on the days indicated, followed by image acquisition. Thirteen mice were used for each group. Data were acquired for individual mice that received a xenograft, and the horizontal bars indicate the median of each group. (B-C) Induction of ASK1 prolongs animal survival. Kaplan-Meier survival curve of mice engrafted with luciferase-expressing ASK1-inducible IM9 transfectants (B) or luciferase-expressing ASK1-inducible H929 transfectants (C). (D) Induction of ASK1 increases apoptosis of MM cells in vivo. Paraffin-embedded lung sections from luciferase-expressing ASK1-inducible–engrafted mice or control TRE H929 transfectant–engrafted mice treated with or without Dox at 20 days after engraftment were subjected to TUNEL assay. *P < .05, **P < .01, ***P < .001.
On the basis of our results that induction of ASK1 diminished MM cell growth in vivo, we wondered whether the reduced cell growth could reflect enhanced apoptosis. We used a TUNEL assay with lung tissues of engrafted mice because of their strong bioluminescence on day 20 (supplemental Figure 3C). Indeed, a pronounced increase in TUNEL+ signals was observed in lung tissues of mice bearing ASK1-inducible H929 transfectants on Dox administration compared with mice without Dox treatment and the control TRE transfectants (Figure 5D).
ASK1-induced apoptosis is involved in the activation of JNK and p38 MAPKs and elevated Bim expression
We next examined the downstream signaling pathways regulated by ASK1-induced apoptosis in long-lived plasma cells. ASK1 activates JNK and p38 MAPKs.12 As expected, increased phosphorylation of JNK and p38 MAPK was observed in Dox-treated ASK1-inducible H929 transfectants but not in control TRE transfectants. Of note, ASK1 was phosphorylated at T838 after Dox treatment for various times (Figure 6A). Bcl-2 family proteins are important apoptotic regulators,33 and the balance between Mcl-1 and Bim is especially crucial for MM cell survival.34 We examined the expression of antiapoptotic and proapoptotic Bcl-2 family proteins after ASK1 induction. We found that total Bim, especially BimEL, which is encoded by an alternatively spliced form of Bim mRNA,35 was significantly increased on the induction of ASK1, whereas the levels of Bcl-2, Bcl-xL, and Mcl-1 did not change (Figure 6B). Phosphorylation of BimEL at serine (S) 65 is required for JNK- or p38-mediated apoptosis,36,37 and phosphorylation of Mcl-1 at S159 potentiates Mcl-1 degradation38 ; we thus checked whether the increased BimEL was phosphorylated at S65 and if Mcl-1 could be phosphorylated at S159 on ASK1 induction. Indeed, the levels of S65-phosphorylated BimEL increased after ASK1 induction; however, Mcl-1 phosphorylation at S159 was not up-regulated (Figure 6B).
ASK1 induces the activation of JNK and p38 MAPK and increases Bim expression. (A) Induction of ASK1 enhances activation of JNK and p38 MAPK. ASK1-inducible (ASK1) and control (TRE) H929 transfectants treated with Dox (1 μg/mL) were harvested on the days indicated, followed by immunoblotting of cell lysates with the indicated antibodies. Tubulin was used as an internal control. Each lowercase p indicates a phosphorylated protein. (B) Induction of ASK1 increases the expression of total Bim and enhances the phosphorylation of BimEL at residue S65. Lysates used in the experiments for panel A were used for immunoblotting with the indicated antibodies. (C) ASK1 is required for the Blimp-1 knockdown–mediated elevation of Bim level. H929 cells were transfected by electroporation with an siRNA that targeted ASK1 mRNA (ASK1i) or a nonspecific control siRNA (ctrli). After 24 hours, cells were transduced by a lentiviral vector to produce an shRNA against Blimp-1 (Blimp-1i) or a scrambled sequence (ctrli) for an additional 4 days. Cells were then harvested for lysate preparation, followed by immunoblotting with the indicated antibodies. (D) ASK1 is required for Blimp-1 knockdown–mediated apoptosis. H929 cells depleted of Blimp-1 or depleted of ASK1 and Blimp-1, as described in panel C, were subjected to annexin V staining followed by flow cytometric analysis.
ASK1 induces the activation of JNK and p38 MAPK and increases Bim expression. (A) Induction of ASK1 enhances activation of JNK and p38 MAPK. ASK1-inducible (ASK1) and control (TRE) H929 transfectants treated with Dox (1 μg/mL) were harvested on the days indicated, followed by immunoblotting of cell lysates with the indicated antibodies. Tubulin was used as an internal control. Each lowercase p indicates a phosphorylated protein. (B) Induction of ASK1 increases the expression of total Bim and enhances the phosphorylation of BimEL at residue S65. Lysates used in the experiments for panel A were used for immunoblotting with the indicated antibodies. (C) ASK1 is required for the Blimp-1 knockdown–mediated elevation of Bim level. H929 cells were transfected by electroporation with an siRNA that targeted ASK1 mRNA (ASK1i) or a nonspecific control siRNA (ctrli). After 24 hours, cells were transduced by a lentiviral vector to produce an shRNA against Blimp-1 (Blimp-1i) or a scrambled sequence (ctrli) for an additional 4 days. Cells were then harvested for lysate preparation, followed by immunoblotting with the indicated antibodies. (D) ASK1 is required for Blimp-1 knockdown–mediated apoptosis. H929 cells depleted of Blimp-1 or depleted of ASK1 and Blimp-1, as described in panel C, were subjected to annexin V staining followed by flow cytometric analysis.
We previously showed that knockdown of Blimp-1 caused apoptosis of MM cells with a concomitant increase in Bim.20 We thus speculated that ASK1 is required for Blimp-1 knockdown–induced apoptosis and Bim elevation. Indeed, Blimp-1 knockdown increased expression of Bim, which was diminished when ASK1 was simultaneously depleted (Figure 6C). Furthermore, knocking down ASK1 and Blimp-1 together efficiently blocked Blimp-1 knockdown–mediated apoptosis in H929 cells (Figure 6D), showing that ASK1 is required for Blimp-1 knockdown–induced Bim elevation and apoptosis.
Discussion
In this study, we identified a new role for ASK1 in apoptotic regulation of plasma cells. ASK1 is activated during differentiation of murine short-lived plasma cells in vitro and contributes to apoptosis of these cells. We hypothesized that ASK1 is directly suppressed by Blimp-1 in long-lived plasma cells to avoid the possibility of activation of stress-induced apoptotic signaling pathways. The physiologic relevance of ASK1 in regulating plasma cell survival was further supported by our findings that Ask1 KO mice produced higher numbers of antigen-specific short-lived and long-lived plasma cells.
The limited lifespan of short-lived plasma cells is attributable to spontaneous apoptosis caused by activation of caspase-12, which is the human caspase-4 counterpart and is involved in ER stress–induced apoptosis.39 Our results showed that ASK1 is phosphorylated, but its expression does not significantly change during differentiation of short-lived plasma cells after treatment with a TD or TI stimulant in vitro. Therefore, ASK1 function may be mainly regulated at the posttranslational level during the generation of short-lived plasma cells, thereby providing a means to rapidly respond to ER stress. Accordingly, short-lived plasma cells generated from B cells of Ask1 KO mice appeared to survive longer, and more antigen-specific short-lived plasma cells were found in Ask1 KO mice after immunization; this was not due, however, to abnormal B-cell development in those mice because Ask1 KO and control mice were comparable for the distribution of various splenic B-cell subsets (data not shown). In malignant counterparts of long-lived plasma cells, however, the regulation of ASK1 may be controlled at the transcriptional level by Blimp-1–mediated suppression. Enforced induction of ASK1 expression was sufficient to induce MM cell death in vitro and in vivo, and the latter led to reduced MM cell number and prolonged the survival of engrafted animals. However, residual ASK1 was still detectable in MM cells, suggesting that the chromatin environment of the ASK1 locus is not completely silenced in MM cells. Supporting this notion, Blimp-1 knockdown in MM cells could reestablish ASK1 expression. In addition, the residual ASK1 could be activated by treating cells with OA; as a consequence, apoptosis was triggered in the MM cell line or in MM cells from patients, indicating that ASK1 function is manipulatable in MM cells. However, why Blimp-1 induction during short-lived plasma cell differentiation was not able to repress Ask1 transcription remains to be mechanistically investigated. It could be that Blimp-1 binding to the Ask1 locus in short-lived plasma cells is not sufficient to repress transcription. Supporting this notion, we found that in short-lived plasma cells generated by stimulation of splenocytes with LPS, the region in mouse Ask1 that contained the Blimp-1 binding site (site 2) still contained high levels of acetylated histone (H)3, a marker for transcriptional activation, compared with those in day 0 culture (supplemental Figure 4).
Aberrant activation of ASK1 is involved in various diseases, such as cardiac hypertrophy, diabetes, and neuronal degeneration.11,40,41 ASK1 is also involved in tumor necrosis factor–induced generation of reactive oxygen species.42 Thus, ASK1 is required for inflammation because bone marrow–derived dendritic cells from Ask1 KO mice exhibit an attenuated inflammatory response.22 Here, we showed that suppression of ASK1 expression or reduction of its activity is required for the survival of short-lived plasma cells, long-lived plasma cells, and MM cells. Particularly, abnormal survival of short-lived or long-lived plasma cells correlates with autoimmune diseases in which autoantibodies are overproduced.43 Therefore, regulation of ASK1 activity could be a potential therapeutic strategy for modulating immune responses, malignant plasma cell survival, or the above-mentioned diseases.
In our previous study, Blimp-1 knockdown–mediated apoptosis of MM cells was accompanied by increased levels of Bim and decreased Mcl-1.20 In the present study, we show that the level of Bim, but not of Mcl-1, is altered in response to ASK1 induction in MM cells, suggesting that an elevated level of Bim after Blimp-1 knockdown has its effect downstream of ASK1 activation. Supporting this notion, we found that the increase in Bim level was reversed when Blimp-1 and ASK1 were simultaneously depleted in MM cells. However, Mcl-1 expression was not sensitive to ASK1 induction. In fact, the regulation of Mcl-1 expression is also controlled by ER stress.44 The Mcl-1 level decreases because of cessation of mRNA translation through phosphorylation of eukaryotic initiation factor 2α,44 whose activation is triggered by the RNA-dependent protein kinase–like ER protein kinase pathway on ER stress.45 We previously showed that Blimp-1 knockdown–induced apoptosis could trigger caspase-4 activation,20 a hallmark for ER stress–induced apoptosis.46 Therefore, taken all together, we think that Blimp-1 knockdown–induced apoptosis may be influenced by 2 pathways in ER stress response: the protein kinase–like ER protein kinase pathway, which leads to a pause of Mcl-1 translation, and the inositol-requiring transmembrane kinase/endonuclease 1 (IRE1) pathway because ASK1 is one of its downstream effectors.45 IRE1 is particularly important in the context of plasma cells because its activation triggers the splicing of X-box–binding protein-1 mRNA, which encodes an active transcription factor.45 X-box–binding protein-1 is required for the production of immunoglobulins47 and for MM cell survival in response to ER stress.48 Accordingly, disruption of IRE1 in mice causes decreased levels of serum immunoglobulins produced by plasma cells.49 In addition to up-regulation of Bim expression, phosphorylation of Bim at S65 was enhanced after forced expression of ASK1 in MM cells. It is known that phosphorylation of S65 in Bim is required for p38 MAPK– or JNK-mediated apoptosis36,37 and for its release from binding to Mcl-1.50 Taken together, the evidence suggests that ASK1 induction–mediated apoptosis of MM cells also is potentiated by the enhanced phosphorylation of Bim at S65 by JNK and p38 MAPK, which act downstream of ASK1; as a consequence, Bim can be released from binding to Mcl-1 to execute apoptosis.
In summary, we here show a new role for ASK1 in regulating apoptosis of normal and malignant plasma cells. We rationalized that long-lived or malignant plasma cells may require sustained suppression of ASK1 by Blimp-1 to overcome the activation of stress-induced apoptosis. Our study suggests the importance of regulating the ASK1 signaling axis for optimal control of plasma cell numbers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr W.-H. Lee for discussion and S.-Y. Yang for technical assistance.
This work was supported by Academia Sinica (AS-99-CDA-L12, K.-I.L.) and the National Science Council (100-2628-B-001-015-MY4, K.-I.L.), Taiwan.
Authorship
Contribution: F.-R.L. and K.-I.L. designed the experiments, analyzed the data, and wrote the paper; F.-R.L., K.-H.H., S.-T.S., and C.-H.C. performed the experiments; and S.-Y.H., A.M., M.H., and H.I. provided important materials.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kuo-I Lin, Genomics Research Center, Academia Sinica, Taipei, 115, Taiwan; e-mail: kuoilin@gate.sinica.edu.tw.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal