In this issue of Blood, Greiner and colleagues describe how peptides derived from the mutated nucleophosmin 1 gene (NPM1mut) can elicit in vitro CD4+ and CD8+ T-cell responses in patients with acute myeloid leukemia, which can lead to antigen-specific lysis of leukemic blasts.1
Acute myeloid leukemia (AML) with normal karyotype is a heterogeneous entity with regard to prognosis. The cases with NPM1mut but without the FMS-like tyrosine kinase 3 internal tandem duplication (FLT3-ITDneg) have a better prognosis. This may be related to the specific immune response that the mutated NPM1 can elicit in AML patients. At first sight, it is difficult to believe that the immune system can offer protection against such an overwhelming disease as AML. Yet, clinical evidence for a T-cell response against AML has come from allogeneic hematopoietic stem cell transplantation, where it is clear that allogeneic T cells in the transplant and in donor lymphocyte infusions can bring about a graft-versus-leukemia effect. Evidence for the importance of an autologous T-cell response against AML has come from the tumor vaccination field. Vaccination against the leukemia-associated antigens2 Wilms tumor protein 1 (WT1),3-6 PR1 (derived from proteinase 3),4 and receptor for hyaluronic acid–mediated motility (RHAMM)7 can bring about clinical antileukemic effects in AML. The clinical response was generally correlated with the T-cell responses elicited.3,4,6 Loss of clinical response has been reported to be associated with decrease or loss of specific T-cell immunity.
But can an antileukemic immune response be elicited in patients not receiving immunotherapy? The answer comes from a vast body of work, demonstrating that, contrary to general belief, certain chemotherapeutic agents can augment immune responses against tumors.8 Chemotherapy thus not only has direct cytotoxic effects on cancerous cells, but can also boost the immunity against them by different mechanisms, including stimulating tumor antigen presentation by dendritic cells to cytotoxic T lymphocytes. This is particularly true of anthracyclines, still the mainstay of treatment of AML, which have been demonstrated to be a prototype of immunogenic chemotherapy.9 It was already known for a while that the antitumoral effect of doxorubicin in certain animal models was strongly reduced if the immune system was not functioning properly.
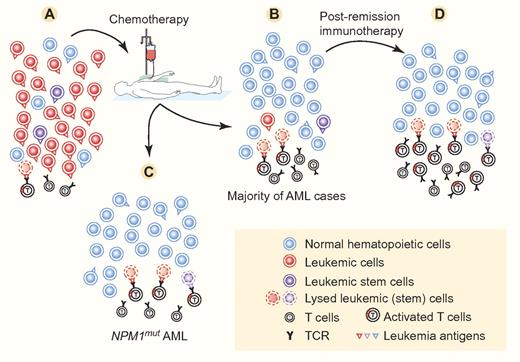
In the case of NPM1mut AML, especially if it is also FLT3-ITDneg, the autologous T-cell response induced by the mutated NPM1 could bring about a significant antileukemic effect directly after chemotherapy (figure panels A and C). At this stage, the number of leukemic cells would significantly be reduced, the immune response could be strengthened, and the stimulated anti-NPM1mut cytotoxic T lymphocytes could mount a final attack against the remaining leukemic cells. This could account for the cures seen with chemotherapy alone in NPM1mut AML. But not all patients with NPM1mutFLT3-ITDneg AML are cured by chemotherapy alone. The findings by Greiner et al theoretically suggest the possibility that postremission immunotherapy directed against NPM1mut could induce cures and/or longer-lasting remissions in this type of AML and maybe even in NPM1mutFLT3-ITDpos AML, especially if there is molecular evidence of residual disease.
In full-blown AML (A), the T cells directed against the leukemia antigens cannot bring about enough lysis of leukemic (stem) cells to bring the disease under control. This is due to the fast dynamics and immunosuppressive mechanisms of AML. Chemotherapy strongly suppresses the leukemic cells and usually allows normal hematopoietic cells to reappear in sufficient numbers (B-C). Chemotherapy can also stimulate the immune response, which may be effective in lysing leukemic cells and stem cells, but usually this is not effective enough to eradicate (minimal) residual disease persisting in a majority of AML cases. In NPM1mut AML (C), especially NPM1mutFLT3-ITDneg AML, it is hypothesized here that T cells, directed against leukemia antigens—especially mutated NPM1—and activated by immunogenic chemotherapy, may be powered enough to bring about complete eradication of AML by lysing all remaining residual leukemic (stem) cells. In case of minimal residual disease (B), complete eradication of leukemic (stem) cells can be brought about by immunotherapy (D). Examples of clinically effective immunotherapy include allogeneic hematopoietic stem cell transplantation followed or not by donor lymphocyte infusions, and vaccination with tumor antigen peptides or with dendritic cells loaded with tumor antigens. Successful immunotherapy against AML has been associated with an increase of T lymphocytes reacting against the leukemia antigens. Leukemia antigens may be present on the surface of normal hematopoietic cells, but these seem to be less susceptible to lysis by antigen-specific T cells. The leukemia antigens present on leukemic cells may be similar to or different from those on normal hematopoietic cells. They are then designated, respectively, as leukemia-associated and leukemia-specific antigens. The cell numbers and leukemia antigen distribution and density indicated in this figure are for schematic purposes only. Professional illustration by Paulette Dennis.
In full-blown AML (A), the T cells directed against the leukemia antigens cannot bring about enough lysis of leukemic (stem) cells to bring the disease under control. This is due to the fast dynamics and immunosuppressive mechanisms of AML. Chemotherapy strongly suppresses the leukemic cells and usually allows normal hematopoietic cells to reappear in sufficient numbers (B-C). Chemotherapy can also stimulate the immune response, which may be effective in lysing leukemic cells and stem cells, but usually this is not effective enough to eradicate (minimal) residual disease persisting in a majority of AML cases. In NPM1mut AML (C), especially NPM1mutFLT3-ITDneg AML, it is hypothesized here that T cells, directed against leukemia antigens—especially mutated NPM1—and activated by immunogenic chemotherapy, may be powered enough to bring about complete eradication of AML by lysing all remaining residual leukemic (stem) cells. In case of minimal residual disease (B), complete eradication of leukemic (stem) cells can be brought about by immunotherapy (D). Examples of clinically effective immunotherapy include allogeneic hematopoietic stem cell transplantation followed or not by donor lymphocyte infusions, and vaccination with tumor antigen peptides or with dendritic cells loaded with tumor antigens. Successful immunotherapy against AML has been associated with an increase of T lymphocytes reacting against the leukemia antigens. Leukemia antigens may be present on the surface of normal hematopoietic cells, but these seem to be less susceptible to lysis by antigen-specific T cells. The leukemia antigens present on leukemic cells may be similar to or different from those on normal hematopoietic cells. They are then designated, respectively, as leukemia-associated and leukemia-specific antigens. The cell numbers and leukemia antigen distribution and density indicated in this figure are for schematic purposes only. Professional illustration by Paulette Dennis.
An additional potential advantage of the T-cell immune response directed against certain leukemia antigens is that it may also be directed against the leukemic stem cells.2 Leukemic stem cells are relatively resistant to chemotherapy,10 accounting at least in part for the (minimal) residual disease persisting after cytotoxic treatment in a majority of AML cases (figure panel B). The chemotherapy resistance of minimal residual disease has led to the development of another type of postremission treatment, that is, immunotherapy, to try to definitively cure AML patients (figure panel D). NPM1mut, a leukemia-specific antigen,2 is expressed in leukemic stem cells,11 making those cells vulnerable to immune eradication, as discussed above.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■