Abstract
There is evidence that dendritic cells (DCs) induce peripheral tolerance. Nevertheless, it is not known whether immature DCs in general are able to tolerize CD4+ T cells or if this is a prerogative of specialized subtypes. Here we show that, when autoantigen presentation is extended to all conventional mouse DCs, immature lymphoid tissue resident DCs are unable to induce autoantigen-specific regulatory T (iTreg) cell conversion. In contrast, this is an exclusive prerogative of steady-state migratory DCs. Because only lymph nodes host migratory DCs, iTreg cells develop and are retained solely in lymph nodes, and not in the spleen. Mechanistically, in cutaneous lymph nodes, DC-derived CCL22 contributes to the retention of iTreg cells. The importance of the local generation of iTreg cells is emphasized by their essential role in preventing autoimmunity.
Introduction
Induction and maintenance of T-cell tolerance toward self-antigens is vital to prevent autoimmunity. To this purpose, many different overlapping and nonoverlapping mechanisms of T-cell tolerization exist, both at central and peripheral levels.1-5
A common vision of how dendritic cells (DCs) contribute to the induction and maintenance of peripheral CD4+ T-cell tolerance is that, in resting conditions, immature DCs, expressing low levels of signal 1 (specificity) and low or no levels of signal 2 (costimulation), are able to induce T-cell unresponsiveness.
However, the effective knowledge concerning the contribution of DCs in inducing and maintaining CD4+ T-cell tolerance in peripheral lymphoid organs derives from the selective analysis of specific DC subpopulations. In particular, CD205+ DCs are able to induce CD4+ T-cell tolerance in conditions of suboptimal activation.6-9 Moreover, steady-state migratory DC (ssmDC) subpopulations from the gut and the skin, phenotypically CD103+ and CD103−, respectively, mediate the conversion of naive T cells into Foxp3+ regulatory T cells (iTreg) in a retinoic acid (RA)–dependent manner.10-13 Because the lymph constantly carries peptides for loading on both migratory and lymphoid tissue resident immature DCs in a dose range suitable for tolerization,14 it cannot be excluded that, in addition to the analyzed populations and in agreement with the common vision, all conventional immature DCs can induce autoantigen specific CD4+ T-cell tolerance in the periphery. Therefore, it was relevant to know whether DCs in general are able to induce T-cell tolerance at the steady state or whether this is a prerogative of specialized subsets. We investigated this question using an experimental system where antigen presentation, in contrast to previous studies, is not a priori confined to a specific DC subpopulation but is extended to all conventional DC subtypes. Specifically, we adopted the 2a T transgenic animal model.15 In this experimental system, T-cell receptor (TCR) transgenic T cells (2a T cells) recognize a portion of the CH3 region (435-451) of the IgG2ab, the Bpep, in association with the MHC molecule, I-Ad. We also generated a mouse model in which the Bpep was presented by CD11c+ cells that include all conventional DC subtypes. By performing a systematic study of the behavior of naive autoantigen-specific T cells after interaction with all conventional CD11c+ DCs in homeostatic conditions, we found that DCs are able to induce CD4+ T-cell tolerance by promoting the conversion of autoantigen-specific naive T cells into iTreg cells. Among the different DC subtypes, ssmDCs possess the unique ability to induce antigen-specific iTreg cells in an RA-dependent manner. Diversely, lymphoid tissue resident DCs do not show this capacity. Therefore, iTreg cells develop solely in lymph nodes and not in the spleen, which does not host the migratory DC subtype. We also show that iTreg cells that are newly generated in lymph nodes do not recirculate but are retained inside the lymph nodes. Specifically, in cutaneous lymph nodes (CLNs) CCL22, produced by CLN DCs, contributes to the retention of iTreg cells that all express the CCL22 receptor CCR4.
Protection from autoimmunity is assured, although the exclusive regional nature of the tolerization process controlled by DCs, as assessed using the herpes stromal keratitis (HSK) autoimmunity model.
Methods
Generation of DC-tg mice
For the construction of the I-Ad β-chain fused to the Bpep, the sequence coding for the Bpep (YFMYSKLRVQKSTWERG) was covalently attached by a 6-residue linker and a thrombin cleavage site to the amino terminus of the I-Ad β-chain as described.16 The expression cassette used for the generation of the DC-tg mice was generated in our laboratory: a 1000-bp EcoRI-DraIII fragment containing the external domain of I-Ad β-chain fused to the Bpep coding sequence was amplified by PCR; Dra III-EcoRI fragment containing intracellular and transmembrane domain of I-Ad β-chain was amplified by RT-PCR using RNA obtained from B-blasts as template; the 2 fragments were digested with DraIII (Promega), ligated, and the resulting fragment was cloned into EcoRI site of a previously generated pBS vector containing the β-globin cassette under the control of the CD11c promoter; the resulting vector was used to obtain the DC-tg trasgenic animals.
Transgenic mice were generated as described.17 In brief, CBA × C57/Bl6 oocytes were microinjected with the genetic material, implanted into pseudopregnant females, and transgenic founder animals were obtained. To assess for the presence of the transgene, 2 mm of tail was digested in 200 μL of digestion buffer (50mM KCl, 10mM Tris-HCl pH 8.3, 1.5mM MgCl2, 0.1 mg/mL gelatin, 0.45% Np40, 0.45% Tween20, 30 μg proteinase K) over night at 56°C followed by incubation at 95°C for 30 minutes. The presence of the transgene was verified by PCR using 2 mL of the extract as template and the following primer pairs: DCtgF (5′-CTCAGAGTACAAAAGAGCACTTGG-3′) and DCtgR (5′-TCCACATGGCAGGTGTAGAC-3′; Primm Srl).
Mice
The 2a T BALB/c Rag2-deficient mice (2a T mice) expressing the transgenic TCR recognizing the 435-451 peptide (Bpep) in the CH3 region of IgG2ab in association with I-Ad have been generated in our laboratory as described elsewhere.18 DC-tg mice were backcrossed on the BALB/c Rag2–deficient background for at least 10 generations. BALB/c and congenic CB-17 mice (expressing the IgG2ab) were obtained from Harlan Italy, and DEREG mice on the BALB/c background were obtained from T. Sparwasser (Twincore, Hannover, Germany). All the animals were maintained in specific pathogen-free conditions. All experiments were carried out in accordance with the relevant laws and institutional guidelines and with approval from the internal ethics committee of the University of Milano-Bicocca.
In vitro Bpep presentation assay
B5HII39 (B5) hybridoma cells19 were grown in IMDM supplemented with 2mM L-glutamine, 100 U/mL penicillin, 100 mg/mL streptomycin, 10% heat-inactivated FBS (all EuroClone), and 50 μM 2-Mercaptoethanol (Sigma-Aldrich).
For the Bpep presentation assay, CD11c− cells, DCs (CD11c+), and macrophages (F4/80+, CD11b+, CD11c−) were sorted from the spleen and peripheral lymph nodes of DC-tg mice using MoFlo cell sorter (purity > 98%); graded cell numbers were cocultured with 105 B5 cells in 200 μL of complete medium. After 24 hours, supernatants were col-lected and IL-2 concentration measured using mouse IL-2 duo-set ELISA (R&D Systems).
Cell preparation and adoptive transfer
To obtain naive TCR-Tg anti-IgG2ab T cells (2a T cells), the spleen and inguinal, axillary, maxillary, brachial, and mesenteric lymph nodes were collected from 2a T mice. Single-cell suspensions were prepared, and red blood cells were lysed, incubating spleen cells in 5 mL of red blood cells lysis buffer (8.29 g/L NH4Cl, 0.037 g/L tetrasodic EDTA, 1 g/L KHCO3, pH 7.3) for 5 minutes on ice. Cell preparations were resuspended in IMDM supplemented with 10% FBS and plated in cell culture-treated plates. After 1 hour, cells in suspension were collected, washed extensively in PBS, and purity of 2a T-cell preparation was evaluated by flow cytomery. DC-tg mice were intravenously injected with 105 2a T cells/200 μL PBS.
To obtain nonlymphopenic recipients, total Vβ14− T cells were purified from the spleen of CB-17 mice. Single-cell suspensions were prepared, and red blood cells were lysed. The cells were then incubated with biotinylated anti-Vβ14, anti-CD11c, anti-B220, anti-CD19, anti-DX5, anti-CD11b, and anti-GR1 antibodies (20 μg/mL, all from BD Biosciences) 20 minutes on ice, washed in PBS, and incubated with streptavidin MicroBeads (Miltenyi Biotec). Labeled cells were negatively selected on LS MACS separation columns according to the manufacturer's instructions. DC-tg mice were intravenously injected with 107 CD4+ cells/200 mL PBS. Two weeks later, blood samples were collected and the presence of CD4+Vβ14− in the blood was evaluated by flow cytometry: animals were used as nonlymphopenic recipients when the percentage of CD4+ cells was > 5%. The 2a T cells were adoptively transferred in these mice as described.
Flow cytometry
Blood samples (50 μL) and single-cell suspensions of 1 × 106 splenocytes or lymph node cells were pelleted and resuspended with the appropriate amount of antibody in 200 μL of PBS, and incubated for 20 minutes on ice in the dark. The cells were than washed once with 1 mL of PBS. When required, secondary reagent incubation in 100 μL of peridinin chlorophyll protein-Cy5.5–conjugated streptavidin (diluted 1/500; Sigma-Aldrich) was performed for 15 minutes on ice in the dark. For FACS analysis, the following antibodies were used: anti-CD4–FITC, anti–peridinin chlorophyll protein, anti-Cy5.5, or anti-PE (RM4-5), anti-CD8α–PE (53-6.7), anti-CD25–PE or anti-biotin (7D4), anti-Vβ14–FITC, or anti-biotin (14-2) were from BD Biosciences; anti-CD25–APC (7D4) was from Southern Biotechnology; anti-CCR4–PE (2G12) was from eBioscience. Intracellular detection of Foxp3 was performed using PE-anti–mouse/Rat Foxp3 staining set (clone FJK16s, eBioscience). CFSE cell labeling (Invitrogen) was performed following the manufacturer's instructions. Data were acquired using a BD FACSCalibur and analyzed with CellQuest (BD Biosciences) or FlowJo (TreeStar) softwares.
Reagents
LE540 (Wako Chemicals) was resuspended in DMSO and stored −20°C; aliquots were diluted in PBS and 100 μg/200 μL PBS 5% DMSO were intraperitoneally injected every 2 days for 20 days. Concerning the CCR4 inhibitor (AF399/42018025), 1.5 μg/200 μL PBS were intravenously injected 3 times every second day starting 18 days after the adoptive transfer. For all of the compounds, analysis was performed 24 hours after the last treatment.
Suppression assay and in vitro restimulation
Responder naive polyclonal T cells were positively selected from the spleen of BALB/c mice using anti-CD4 microbeads and MACS LS columns (Miltenyi Biotec). Cells were labeled with 1 μM CFSE. Splenocytes were obtained from the spleen of BALB/c mice and were depleted of T cells using biotinylated anti-CD3ϵ and anti–TCRβ-chain antibodies (BD Biosciences), streptavidin-microbeads, and MACS LS columns (Miltenyi Biotec).
To analyze the suppressive capacity, cells were recovered from the spleen or lymph nodes of transferred animals and were split in 2 samples: one of these was depleted of CD25+ cells using biotinylated anti-CD25 antibody (BD Biosciences) and magnetic streptavidin microbeads (Dynal). Then, CD4+ T cells were positively purified using anti-CD4 microbeads and MACS MS columns (Miltenyi Biotec).
Responder CFSE-labeled T cells were cocultured with putative Tregs at a 1:1 ratio and splenocytes in 96-well, round-bottom plate in complete medium supplemented with 0.6 μg/mL of purified anti-CD3ϵ (145.2C11). Proliferation of CFSE-labeled cells was assessed by flow cytometry after 72 hours of coculture.
In vitro 2a T restimulation
To assess the functionality of 2a T cells after the primary response, T cells recovered from the spleen or lymph nodes of transferred mice were purified, as illustrated in the previous paragraph, and cocultured with splenocytes depleted of T cells as described in “Suppression assay and in vitro restimulation” either loaded or not with 1 μg/mL of Bpep. After 48 or 72 hours, supernatants were collected and IFN-γ production was evaluated by ELISA using BD OptiEIA mouse–IFN-γ kit.
In vivo proliferation assay
Purified 2a T cells (5 × 105) were CFSE labeled and intravenously injected in the animals. After 48 or 72 hours, spleen and CLNs of injected animals were collected and proliferation of CFSE-labeled cells was assessed by flow cytometry, on gated CD4+Vβ14+ cells.
In vitro conversion assay
The 2a T cells were sorted by negative selection of CD11c+, B220+, DX5+, CD11b+ cells from a pool of spleen and lymph node single-cell suspensions. For the purification of CLN resident DCs (MHC class IIlowCD11c+), ssmDCs (MHC class IIhighCD11c+), and spleen resident DCs (MHC class IIint/highCD11c+) from BALB/c mice, spleens and lymph nodes have been mechanically reduced to single-cell suspensions. Cells where stained with the following antibodies: FITC-conjugated anti–MHC II (2g9 clone from BD Biosciences), biotin-conjugated anti-CD11c (HL3 clone from BD Biosciences) and allophycocyanin-conjugated B220 (to exclude plasmacytoid DCs). Biotinylated antibodies where detected with PE–Texas Red–conjugated streptavidin (BD Biosciences). DC subpopulations were then sorted with MoFlo high-speed sorting (purity > 90%). Propidium iodide-positive cells were excluded (for gating strategy, see supplemental Figure 5, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The 2a T cells (105) and MHC class IIlow/highCD11c+ cells (2 × 104) were cocultured in 96-well plates in a final volume of 200 μL of complete medium in the presence of Bpep (1 μg/mL). In some experiments, 1 μg LE540 (Wako) and 3 ng/mL rhTGF-β1 and 3 ng/mL TGF-β2 were added. After 5 days, cells were collected and analyzed for the expression of Foxp3 and CD25.
Microarray
CD11c+ cells were sorted from the spleen or lymph nodes of BALB/c mice using MoFlo (purity > 98%). Total RNA was extracted by the double-extraction protocol: RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction (TRIzol Invitrogen) followed by a QIAGEN RNeasy clean-up procedure
Because of the small amount of the cells, 20 ng of QIAGEN RNA carrier was used in every extraction. Total RNA concentration was calculated by Quant-IT RiboGreen (Invitrogen). Total RNA integrity was assessed by Agilent Bioanalyzer (Pico kit), and the RNA Integrity Number was calculated. Only high-quality RNA preparations, with RNA Integrity Number greater than 8.4, were used for microarray analysis.
Biotinylated cDNA was prepared from 2 ng of total RNA and fragmented according to the Nugen protocol (WT-Ovation Pico RNA Amplification System). A total of 5 μg of biotinylated cRNA were hybridized to the Affymetryx GeneChip Mouse Genome 430A Version 2.0 arrays. Data handling was mainly done using Bioconductor Version 2.6.20,21 The Robust Multichip Average22 method was used to calculate probe set intensity. To filter out noisy data before the selection of differentially expressed genes a filter was applied based on a interquartile range > 0.25. The identification of differentially expressed genes was addressed using Linear Models for Microarray Data23 and a discovery rate correction of the P value (Benjamini-Hochberg). The corrected P value selected in the analysis is P ≤ .01. All microarray data are available in the Gene Expression Omnibus under accession no. GSE39022.
Ocular infection and scoring of HSK
Corneas of anesthetized mice were scarified using a sterile gauge needle before infection with HSV-1 (5 × 104 PFU) in the right eye, and disease severity was scored on days 7 and 10 as described24 based on the degree of corneal opacity: ≤ 25% of cornea, 1; ≤ 50%, 2; ≤ 75%, 3; and ≤ 100%, 4. At day 10, the animals were killed for ethical reasons and the cornea analyzed by FACS for the presence of iTreg cells.
Cornea single-cell suspensions
Corneas have been digested with Liberase TM (Roche Diagnostics) for 60 minutes at 37°C. A minimum of 6 corneas for digestion have been used. Single-cell suspensions have been used for FACS analysis.
Statistical analysis
Means were compared by paired or unpaired t tests. Data are expressed and plotted as mean ± SE values unless indicated. Sample sizes for each experimental condition are provided in the figures and the respective legends.
Results
Experimental model
To obtain an animal model in which all of the DC subpopulations present the selected class II restricted self-peptide, a transgenic mouse expressing the Bpep covalently linked to the β-chain of the I-Ad molecule under the control of the CD11c promoter was produced (DC-tg animals; supplemental Figure 1). In agreement with previous results,25,26 CD11c regulatory region limited Bpep presentation to conventional DCs (supplemental Figure 2A), whereas plasmacytoid DCs were unable to present it (supplemental Figure 2A-B).
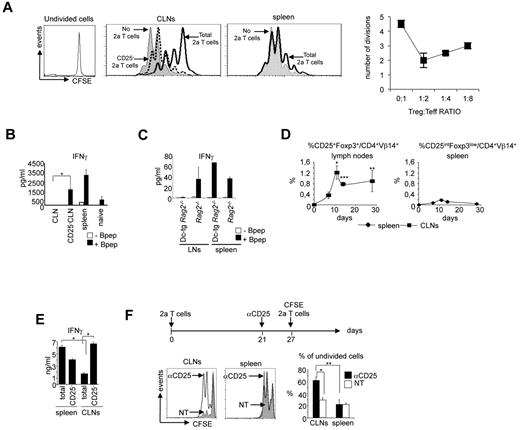
Bpep presentation occurred efficiently in vivo in secondary lymphoid organs. Initially, we focused our analysis on 2 secondary lymphoid organs clearly described to be populated by different DC subtypes: namely, the spleen, exclusively populated by lymphoid tissue resident DC subsets, which arrive there as precursors by a hematogenous route, and CLNs, in which, diversely from the spleen, ssmDCs represent an abundant population.3,27 Subsequently, the analysis was extended to other areas. Adoptive transfer of CFSE-labeled naive anti-Bpep TCR transgenic T cells (2a T cells) into DC-tg mice showed a comparable antigen-driven expansion between spleen and CLNs (brachial, inguinal, and axillary; Figure 1A). Homeostatic proliferation in Rag2-deficient recipients was negligible (Figure 1A).
Generation of CD25+Foxp3+ 2a T cells in CLNs and not in the spleen. (A) Expansion of 2a T cells in the spleen and CLNs of DC-tg Rag2-deficient (DC-tg) or Rag2-deficient (ntg) mice at the indicated time points after transfer. The percentage of divided cells is shown. One representative experiment of 6 is shown. (B) Flow cytometry of CD25 and Foxp3 expression on 2a T cells before transfer. (C) Kinetics of 2a T-cell expansion in spleen, CLNs, and blood of DC-tg Rag2-deficient (DC-tg) or Rag2-deficient control mice (ntg) at the indicated time points after transfer. Bottom right panel: IFN-γ serum levels measured at the indicated days after transfer in DC-tg Rag2-deficient (DC-tg) or Rag2-deficient control mice (ntg). (D) Top panels: kinetics of CD44 and CD25 expression by 2a T cells in spleen and CLNs of DC-tg Rag2-deficient recipient mice at the indicated time points after transfer. Top panels: CD25 and Foxp3 expression by 2a T cells (CD4+Vβ14+ cells) in CLNs and spleen 3 weeks after transfer in DC-tg Rag2-deficient mice. (C-D) Data are mean ± SEM of 3 independent experiments performed using at least 3 mice per group. **P < .01.
Generation of CD25+Foxp3+ 2a T cells in CLNs and not in the spleen. (A) Expansion of 2a T cells in the spleen and CLNs of DC-tg Rag2-deficient (DC-tg) or Rag2-deficient (ntg) mice at the indicated time points after transfer. The percentage of divided cells is shown. One representative experiment of 6 is shown. (B) Flow cytometry of CD25 and Foxp3 expression on 2a T cells before transfer. (C) Kinetics of 2a T-cell expansion in spleen, CLNs, and blood of DC-tg Rag2-deficient (DC-tg) or Rag2-deficient control mice (ntg) at the indicated time points after transfer. Bottom right panel: IFN-γ serum levels measured at the indicated days after transfer in DC-tg Rag2-deficient (DC-tg) or Rag2-deficient control mice (ntg). (D) Top panels: kinetics of CD44 and CD25 expression by 2a T cells in spleen and CLNs of DC-tg Rag2-deficient recipient mice at the indicated time points after transfer. Top panels: CD25 and Foxp3 expression by 2a T cells (CD4+Vβ14+ cells) in CLNs and spleen 3 weeks after transfer in DC-tg Rag2-deficient mice. (C-D) Data are mean ± SEM of 3 independent experiments performed using at least 3 mice per group. **P < .01.
Conversion of naive T cells into iTreg cells occurs in the lymph nodes and not in the spleen
To investigate whether CD11c+ DCs presenting a self-peptide, in homeostatic conditions, were able to tolerize naive antigen-specific CD4+ T cells in lymph node and spleen, the fate of 2a T cells after the encounter of antigen-presenting DCs was investigated. Naive (CD44low; data not shown) CD4+Vβ14+CD25− 2a T cells sorted from Rag2-deficient TCR transgenic mice were transferred into DC-tg or nontransgenic mice as a control. As shown in Figure 1B, purified naive cells used for the adoptive transfer experiments were Foxp3-negative. Cell behavior was followed over time (Figure 1C). We initially used DC-tg mice on Rag2-deficient background as recipients to formally exclude some predictable complications of the system: (1) the possibility that anti-Bpep Treg cells, originated in the thymus (data not shown) because of the forced presentation of the Bpep by DCs,28 could suppress 2a T-cell responses immediately after transfer; and (2) the possibility that the endogenous IgG2aa, whose 435-451 sequence (Apep) is very close to the Bpep and could be presented by B cells, could bias the results by leading to a suboptimal activation or to the inactivation of 2a T cells.
During the first 2 weeks after transfer, a robust expansion of 2a T cells was observed in spleen, lymph nodes, and blood of DC-tg mice. After a decrease, the amount of 2a T cells reached a plateau in the blood and both types of peripheral lymphoid organs (Figure 1C). Moreover, IFN-γ serum levels could be detected starting from 4 days after the transfer with a peak at day 7 (Figure 1C). The expression of surface markers, including CD44 and CD25, indicated a robust antigen-specific T-cell response (Figure 1D). CD25 was transiently up-regulated on 2a T cells in CLNs and spleen very early after transfer (3-5 days). Subsequently, it was completely down-regulated in the spleen, whereas a discrete population of 2a T cells maintained CD25 expression over time in CLNs (Figure 1D). Interestingly, a significant percentage of these cells also expressed the Treg cell marker, Foxp3 (Figure 1D). We then analyzed different regions. CD25+Foxp3+ cell differentiation was not limited to CLNs but was apparent in all the different types of lymph node analyzed. In mesenteric lymph nodes, the efficiency of Foxp3+ cell generation was comparable with previous data10,12 (supplemental Figure 3). To determine whether CD25+Foxp3+ 2a T cells present in CLNs had, indeed, regulatory properties, their suppressive activity was investigated. In vitro, 2a T cells recovered from CLNs of DC-tg mice 4 weeks after transfer were able to suppress anti-CD3 induced polyclonal T-cell proliferation and antigen-induced IFN-γ production (Figure 2A-B). This activity was entirely the result of the CD25+ cells because it was completely abolished upon CD25+ cell depletion. In contrast, 2a T cells recovered from the spleen did not display any regulatory activity (Figure 2A) and fully responded to restimulation (Figure 2B). Similar results were obtained after the transfer of naive polyclonal BALB/c CD4+ T cells: only T cells from spleen and not T cells from lymph nodes could be restimulated in vitro in response to the Bpep (Figure 2C). This indicated that iTreg cells developed solely in the lymph nodes and not in the spleen.
CD25+Foxp3+ 2a T cells generated in CLNs have suppressing activity in vitro and in vivo. (A) CD25+ 2a T cells recovered from CLNs of DC-tg Rag2-deficient mice inhibit mitogen-induced proliferation of polyclonal T cells. CFSE-labeled polyclonal T cells were stimulated with an anti-CD3ϵ monoclonal antibody and syngeneic splenocytes in the presence or not of 2a T cells recovered from spleen or CLNs of DC-tg Rag2-deficient mice 4 weeks after transfer. Polyclonal T-cell proliferation was assessed by flow cytometry 72 hours later. Where indicated, recovered 2a T cells were depleted of the CD25+ population. Left panels: histograms of CFSE-labeled polyclonal T-cell proliferation. Right panel: quantitative analysis of the efficiency of inhibition of naive anti-CD3 stimulated T cells (Teff) cultured at the indicated ratio with CD25+ 2a T cells (Treg). Data are mean ± SD of 3 independent experiments. (B) IFN-γ production by 2a T cells, recovered from CLNs or spleen of DC-tg Rag2-deficient mice 3 weeks after transfer, on in vitro restimulation in the presence of splenocytes loaded or not with the Bpep. Where indicated, 2a T cells recovered from CLNs were depleted of the CD25+ population (CD25−CLN). The activation of naive 2a T cells is also shown. (C) IFN-γ production by polyclonal BALB/c T cells, recovered from CLNs or spleen of Rag2-deficient and DC-tg Rag2-deficient mice 3 weeks after transfer, on in vitro restimulation in the presence of splenocytes loaded or not with the Bpep. (D) Kinetics of CD25+Foxp3+ 2a T-cell generation in CLNs and kinetics of CD25intFoxp3low 2a T-cell generation in the spleen of DC-tg Rag2-deficient mice. (E) IFN-γ production by 2a T cells, recovered from CLNs or spleen of DC-tg Rag2-deficient mice 10 days after transfer, on in vitro restimulation in the presence of splenocytes loaded with the Bpep. Where indicated, 2a T cells recovered from CLNs and spleen were depleted of the CD25+ population (CD25−). (A-C,E) Data are mean ± SEM of 3 independent experiments performed by pooling cells derived from 3 mice per group. (D) Data are mean ± SEM of 3 independent experiments performed using at least 3 mice per group. (F) Top diagram: schematic representation of the adoptive transfer experiment. DC-tg Rag2-deficient mice were first transferred with naive 2a T cells; 3 weeks later, reconstituted mice were either nontreated (NT) or treated with the anti-CD25 blocking antibody, PC61 (labeled as αCD25), and one week later transferred with CFSE-labeled 2a T cells. Bottom left panels: in vivo proliferation of CFSE-labeled 2a T cells measured 48 hours after transfer. Bottom right panel: quantitative analysis of the frequency of undivided cells. Data are mean ± SD of 4 mice analyzed in 2 independent experiments. *P < .05; **P < .01; ***P < .005.
CD25+Foxp3+ 2a T cells generated in CLNs have suppressing activity in vitro and in vivo. (A) CD25+ 2a T cells recovered from CLNs of DC-tg Rag2-deficient mice inhibit mitogen-induced proliferation of polyclonal T cells. CFSE-labeled polyclonal T cells were stimulated with an anti-CD3ϵ monoclonal antibody and syngeneic splenocytes in the presence or not of 2a T cells recovered from spleen or CLNs of DC-tg Rag2-deficient mice 4 weeks after transfer. Polyclonal T-cell proliferation was assessed by flow cytometry 72 hours later. Where indicated, recovered 2a T cells were depleted of the CD25+ population. Left panels: histograms of CFSE-labeled polyclonal T-cell proliferation. Right panel: quantitative analysis of the efficiency of inhibition of naive anti-CD3 stimulated T cells (Teff) cultured at the indicated ratio with CD25+ 2a T cells (Treg). Data are mean ± SD of 3 independent experiments. (B) IFN-γ production by 2a T cells, recovered from CLNs or spleen of DC-tg Rag2-deficient mice 3 weeks after transfer, on in vitro restimulation in the presence of splenocytes loaded or not with the Bpep. Where indicated, 2a T cells recovered from CLNs were depleted of the CD25+ population (CD25−CLN). The activation of naive 2a T cells is also shown. (C) IFN-γ production by polyclonal BALB/c T cells, recovered from CLNs or spleen of Rag2-deficient and DC-tg Rag2-deficient mice 3 weeks after transfer, on in vitro restimulation in the presence of splenocytes loaded or not with the Bpep. (D) Kinetics of CD25+Foxp3+ 2a T-cell generation in CLNs and kinetics of CD25intFoxp3low 2a T-cell generation in the spleen of DC-tg Rag2-deficient mice. (E) IFN-γ production by 2a T cells, recovered from CLNs or spleen of DC-tg Rag2-deficient mice 10 days after transfer, on in vitro restimulation in the presence of splenocytes loaded with the Bpep. Where indicated, 2a T cells recovered from CLNs and spleen were depleted of the CD25+ population (CD25−). (A-C,E) Data are mean ± SEM of 3 independent experiments performed by pooling cells derived from 3 mice per group. (D) Data are mean ± SEM of 3 independent experiments performed using at least 3 mice per group. (F) Top diagram: schematic representation of the adoptive transfer experiment. DC-tg Rag2-deficient mice were first transferred with naive 2a T cells; 3 weeks later, reconstituted mice were either nontreated (NT) or treated with the anti-CD25 blocking antibody, PC61 (labeled as αCD25), and one week later transferred with CFSE-labeled 2a T cells. Bottom left panels: in vivo proliferation of CFSE-labeled 2a T cells measured 48 hours after transfer. Bottom right panel: quantitative analysis of the frequency of undivided cells. Data are mean ± SD of 4 mice analyzed in 2 independent experiments. *P < .05; **P < .01; ***P < .005.
In a kinetic analysis, we determined that CD25+Foxp3+ 2a T cells first appeared in CLNs approximately 5 days after transfer and eventually persisted (Figure 2D). Diversely, in the spleen, we could only document the transient appearance of a CD25intFoxp3low 2a T-cell population (Figure 2D), which did not show suppressive activity. Indeed, when the spleen cells were rechallenged in vitro with the Bpep, 10 days after transfer, the presence of CD25int T lymphocytes did not influence the production of IFN-γ by 2a T cells. In contrast, CD25+Foxp3+ cells of CLNs showed suppressive activity (Figure 2E).
These in vitro results suggested that induction of Treg cells specifically occurred in CLNs and not in the spleen. An in vivo analysis confirmed this split situation. Indeed, CFSE labeled 2a T cells, injected into DC-tg animals 4 weeks after the first 2a T cell transfer, proliferated in the spleen but not in CLNs (Figure 2F). The CLN inhibitory activity could be removed by inhibiting CD25+ cells, indicating that these cells were responsible for the suppression. Thus, in agreement with Sun et al,12 we found that splenic DCs were clearly inefficient in inducing iTreg cell conversion, in contrast to what happens in the CLNs.
The lack of iTreg cell generation in the spleen was not the result of the lymphopenic environment. iTreg cells did not differentiate in the spleen under nonlymphopenic conditions as well (supplemental Figure 4). On the other hand, a more efficient generation of iTreg cells was observed in CLNs of nonlymphopenic hosts (supplemental Figure 4), suggesting that T cells can influence the efficiency of DCs to induce iTreg cell conversion.
ssmDCs have the exclusive capacity to induce iTreg cell conversion via RA production
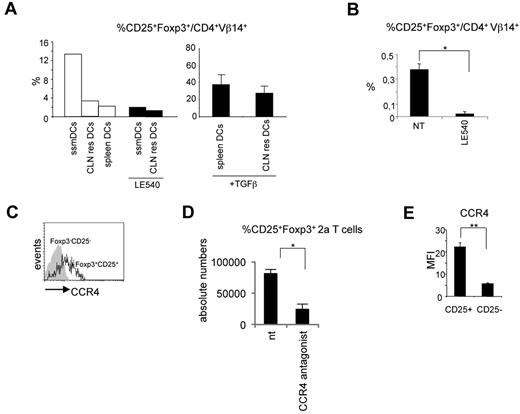
The observed results posed the question why iTreg cells differentiate only in lymph nodes. To investigate this question, we reasoned that the major difference between the spleen and lymph nodes could be represented by ssmDCs. This DC subpopulation could therefore be responsible for the conversion of iTreg cells. To explore this possibility, migratory and resident DCs from CLNs and spleen DCs all purified from wild-type BALB/c mice were cocultured with naive 2a T cells in the presence of the Bpep. Migratory and resident DCs were selected based on the level of MHC class II expression (high levels for the migratory and low levels for the resident cells) as previously described.29,30 Spleen DCs were MHC classIIint/high. The purity of sorted populations was > 90% (supplemental Figure 5). iTreg cell conversion was then evaluated. Only when the antigen was presented by CLN migratory DCs, a fraction of 2a T cells converted into iTreg cells (Figure 3A). As control, the addition of TGF-β rendered spleen DCs and CLN resident DCs capable of inducing iTreg cells (Figure 3A).
Migratory DCs are responsible for iTreg cell conversion. (A) In vitro differentiation of CD25+Foxp3+ 2a T cells induced by migratory (MHC class IIhighCD11c+, ssmDCs), CLN resident (MHC class IIlowCD11c+, CLN res DCs), or spleen resident (MHC class IIint/highCD11c+, spleen DCs) DCs from wild-type BALB/c mice loaded with the Bpep. Where indicated, the RA receptor inhibitor LE540 and TGF-β have been added to the cocultures. The experiment has been repeated 3 times with similar results. (B) Inhibition of CD25+Foxp3+ 2a T-cell generation in vivo by the LE540. DC-tg Rag2-deficient animals were adoptively transferred with naive 2a T cells and treated every second day with the inhibitor for 3 weeks. The percentage of CD25+Foxp3+ 2a T cells on the total 2a T-cell population was determined by cytofluorimetric analysis 3 weeks after transfer. Data are mean ± SEM of 4 mice analyzed in 2 independent experiments. (C) Flow cytometry of the CCR4 expression by the indicated lymph node populations of 2a T cells 2 weeks after transfer into DC-tg Rag2-deficient mice. The experiment was repeated 3 times with similar results. (D) Reduction in the numbers of CD25+Foxp3+ 2a T cells in the lymph nodes of transferred mice after treatment with the CCR4 inhibitor, AF399/42018025. DC-tg Rag2-deficient mice were transferred with naive 2a T cells and treated with the CCR4 inhibitor. Absolute numbers of CD25+Foxp3+ 2a T cells were then assessed by flow cytometry. NT indicates nontreated mice. Data are mean ± SEM of 4 mice analyzed in 2 independent experiments. (E) Flow cytometric analysis of CCR4 expression by CD25+Foxp3+ 2a T cells generated in vitro as described in panel A. CCR4 mean fluorescence intensity (MFI) on CD25+Foxp3+ and CD25−Foxp3− 2a T cells after coculture with ssmDCs from BALB/c mice in presence of the Bpep. The experiment was repeated twice with similar results. *P < .05; **P < .01.
Migratory DCs are responsible for iTreg cell conversion. (A) In vitro differentiation of CD25+Foxp3+ 2a T cells induced by migratory (MHC class IIhighCD11c+, ssmDCs), CLN resident (MHC class IIlowCD11c+, CLN res DCs), or spleen resident (MHC class IIint/highCD11c+, spleen DCs) DCs from wild-type BALB/c mice loaded with the Bpep. Where indicated, the RA receptor inhibitor LE540 and TGF-β have been added to the cocultures. The experiment has been repeated 3 times with similar results. (B) Inhibition of CD25+Foxp3+ 2a T-cell generation in vivo by the LE540. DC-tg Rag2-deficient animals were adoptively transferred with naive 2a T cells and treated every second day with the inhibitor for 3 weeks. The percentage of CD25+Foxp3+ 2a T cells on the total 2a T-cell population was determined by cytofluorimetric analysis 3 weeks after transfer. Data are mean ± SEM of 4 mice analyzed in 2 independent experiments. (C) Flow cytometry of the CCR4 expression by the indicated lymph node populations of 2a T cells 2 weeks after transfer into DC-tg Rag2-deficient mice. The experiment was repeated 3 times with similar results. (D) Reduction in the numbers of CD25+Foxp3+ 2a T cells in the lymph nodes of transferred mice after treatment with the CCR4 inhibitor, AF399/42018025. DC-tg Rag2-deficient mice were transferred with naive 2a T cells and treated with the CCR4 inhibitor. Absolute numbers of CD25+Foxp3+ 2a T cells were then assessed by flow cytometry. NT indicates nontreated mice. Data are mean ± SEM of 4 mice analyzed in 2 independent experiments. (E) Flow cytometric analysis of CCR4 expression by CD25+Foxp3+ 2a T cells generated in vitro as described in panel A. CCR4 mean fluorescence intensity (MFI) on CD25+Foxp3+ and CD25−Foxp3− 2a T cells after coculture with ssmDCs from BALB/c mice in presence of the Bpep. The experiment was repeated twice with similar results. *P < .05; **P < .01.
It has been shown that migratory CLN DCs express the aldehyde dehydrogenase, RALDH2,13 which catalyzes the synthesis of RA from retinaldehyde. RA, the active derivative of vitamin A (retinol), has been extensively shown to be one of the active molecules responsible, either directly31 or indirectly,32 for the conversion of iTreg cells in mesenteric and CLNs.10,12,33 Therefore, we predicted that migratory DCs could induce iTreg conversion in an RA-dependent manner. Accordingly, we observed that treatment with the RA receptor inhibitor, LE540,34 significantly abrogated the iTreg cell conversion (Figure 3A).
Based on these in vitro results, we investigated whether RA was involved also in vivo in the conversion of antigen-specific Foxp3+CD25+ T cells. DC-tg mice were transferred with naive 2a T cells and systemically treated for 3 weeks with LE540. As shown in Figure 3B, a significant reduction in the percentage of iTreg cells was observed in CLNs of treated recipients. Because RALDH2 expression is restricted to migratory DCs,13,35 together these results indicate that migratory and not lymphoid tissue resident DCs are able to induce iTreg cell conversion under steady-state conditions with a mechanism dependent on RA.
CCL22 contributes to the retention of iTreg cells in CLNs
A second question to answer was why newly generated iTregs persist only within lymph nodes. Indeed, the absence of differentiated iTregs in the spleen and circulation (data not shown) suggested that these iTregs do not recirculate among different lymphoid organs.
To investigate this question, we analyzed the differences between CD11c+ cells of CLNs and spleen from resting wild-type BALB/c animals by performing a comparative global gene expression analysis. Genes showing a fold change of at least 3 in the level of expression in lymph node versus spleen DCs were considered differentially expressed. The total list of differentially expressed genes is shown in supplemental Table 1. Strikingly, Aldh1a2 (coding RALDH2) resulted on the top of the list of genes specifically expressed in lymph nodes DCs, thus substantiating our finding that exclusively migratory DCs expressing RALDH2 were involved in the conversion of antigen-specific iTreg cells.
The other gene that was far more expressed in lymph nodes compared with spleen was Ccl22. We thus focused our attention on CCL22, a chemokine whose receptor, CCR4, is particularly highly expressed in Treg cells,36 and we hypothesized that CCL22 was involved in the homing of iTreg cells in CLNs.
We first confirmed CCR4 expression by CD4+Vβ14+CD25+ Foxp3+ 2a T cells (Figure 3C). Second, we tested the effect exerted by a CCR4 antagonist on Treg cell homing. DC-tg mice adoptively transferred with 2a T cells were systemically treated with the potent CCR4 antagonist, AF399/42018025,37 to break down the possible CCL22-mediated mechanism of retention, and Treg cells enumerated 3 days later. As shown in Figure 3A,D, a strong reduction in Foxp3+CD25+ 2a T-cell numbers was observed in CLNs.
Notably, ssmDCs from CLNs were able to induce CCR4 expression on the converted iTreg 2a T cells in vitro (Figure 3E). The converted iTreg cells obtained in vitro after DC exposure were the only T-cell population that was expressing CCR4, indicating that ssmDCs have not only the exclusive capacity to induce iTreg cell conversion but also the exclusive capacity to induce CCR4 expression. This is a further indication of the homing function of this receptor. Overall, these results indicate that DC-derived CCL22 contributes to the retention of iTreg cells in CLNs.
Newly generated iTreg cells protect from autoimmunity
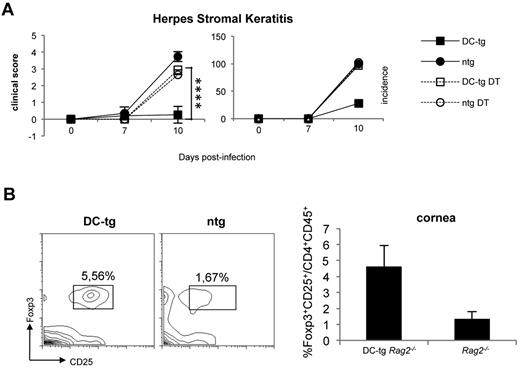
To investigate whether newly generated iTreg cells in DC-tg mice could protect from autoimmunity, we took advantage of the HSK model. HSK is a CD4+ T cell–mediated autoimmune disease of the eye that can be induced in susceptible mice by ocular infection with HSV-1.38 It has been proven that CD4+ T cells responsible for HSK cross-recognize a corneal antigen, the IgG2ab Bpep and a peptide of the viral UL6 protein.39 Ighb animals, such as CB-17 mice, are resistant to HSK, and their resistance has been attributed to the tolerant state of T cells versus the Bpep. Conversely, BALB/c (Igha) mice are to a certain degree susceptible to the disease because keratogenic T anti-IgG2ab T cells are not fully tolerized because of the absence of the Bpep in this strain. We reasoned that DC-tg mice, once reconstituted with polyclonal naive BALB/c CD4+ T lymphocytes containing keratogenic T cells, should develop resistance to HSK a few weeks after T-cell transfer because of iTreg cell conversion. Therefore, we transferred polyclonal naive CD4+ T cells from DEREG (BALB/c) mice into DC-tg Rag2−/− and Rag2−/− mice. DEREG animals carry a diphtheria toxin receptor-eGFP transgene under the control of the Foxp3 promoter, thereby allowing specific depletion of Foxp3+ Treg by diphtheria toxin administration.40 Recipient mice treated or not with diphtheria toxin (therefore depleted or not of iTreg cells) were infected with HSV-1 (strain KOS) and HSK incidence and severity was scored 7 and 10 days after ocular infection. As shown in Figure 4A, in agreement with the prediction, DC-tg mice were totally protected from HSK and the protection was iTreg cell dependent because their ablation rendered the animals strongly susceptible. Moreover, HSK protection correlated with iTreg cell accumulation at the cornea level (Figure 4B).
Newly generated iTreg cells protect from autoimmunity. (A) DC-tg Rag2−/− (DC-tg) or Rag2−/− control (ntg) mice were adoptively transferred with polyclonal CD4+ DEREG T cells. Three weeks after transfer, recipient mice treated or not with diphtheria toxin were infected with HSV-1 and incidence and severity of the disease scored on days 7 and 10 after treatment. Note that deletion of Foxp3+ cells in the recipient DC-tg mice makes them strongly susceptible to HSK. Data are mean ± SEM of 6 mice per group. (B) Left panels: percentage of CD25+Foxp3+ on CD4+CD45+ cells present in the corneas of DC-tg Rag2−/− (DC-tg) or Rag2−/− (ntg) recipient mice at day 10 after HSV-1 ocular infection. Right panel: quantification of the cytofluorimetric analysis. Data are mean ± SEM calculated on 3 different mice. ****P < .001.
Newly generated iTreg cells protect from autoimmunity. (A) DC-tg Rag2−/− (DC-tg) or Rag2−/− control (ntg) mice were adoptively transferred with polyclonal CD4+ DEREG T cells. Three weeks after transfer, recipient mice treated or not with diphtheria toxin were infected with HSV-1 and incidence and severity of the disease scored on days 7 and 10 after treatment. Note that deletion of Foxp3+ cells in the recipient DC-tg mice makes them strongly susceptible to HSK. Data are mean ± SEM of 6 mice per group. (B) Left panels: percentage of CD25+Foxp3+ on CD4+CD45+ cells present in the corneas of DC-tg Rag2−/− (DC-tg) or Rag2−/− (ntg) recipient mice at day 10 after HSV-1 ocular infection. Right panel: quantification of the cytofluorimetric analysis. Data are mean ± SEM calculated on 3 different mice. ****P < .001.
These results indicate that tolerance induced by migratory DCs via iTreg cell generation confers protection from autoimmunity and strongly suggest that iTreg cell mediated autoimmunity protection occurs locally in the nonlymphoid organs.
Discussion
Using an experimental system in which antigen presentation is not confined to a particular DC subset but is extended to all CD11c+ DCs, we have demonstrated that lymphoid tissue resident DCs are not able to induce iTreg cells. Steady-state migratory DCs have, instead, the privileged capacity to induce self–antigen-specific iTreg cell conversion and autoimmunity protection. This ssmDC function is RA-mediated.
Because the only DCs able to convert naive T cells into iTreg cells come from nonlymphoid tissues, we can hypothesize that immature DCs are not generally able to tolerize T cells by default (simply because of the absence of signal-2, the costimulation) but need to receive a specific conditioning. At the steady state, the appropriate signals that render DC tolerogenic (or alternatively activated41 ) may be delivered by the organs/tissues where migratory DCs reside. For instance, tissue-specific stimuli could induce the expression of RALDH2 essential for RA synthesis. In the intestine, it has been proposed that GM-CSF, presumably produced by lamina propria macrophages or eosinophil-like cells, and RA, produced by ALDH1A1+ intestinal epithelial cells from dietary vitamin A or blood-derived retinol, may represent the tissue-derived stimuli that elicit Aldh1a2 expression by migratory DCs.13,42 The same environmental factors may be also responsible for DC conditioning in the skin. Moreover, alternatively matured DCs able to induce the differentiation of IL-10–producing cells with regulatory functions can be generated on exposure to suboptimal doses of TNF-α or by disrupting E-cadherin–mediated adhesion.43,44
In addition to endogenous signals, exogenous signals can alternatively activate DCs. A well-characterized exogenous signal is the anti-DEC205,45,46 which leads to a suboptimal DC activation.6 Targeting the antigens to DCs via the CD205 receptor is a very efficient system for antigen-specific iTreg cell induction and autoimmunity protection.4,46 Other exogenous signals may be some Toll-like receptor (TLR) stimuli, such as TLR2 agonists.47
Given that among the conventional DCs only ssmDCs are able to induce tolerance in the periphery via iTreg cell generation and that ssmDCs constantly deliver tissue-sequestered antigens to draining lymph nodes, we can predict that DCs are specialized to induce tolerance to tissue-sequestered antigens. This hypothesis is substantiated by the recent finding that iTreg cells have a TCR repertoire that only partially overlaps with the repertoire of nTreg cells.48 Moreover, the 2 Treg cell subsets have very similar effector mechanisms but are functionally complementary and nonredundant,48 indicating that their major difference resides in their TCR diversity. Therefore, the multiple peptides that drive the differentiation of nTreg cells in the thymus should partially differ from the peptides that drive the generation of iTreg cells in the periphery. The peptides that most likely never reach the thymus are exactly tissue-sequestered antigens. ssmDCs may be the cells that contribute to the diversification of the antigen-specificity of iTreg cells compared with nTreg cells because they are the cells that present tissue-sequestered antigens to the adaptive immune system to prevent autoimmune reactions. Nevertheless, there is also a certain overlapping in the specificities of the 2 Treg cell subtypes. Therefore, we can hypothesize that other cells, in addition to conventional DCs, contribute to the conversion of Treg cells in peripheral lymphoid organs. The repertoire of peptide presented by these additional cells, including for instance plasmacytoid DCs, may not differ from the repertoire of peptide presented in the thymus. We predict that, if iTreg cells differentiate in the spleen (thanks to cells different from conventional DCs), their TCR repertoire could in large part overlap with the repertoire of nTreg cells.
In this view, it is important that the iTreg cells induced locally by DCs do not recirculate but remain localized in the lymph nodes. Because their specificity may be directed to tissue-sequestered antigens, their regulatory activity may be locally required. The possibility that the newly generated iTreg cells not only home in the lymph node but also in the organ/tissue where ssmDCs originate should be also taken into account. A clear drop in the percentage of iTreg cells in the lymph nodes is, indeed, observable very soon after generation (Figure 2D). As DC-induced iTreg cells are not found in circulation, this observation is compatible with a relocalization of these cells into the nonlymphoid tissue.49 Leukocyte trafficking is critically regulated by chemokines, and Treg cells have been previously shown to express CCR4. We have found that ssmDCs from CLNs specifically induce CCR4 expression by iTreg cells. Therefore, their localization in CLNs is regulated by the abundant CCL22 (CCR4 ligand) production by lymph node DCs.50
It has been shown that TCR ligand potency influences the differentiation of iTreg cells in vivo.51 Moreover, the relevance of TCR avidity in determining the fate of T cells has been repeatedly proposed.52,53 Therefore, we cannot exclude that DCs can tolerize T cells by other means, such as anergy or TCR down-regulation instead of iTreg cell conversion, in systems in which the efficiency of antigen presentation and therefore the avidity of cognate antigen recognition by autoreactive T cells is much higher or in conditions in which autoreactive T cells physiologically compete with other T cells.
Interestingly, the small number of iTreg cells generated in the lymph nodes is sufficient to protect from autoimmunity. In the HSK experimental system, DC-tg recipient mice are totally protected from disease, unless iTreg cells are ablated. Interestingly, disease protection correlates with the recruitment of iTreg cells to the infected corneas. Therefore, during the viral-induced inflammatory process, iTreg cells acquire the ability to migrate to the corneas (otherwise totally free of immune cells) where they presumably exert their suppression activity.
In conclusion, some tissue-sequestered antigens may escape the penalties of thymus exclusion for specific tolerance induction using RALDH2+ migratory DCs. These cells function as antigen shuttles to tissue draining lymph nodes that become, in this way, additional and exclusive sites of iTreg cell conversion in noninflammatory conditions. In this scenario, the retention of ssmDC-induced iTreg cells at the site of conversion is consistent with their function to keep at bay autoreactive T cells where the cognate antigen is preferentially transported.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank G. Kollias (Biomedical Sciences Research Center Alexander Fleming, Athens, Greece) for the generation of DC-tg mice and T. Sparwasser (Twincore, Hannover, Germany) for DEREG mice.
This work was supported by the European Union FP7 Program (TOLERAGE: HEALTH-F4-2008-202156; ENCITE: HEALTH-F4-2008-201842; and ALLFUN: HEALTH-F2-2010-260338), the Associazione Italiana per la Ricerca sul Cancro, the Italian Ministry of Education and Research (PRIN2009), the Fondazione Cariplo (grant 2010-0678), and the Regione Lombardia (ASTIL and LIIN projects).
Authorship
Contribution: C.V., F.M., A.B., and S.B. performed the experiments; F.Z. performed microarray analyses; J.B. and G.R. provided advice; I.Z. designed and performed the experiments and analyzed the data; and F.G. conceived the project, designed the experiments, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for C.V. is Molecular Immunology Unit, Department of Experimental Oncology and Molecular Medicine, AmadeoLab, Fondazione IRCCS Istituto Nazionale Tumori, Milan, Italy. The current affiliation for F.Z. is Singapore Immunology Network, Singapore.
Correspondence: Francesca Granucci, Department of Biotechnology and Bioscience, University of Milano-Bicocca, Piazza della Scienza 2, 20126 Milan, Italy; e-mail: francesca.granucci@unimib.it; and Ivan Zanoni, Department of Biotechnology and Bioscience, University of Milano-Bicocca, Piazza della Scienza 2, 20126 Milan, Italy; e-mail: ivan.zanoni@unimib.it.
References
Author notes
C.V., F.M., and A.B. contributed equally to this study.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal