In this issue of Blood, Wang et al reveal that ligation of Ly6G on murine neutrophils inhibits neutrophil recruitment, providing the first evidence of a function for this molecule.1
Gr-1, and more specifically RB6-8C5, the monoclonal antibody that recognizes Gr-1, has been a very important tool for immunologists investigating neutrophil function in murine models. RB6-8C5 binds to 2 members of the Ly6 family of leukocyte-expressed markers, Ly6C and Ly6G. These molecules are small GPI-linked proteins on the surface of mouse neutrophils.2 The discovery that high doses of RB6-8C5 are very effective at removing neutrophils from the circulation gave researchers a convenient and reproducible approach for assessing the contribution of neutrophils to experimental models of inflammation.3,4 More recently, since the advent of highly sensitive forms of in vivo confocal imaging, anti–Gr-1, administered at much lower nondepleting doses, has been an equally useful tool for labeling endogenous neutrophils, enabling intravital microscopy-based assessment of their behavior in vivo.5-7 However, while evidence is emerging of a role for Ly6C in controlling homing of CD8+ T cells,8 little if anything is known about the actions of Ly6G. So the question arises, what does this mole-cule do?
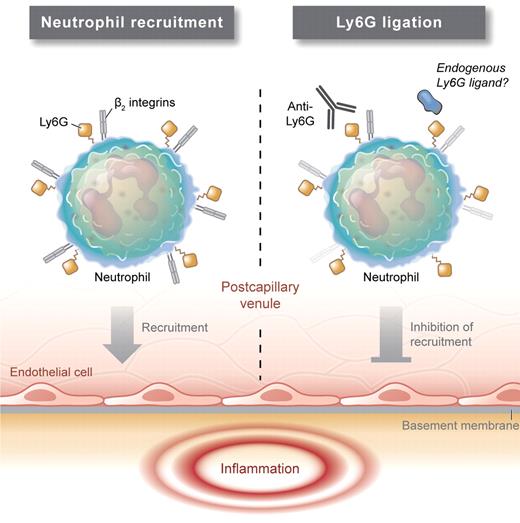
Wang et al did not set out to discover the role of this molecule either, but to investigate the effects of submaximal neutrophil depletion. To their surprise they observed that at low doses that did not markedly alter the number of circulating neutrophils, anti–Gr-1 treatment was associated with an almost complete inhibition of neutrophil entry into inflamed tissues. Investigating this more closely, they observed similar effects using the Ly6G-specific antibody 1A8, which caused a striking reduction in neutrophil infiltration in a model of arthritis, without depleting circulating neutrophils. 1A8 was even able to reduce joint inflammation therapeutically when administered at the peak of the response. These findings indicate that antibody ligation of Ly6G had the unexpected effect of inhibiting neutrophil recruitment. In investigating the mechanism of this response, the authors excluded effects on neutrophil apoptosis or initial chemokine receptor signaling. In contrast, they observed that anti-Ly6G reduced the ability of neutrophils to respond to chemotactic stimuli. To explain this finding, they used confocal microscopy, coimmunoprecipitation, and fluorescence lifetime imaging to generate evidence of a direct association between Ly6G and the β2 integrins CD11a and CD11b. Moreover, they observed that Ly6G ligation reduced expression and function of these molecules. These effects were not associated with traditional readouts of neutrophil activation, such as L-selectin shedding, although they did require an intact Fc portion of the antibody. Together these studies demonstrate a novel association between Ly6G and neutrophil β2 integrins and indicate that Ly6G may act to control surface levels and function of these important adhesion molecules, and thereby the recruitment capabilities of neutrophils.
The mechanism underlying this response remains to be determined. As Ly6G is a GPI-linked protein and therefore cannot directly signal into the cell (see figure), this raises the question as to whether Ly6G associates with other extracellular binding partners that might contribute to its effects on the β2 integrins. However, while not fully delineating the mechanism, a number of issues make this an important study. At a technical level, it raises a cautionary note for researchers using anti–Gr-1 or anti-Ly6G to identify neutrophils in vivo to be vigilant in their assessment of potential artifacts associated with their imaging methodology. Secondly, it raises the question as to the existence of as yet-unidentified endogenous ligands that might mediate similar effects to the anti-Ly6G antibody used here. Finally, it reveals a novel function for this poorly understood molecule that, if it could be translated to human biology, may be therapeutically relevant. Ly6G is only present in mice, but human neutrophils express the structurally related molecule CD177, a member of the Ly6/uPAR (urokinase plasminogen activator receptor) family. Interestingly, antibodies against CD177 have been shown to inhibit neutrophil transmigration across an endothelial monolayer, potentially by interfering with an interaction between Ly6G and PECAM-1.9 While murine Ly6G and human CD177 are unlikely to function identically, the findings from the murine and human systems identify these molecules as worthy of further investigation for their potential as novel therapeutic targets.
β2 integrins LFA-1 and Mac-1 are expressed at high levels on neutrophils, where they mediate important functions in neutrophil recruitment. Ly6G, a GPI-linked protein, is also present at high levels on the neutrophil surface, although its function is unknown. Wang et al show that Ly6G is co-localized with β2 integrins, and that antibody ligation of Ly6G reduces β2 integrin expression and inhibits neutrophil recruitment. The existence of an endogenous ligand for Ly6G is yet to be demonstrated. Professional illustration by Kenneth X. Probst.
β2 integrins LFA-1 and Mac-1 are expressed at high levels on neutrophils, where they mediate important functions in neutrophil recruitment. Ly6G, a GPI-linked protein, is also present at high levels on the neutrophil surface, although its function is unknown. Wang et al show that Ly6G is co-localized with β2 integrins, and that antibody ligation of Ly6G reduces β2 integrin expression and inhibits neutrophil recruitment. The existence of an endogenous ligand for Ly6G is yet to be demonstrated. Professional illustration by Kenneth X. Probst.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal