To the editor:
Recent discussions have been published on the flow cytometric analysis of cell-derived microparticles (MPs) and the confounding effects of insoluble immune complexes (ICs) on the interpretation of data,1-3 with György et al stating that MPs and ICs share biophysical light scatter properties when analyzed by flow cytometry (FCM).1
Although MPs and ICs overlap in size, we propose not all MPs would share biophysical light scatter properties with ICs because of the different constituents of each: MPs being a lipid-bilayer vesicle, with derived cellular components bound and internal to the vesicle, which is plastic in nature,1,4 whereas ICs are a result of multimolecular antibody-antigen bindings with a packed, rigid composition and are less prone to morphological change.1,5 The size of cell-derived MPs range from 0.1-1 micron in diameter,6 placing analysis at the very limits of flow cytometry (FCM) detection. Collecting at these detection limits, small differences in instrument design, maintenance, and operation can lead to contrasts in quality of data. Although FCM is used extensively for this application, standardization is very much in its infancy.7
Following procedures described by György et al,1 artificial, insoluble ICs were created, cell-derived MPs were isolated from the serum of healthy donors after an established protocol.6 Both samples were analyzed by FCM and Dynamic Light Scatter (DLS). In addition, György et al proposed antibody aggregation of certain antibodies leads to microvesicle-mimicking signals.2 We followed procedures as described2 to investigate the signal of antibody aggregate versus MPs.
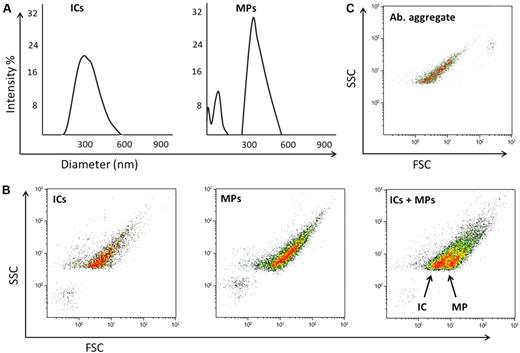
As the DLS data shows (Figure 1A), the MPs and ICs overlap in size, but when analyzed by FCM the populations are distinct (Figure 1B). In addition, we did not see antibody aggregates produce a microvesicle-mimicking signal, but they did coincide with the IC population (Figure 1C), which is not unexpected based on the comparable constituents.
DLS and FCM analysis of IC and MP samples. (A) DLS analysis of ICs and MPs, showing both samples are of overlapping size. (B) FCM analysis of ICs, MP, and MPs spiked with ICs. It can be seen the ICs and MPs are distinguished by light scatter alone. (C) FCM analysis of antibody (Ab) aggregate, where Ab aggregate overlaps with the IC population but does not create microvesicle-mimicking signals. For FCM analysis, a Coulter Epics XL instrument was used for acquisition, triggering of side scatter and using a Nano Fluorescent Particle Size Standard Kit; NFPPS-52-4K (Spherotech) for instrument calibration (data not shown). For DLS, a Coulter N4 plus instrument was used, acquiring the data at 90° angle, experiments carried out at 22°C.
DLS and FCM analysis of IC and MP samples. (A) DLS analysis of ICs and MPs, showing both samples are of overlapping size. (B) FCM analysis of ICs, MP, and MPs spiked with ICs. It can be seen the ICs and MPs are distinguished by light scatter alone. (C) FCM analysis of antibody (Ab) aggregate, where Ab aggregate overlaps with the IC population but does not create microvesicle-mimicking signals. For FCM analysis, a Coulter Epics XL instrument was used for acquisition, triggering of side scatter and using a Nano Fluorescent Particle Size Standard Kit; NFPPS-52-4K (Spherotech) for instrument calibration (data not shown). For DLS, a Coulter N4 plus instrument was used, acquiring the data at 90° angle, experiments carried out at 22°C.
Following the protocol from Amabile et al,3 who propose systematic triton lysis as a control to prove the vesicular nature of the MP population, we agree that the use of triton lysis can be a validating step for many MP-IC applications by FCM, as we also saw MP population dissolve from the MP gate. However, using the triton lysis we observed an impact on the IC population (data not shown). Under our conditions, as both populations are distinguishable by light scatter alone, the use of triton lysis in this instance added an unnecessary and complicating step.
Here we have shown that MPs and ICs do not share biophysical light scatter properties when analyzed by FCM, but because of the small size of both, and the limits of some FCM instruments, maintenance, and operation, they may not appear discrete.
Authorship
Acknowledgments: The authors thank Greg Noel and Xiaoyang Qi for technical assistance. Flow cytometry was performed at Shriners Hospital for Children supported by a grant from the Shriners of North America SSF 84070.
Contribution: P.H. designed and performed the experiments and wrote the manuscript; C.T.R. designed and performed the experiments and evaluated the manuscript; A.O. evaluated the experiments and the manuscript; and G.B. supervised the study, designed experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr George Babcock, Cincinnati Shriners Hospital for Children, 3229 Burnet Ave, Cincinnati, OH 45229-3095; e-mail: babcocgf@ucmail.uc.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal