In this issue of Blood, Usmani et al provide important information on second primary malignancies in patients treated with thalidomide and lenalidomide in the Arkansas total therapy trials.1
It took more than a decade for the association between melphalan exposure and acute myeloid leukemia to become apparent (see figure).2 It has taken about the same amount of time since the introduction of lenalidomide for the risks of second primary malignancies (SPM) with this drug to be recognized as something that warrants careful attention. Three recent randomized placebo-controlled trials have found a 2- to 3-fold increase in the risk of SPMs with the use of lenalidomide as maintenance therapy for patients with multiple myeloma.3–5 The SPMs included acute leukemias as well as solid tumors. In each of these trials, the risk of SPMs (excluding nonmelanoma skin cancers) was 2% to 3% in the placebo group versus 7% to 8% in the lenalidomide group. These differences were statistically and clinically significant and stunned most myeloma researchers because lenalidomide was considered one of the safest antimyeloma drugs, with an excellent overall side-effect profile. Since the initial reports surfaced there has been a virtual explosion of studies reporting on the risk of SPMs in multiple myeloma6–9 and the precursor premalignant stage of monoclonal gammopathy of undetermined significance.10
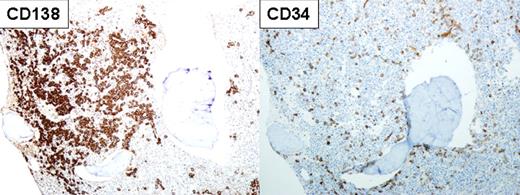
Immunostaining of the bone marrow (100× magnification) in a patient with multiple myeloma and therapy-related acute myeloid leukemia showing myeloma cells staining for CD138 (left panel) and leukemic blasts staining for CD34 (right panel) in the same specimen. See Figure 1 (top panel) in the article by Usmani et al that begins on page 1597.
Immunostaining of the bone marrow (100× magnification) in a patient with multiple myeloma and therapy-related acute myeloid leukemia showing myeloma cells staining for CD138 (left panel) and leukemic blasts staining for CD34 (right panel) in the same specimen. See Figure 1 (top panel) in the article by Usmani et al that begins on page 1597.
Here, Usmani and colleagues provide additional information on the risk of SPMs associated with prolonged therapy with lenalidomide and thalidomide.1 Of note, one of the trials they investigated, Total Therapy 2 (TT2), represents the only other randomized trial (aside from the 3 lenalidomide maintenance trials) with prolonged drug exposure and an appropriate control arm that could be used to interrogate the association of immunomodulatory agents and melphalan with SPMs. Their study shows a trend to increased risk of solid SPMs in the thalidomide arm of TT2 compared with the control arm, although the difference did not achieve statistical significance. No increase was noted in the risk of hematologic SPMs with thalidomide therapy. The authors also report an overall rate of SPMs in the total therapy trials that approached the rate seen in the lenalidomide maintenance trials. The lack of a control arm and shorter duration of follow-up make this study less suitable to examine the association of lenalidomide with SPMs.
How should we interpret the plethora of information on SPMs in myeloma? First, although the various studies of SPMs in myeloma that have ensued in the wake of the lenalidomide maintenance trials are of considerable importance, it is important to recognize their place in the big picture, and not allow the vast amount of information to create a smokescreen. Just as randomized, placebo-controlled trials are the gold standard to determine treatment efficacy, they are just as powerful and credible in determining the specific risks associated with therapy. Thus, no amount of uncontrolled observational studies can mitigate or negate the association between lenalidomide and SPMs seen in 3 randomized trials. The appropriate control population to compare the risk of SPMs is the placebo arm of these trials, not the Surveillance, Epidemiology, and End Results (SEER) registry or rates seen in other trials. Second, although the risk of SPMs with lenalidomide appears to be real, such risks have only been seen in the context of melphalan exposure and in the setting of prolonged maintenance therapy. So far, no increase in risk has been reported from studies that used lenalidomide without accompanying melphalan exposure, or in studies in which the median duration of therapy was short, as in the case of relapsed refractory myeloma. This suggests that an initial strategy to counter the risk would be to simply limit exposure to concurrent melphalan as much as possible, and to limit the duration of lenalidomide maintenance (if employed) to 2 years. Third, as always, there is a need to balance risks and benefits. For the approved indication of lenalidomide in patients with relapsed myeloma, the benefits clearly outweigh the risks. Similarly, in frontline therapy without concurrent melphalan, regimens such as lenalidomide plus dexamethasone remain safe and effective options. In contrast, more care is needed for the use of lenalidomide in the maintenance setting where there is antecedent high-dose melphalan exposure coupled with the prospect of prolonged use. Here more data are needed on the type of patients most likely to benefit from maintenance, as well as the optimal duration of therapy. Finally, the study by Usmani and colleagues suggests that the risk of SPMs, at least solid tumors, may be an issue with thalidomide as well. It is hard to determine whether the risk is different in magnitude compared with lenalidomide because the comparisons are not randomized. Further, the number and variety of drugs used in the Total Therapy trials makes it harder to isolate the effect of individual drugs on SPMs. Although it is tempting to be reassured by the statistically insignificant P values, the slope of the incidence curves is a call to caution and the need for more follow-up.
Given the remarkable progress made in myeloma therapy in the past decade, therapy-induced SPMs represent a small but serious bump in the road. Identification of the biologic mechanisms involved, associated risk factors, and strategies for prevention will be of critical importance. SPMs are serious cancers that may be a necessary consequence of life-saving treatment in myeloma, but in terms of importance and the need to eliminate as much as possible, they are second to none.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal