Abstract
Venous thromboembolism is a significant cause of illness and death worldwide. Large bodies of evidence support the heightened risk status of hospitalized medical patients, and that prophylactic measures significantly reduce the risk of thrombosis, yet these patients often fail to receive adequate prophylactic therapy. This failure may be accounted for by a lack of awareness of the relevant indications, poorly designed implementation systems, and clinical concerns over the side effects of anticoagulant medications. This article briefly summarizes our understanding of the clinical factors relevant to the evaluation of venous thromboembolism risk in hospitalized medical patients. We describe our approach to the use of thromboprophylaxis, through which we aim to minimize the disease burden of this under-recognized and preventable pathology.
Introduction
Venous thromboembolism (VTE), defined as deep venous thrombosis (DVT) or pulmonary embolism (PE), is associated with a significant disease burden worldwide. Hospitalized medical patients face a significant risk of VTE, with 42% at moderate or high risk according to American College of Chest Physicians criteria. As many as 10% to 20% of hospitalized medical patients can be expected to develop a VTE secondary to hospitalization.1,2 Autopsy data suggest that VTE contributes to more than 10% of deaths among hospitalized medical patients.3–5
Effective VTE prophylaxis can reduce the risk of VTE in this group by half.6,7 Although increasing adoption of VTE prevention strategies appears to have caused a decline in VTE incidence over time, this has disproportionately benefited surgical rather than medical patients. The period 1966 to 2000 saw a 71% reduction in autopsy-detected fatal PE rates among surgical patients, whereas among medical patients this decline was only 18%.8,9 VTE prophylaxis use rates remain grossly inadequate, with less than 40% of hospitalized medical patients worldwide receiving appropriate prophylaxis.1
Several factors may contribute to the inadequate use of preventive therapy. Medical patients are often complex, with multiple comorbidities and significant bleeding risk factors, which may cause reluctance among clinicians to prescribe thromboprophylaxis. Although surgery is widely recognized as a risk factor for VTE, the risk factors that occur among medical patients are more diverse and may be less commonly identified. Because more than 75% of hospital-based fatal PE events occur in medical patients, this is a deficiency in urgent need of redress.9 Multiple clinical guidelines, protocols, and risk assessment models are available to support decision making, and evidence suggests that their adoption significantly increases use of VTE prophylaxis and reduces VTE events.10–14
In this article, we describe our approach to VTE risk assessment in hospitalized medical patients and the basic data required for clinical risk stratification. We think that many medical patients would benefit from VTE prophylaxis, and we favor a simple exclusion-based checklist for initial assessment of VTE risk.
Risk factors
Virchow triad has long provided the foundation for commonly used approaches to VTE risk. Although stasis, endothelial injury, and a hypercoagulable state clearly interact to affect VTE risk, among hospitalized medical patients it is the alterations in circulating mediators of coagulation that probably play the most discriminating role. Despite increasing knowledge of the relative contribution of various VTE risk factors to VTE incidence, the molecular models linking these remain incomplete. Increasing amounts of data support the key role of inflammatory markers and tissue factor in activation of the coagulation cascade.15–17
Extensive reviews have described the numerous known VTE risk factors.12,18 We present a brief review of the data we find most relevant in clinical evaluation of VTE risk among hospitalized medical patients. The key risk factors for VTE are summarized in Table 1.12
VTE risk factors
| Acute illness . | Clinical . | Genetic . |
|---|---|---|
| Acute MI | Age > 60 y | Antithrombin deficiency |
| Acute heart failure (NYHA class III or IV) | History of VTE | Protein C deficiency |
| Acute infectious disease | History of malignancy | Protein S deficiency |
| Acute respiratory disease | Known thrombophilia | Hyperhomocysteinemia |
| Acute rheumatic disease | Extreme limitation in mobility | Raised prothrombin levels |
| Autoimmune diseases | Estrogen therapy or pregnancy | Factor V Leiden |
| Ischemic stroke | Obesity | Raised factor VIII levels |
| Thrombocytosis | Hyperfibrinogenemia | |
| Varicose veins | ||
| Chronic kidney disease or nephrotic syndrome |
| Acute illness . | Clinical . | Genetic . |
|---|---|---|
| Acute MI | Age > 60 y | Antithrombin deficiency |
| Acute heart failure (NYHA class III or IV) | History of VTE | Protein C deficiency |
| Acute infectious disease | History of malignancy | Protein S deficiency |
| Acute respiratory disease | Known thrombophilia | Hyperhomocysteinemia |
| Acute rheumatic disease | Extreme limitation in mobility | Raised prothrombin levels |
| Autoimmune diseases | Estrogen therapy or pregnancy | Factor V Leiden |
| Ischemic stroke | Obesity | Raised factor VIII levels |
| Thrombocytosis | Hyperfibrinogenemia | |
| Varicose veins | ||
| Chronic kidney disease or nephrotic syndrome |
NYHA indicates New York Heart Association.
Acute medical illness
Acute congestive heart failure (New York Heart Association class III or IV) and acute respiratory disease (respiratory failure or an exacerbation of chronic obstructive pulmonary disease) are well recognized as risk factors for VTE.19,20 The full pathophysiologic mechanism of this association is unclear, although recent evidence has highlighted the role of localized hypoxia in the release of procoagulant factors.21
Medical conditions associated with an inflammatory response (acute infectious disease, rheumatologic disorders, and inflammatory bowel disease) have shown a clear association with VTE risk.18,22,23 The interplay between circulating inflammatory and coagulant mediators is increasingly clear and provides a strong rationale for the observed association.24 Studies have confirmed a link between circulating C-reactive protein levels and VTE.25,26 The presence of either an autoimmune disease or the antiphospholipid antibody syndrome, or both, similarly conveys a significant VTE risk (relative risk [RR] = 3-10).27–29
Arterial thrombotic disease, namely, acute myocardial infarction or ischemic stroke, is intuitively likely to be associated with a prothrombotic state and therefore to convey a risk of VTE. A clear, somewhat weak (2- to 3-fold) increase in VTE rates has been demonstrated in association with arterial disease and arterial risk factors.30 Studies have confirmed a high incidence of DVT after myocardial infarction31,32 and ischemic stroke.33,34
These categories of acute medical illness are recognized by American College of Chest Physicians guidelines as significant VTE risk factors.7 Given the association of these conditions with substantially higher VTE incidence, we consider them strongly supportive of pharmacologic VTE prophylaxis.
Age
Age is perhaps the most well-established VTE risk factor.35 Causality is unclear; age is correlated with other known VTE risk factors, such as decreasing mobility, cancer, and other medical illness.36 Laboratory studies have confirmed significant increases in coagulation factor concentrations with age.37 Various studies have shown that the risk increases exponentially with age, and age more than 60 years has specifically been associated with a significantly higher risk of VTE (hazard ratio [HR] = 1.8; 95% confidence interval [CI], 1.2-2.7) in cohorts of general medical patients and in clinical trials.13,18 Many clinical trials supporting the use of thromboprophylaxis include only patients aged older than 40 years38,39 or older than 60 years.40 However, a practical approach should extrapolate such data to patients younger than 40 years when other established risk factors are present.
Prior VTE
A past medical history of VTE is a strong predictor of future VTE events (HR = 4.7; 95% CI, 3.0-7.2),13 with 3-year recurrence rates ranging from 15% to 25%.41–43 This risk is highest when the initial VTE was unprovoked or precipitated by a known and persistent risk factor.41,44 An initial VTE provoked by a transient risk factor, such as surgery or trauma, is less likely to recur. Some prior studies suggested that recurrent VTE after a DVT was most likely to be in the form of another DVT (86%), and the same was true of PE (66%).45 This finding has not been borne out by recent clinical trials, in which no significant difference in the rates of DVT versus PE after an initial DVT was found.46 A previous history of VTE has been associated with increased risk of VTE in medical patients and should be considered strongly supportive of thromboprophylaxis.18
Inherent thrombophilia
VTE has a strong heritable component, with a more than 2-fold increase in VTE incidence among those with a family history.47,48 A known thrombophilia is associated with an HR of 3.5 (95% CI, 1.1-11) for venous thromboembolism.13 Multiple genetic studies have identified specific loci of association (Table 1). A racial component is also well characterized, with white and black patients at significantly higher risk than Asian and Hispanic patients.49 The full risk conveyed by a history of first-degree relative with VTE is not fully explained by these known associations, suggesting that further genetic loci remain to be identified.47
Although data concerning medical patients specifically are not available, in other patient populations the antithrombin III deficiency and protein C deficiency have proven most significantly predictive of VTE events.29 Generally, however, the predictive value of identified thrombophilia is quite poor, and we do not conduct primary screening for the known genetic VTE risk factors, in accordance with published guidelines.29,50 Occasionally, however, patients present with a documented thrombophilia found on screening. Although there is no direct evidence, a history of thrombophilia or a family history of VTE should be considered a significant VTE risk factor among hospitalized medical patients.
Stasis
Immobility has long been associated with VTE and yet remains poorly defined. Marked immobility has been associated with VTE in medical studies examining short-term periods (6-14 days).39,51 This was also seen in the EXCLAIM study of prolonged thromboprophylaxis.52 Immobilization for 7 or more days has been linked with an HR of 1.9 (95% CI, 1.3-2.7) for VTE.13 The SIRIUS trial among outpatients defined immobility as total confinement to bed and armchair and found an odds ratio (OR) of 5.6 for VTE in such patients.53 Paraplegia and acute spinal cord injury convey an extremely elevated risk of VTE, with VTE rates reaching 60% to 100% of patients.54 We strongly think that patients effectively confined to bed with an acute medical illness as defined in “Acute medical illness,” should be considered at high risk of VTE. Other medical illnesses should be considered on a case-by-case basis. Quantification of the VTE risk in patients with moderately reduced ambulation is more difficult, but such immobility probably also modulates VTE risk.
Cancer
Active malignancy may increase VTE incidence more than 6-fold.55 This association with VTE persists in groups of medical patients with cancer after adjustment for cofounders (HR = 2.8; 95% CI, 1.9-4.2).13 Most of the evidence in this area arises from 2 sources: general internal medical patients with cancer and patients with cancer undergoing therapy.18,52,56 Apart from being linked to endothelial dysfunction and stasis, malignancies are known to be associated with multiple abnormalities in clotting factor concentrations.57 Tumors are known to release significant quantities of tissue factor directly58 ; associations have also been noted with decreased tissue plasminogen activator and antithrombin III levels, and increased plasminogen activator inhibitor-1.57 This risk varies significantly with the type of cancer and with the use of chemotherapy.59 Tumors posing the highest risk of VTE include gastric and pancreatic malignancies, whereas lymphoma, gynecologic tumors and tumors of the lung, bladder, and testes pose an intermediate risk. Colorectal, breast, and prostate tumors appear to have a lower but moderate risk of VTE.60 Myeloproliferative disorders similarly convey significant VTE risk, and this includes essential thrombocythemia, even in the absence of progression to leukemia. Active malignancy also increases the bleeding risk associated with pharmacologic thromboprophylaxis,61 further complicating management decisions in this patient group. Given the complex nature of VTE assessment in these patients, specific guidelines have been published, and management decisions are typically undertaken by clinicians specializing in the field.62 Despite the inherent risks, and in the absence of other risk factors for bleeding, thromboprophylaxis is likely to result in a significant net benefit among hospitalized medical patients with active malignancy.
Obesity
Obesity is known to increase VTE risk among medical patients more than 2-fold.53,63 Once again, numerous confounding factors make causality difficult to determine. The role of circulating factors, such as leptin and adiponectin, has only begun to be explored64,65 ; and as with arterial thrombosis, abdominal adiposity and the metabolic syndrome appear to be more strongly associated than body mass index readings.66
Estrogen
VTE risk is increased by pregnancy, hormone-replacement therapy, estrogen-containing oral contraceptive pills, and selective estrogen receptor modulators, suggesting a key interplay between this hormone and thrombosis.67,68 Hormone replacement therapy is known to increase serum concentration of procoagulant factors, although the full pathophysiology remains incompletely understood.69 We have no direct evidence of hormonal factors causing VTE in medical patients, but it seems likely that they would contribute to risk.
Chronic kidney disease
All forms of chronic kidney disease, ranging from microalbuminuria to end-stage renal disease, have been linked with increased VTE risk.70–72 Circulating procoagulant factor concentrations are elevated in this group, whereas concentrations of fibrinolytic proteins appear decreased,72 suggesting a causative role beyond that of proteinuria alone. The frequent presence of central venous catheters further increases VTE risk, with thrombosis occurring in between 33% and 59% of patients with a central venous catheter.73
Varicose veins
D-dimer
Two papers have described an association between elevated D-dimer levels around the time of admission and the subsequent development of VTE in this patient group.74,75 We have described a strong independent association between D-dimer levels of greater than or equal to twice the upper limit of normal and the subsequent development of VTE in this patient group. This independent association was stronger than that seen for advanced age (> 75 years) and cancer.76
Risk stratification
Although several quantitative risk assessment models have been developed, none has gained widespread acceptance. Indeed, validation studies have generally failed to replicate the ability of such models to accurately stratify risk of VTE.77 For this reason, and for ease of use, many clinical guidelines simply divide medical patients into at-risk and not-at-risk categories based on the presence of at least one or 2 risk factors.50 Broadly, we agree with this rationale and do not routinely use quantitative models to predict VTE risk in clinical practice. We similarly view the presence of a single risk factor, when combined with hospitalization and the associated reduction in mobility, as sufficient to justify prophylaxis in the majority of cases.
Despite such simplifications, we think that an appreciation of the summative nature of VTE risk factors is important. Several quantitative risk prediction scores have been developed recently for use in hospitalized medical patients, which illustrate this point.13,78,79 The latest American College of Chest Physicians guidelines have adopted the Padua Prediction Score.78 Although none has any clear advantage, the key feature of all is that risk increases rapidly with summation of even a few risk factors. For example, the risk score developed by Spyropoulos et al quantifies risk on a scale of 0 to 12, based on the presence of 7 key risk factors.13 The predicted 3-month VTE risk ranged from 0.4% for a score of 0, to 7.2% for a score more than 4.
VTE prophylaxis
The choice of VTE prophylaxis is fairly limited and therefore undemanding. A longstanding cornerstone of thromboprophylaxis is the use of unfractionated heparin (UFH), low molecular weight heparin (LMWH), or fondaparinux. Mechanical prophylaxis has not generally been tested in medical patients, except in those with ischemic stroke where the results have been negative.80 Graduated compression stockings (GCSs) or intermittent pneumatic compression (IPC) are used, particularly when bleeding risk prevents use of anticoagulants. The use of antiplatelet agents, such as aspirin, is not recommended for VTE prophylaxis as there is no evidence of their efficacy and they increase the risk of bleeding.
Mechanical prophylaxis
Mechanical prophylaxis using GCS or IPC is often reserved for patients in whom anticoagulation is contraindicated. Although IPC is cumbersome and may limit mobility, potentially resulting in a counterproductive increase in VTE risk, GCSs are generally well tolerated. The side effects of GCS are however significant, particularly in stroke patients.80 Although conclusive evidence supporting the efficacy of GCS and IPC in medical patients is lacking, data from surgical studies have provided a rationale for the use of GCS and IPC to prevent DVT only.81 Extrapolation from surgical studies means that some recommendations favor IPC use.82
Anticoagulation
The historically prominent role of UFH has been superseded by LMWHs, as numerous trials have demonstrated their comparable efficacy and improved side effect profile.83 Three large trials have validated the use of LMWH or fondaparinux for VTE prophylaxis in medical patients. The MEDENOX trial evaluated enoxaparin once daily and found a significant decrease in VTE events (RR = 0.37). There was no mortality benefit or any significant increase in bleeding risk.39 The PREVENT trial studied dalteparin and similarly found a significant reduction in VTE events (RR = 0.55), but a nonsignificant increase in bleeding complications and no mortality benefit.38 Finally, the ARTEMIS trial evaluated fondaparinux and revealed a 47% RR reduction in VTE events, with no difference in bleeding or mortality rates.40 Two large meta-analyses have pooled these and other results and confirmed a significant reduction in VTE events, particularly PE and fatal PE, but without an overall mortality benefit.6,84
An unresolved issue is the optimal duration of VTE prophylaxis. The 3 large trials of LMWHs (MEDENOX, PREVENT, and ARTEMIS) all involved protocols of between 6 and 14 days of prophylaxis. A recent trial has shown that a protocol of extending prophylaxis with LMWH by 28 days (beyond an initial 10 days) resulted in a 38% RR reduction in VTE events.52 However, there was an increase in major bleeding rates, with an RR of approximately 2.5. Furthermore, the benefits were limited to those with significant immobility, those older than 75 years, and women. Further data are needed before routine use of extended duration prophylaxis can be adopted in medical patients.
The newer generation of anticoagulants (apixaban, dabigatran, and rivaroxaban) were set to radically change the VTE prophylaxis landscape in both medical and surgical patients. Encouraging results have been reported in orthopedic surgery patients, and these new drugs seemed to be the ideal agents for extended duration prophylaxis in medical patients.85 Two studies have recently been reported, and in both safety has proven to be a concern. The ADOPT trial, comparing an extended course of apixaban to a standard course of enoxaparin in medical patients, reported a nonsignificant decrease in VTE-related mortality but a significant increase in bleeding risk (RR = 2.6).86 The MAGELLAN trial evaluated an extended course of rivaroxaban against a standard course of enoxaparin among hospitalized medical patients. Initial data revealed that rivaroxaban was noninferior at day 10 and superior at days 30 to 35 with regards to VTE prevention; however, clinically relevant bleeding rates were increased in the rivaroxaban arm with an RR of 2.3 at day 10 and an RR of 3.0 at days 30 to 35.87 Further data are required before clinical use of any of these newer agents among medical patients for either short-term or extended prophylaxis.
Complications
The primary adverse effect of anticoagulation is the increased risk of major bleeding, as defined by fatal bleeding, symptomatic bleeding in a critical area or organ, or bleeding causing a decrease in hemoglobin concentration of more than 20 g/L or requiring transfusion of 2 or more units of blood.88 Although clinical trials have lacked power to detect a significant increase in hemorrhage rates among medical patients, one meta-analysis confirmed a significant risk associated with the use of prophylactic anticoagulation. The absolute risk increase remains small (0.5%); however, this must be considered clinically significant.6 United Kingdom guidelines list 8 conditions (Table 2) that increase bleeding risk and advise a careful weighing of bleeding versus VTE risk (ie, these are not absolute contraindications).89 An analysis of the IMPROVE registry resulted in a bleeding risk score, with the factors most predictive of bleeding being active gastroduodenal ulcer, bleeding in the preceding 3 months, thrombocytopenia (< 50 × 109/L), and advanced age (> 85 years).90 Bleeding risk was also significantly increased by hepatic failure (with INR > 1.5), renal failure (with GFR < 30 mL/min per m2), admission to the intensive care unit, presence of a central venous catheter, rheumatic disease, cancer, and male sex. Use of LMWH was associated with a nonsignificant increase in bleeding rates, adding an absolute 0.5% risk of bleeding compared with no anticoagulant therapy, identical to that found in the meta-analysis.6 The use of mechanical prophylaxis was associated with a highly significant increase in bleeding complications.90 This probably reflects their initial assessment as high bleeding risk that would lead to the use of mechanical rather than pharmacologic prophylaxis.
Absolute and relative contraindications to anticoagulant prophylaxis
| Active bleeding* |
| Lumbar puncture/epidural/spinal anesthesia within the previous 4 hours or expected within the next 12 hours* |
| Concurrent use of anticoagulants known to increase the risk of bleeding (eg, warfarin with INR > 2)* |
| Acquired bleeding disorders (eg, acute liver failure) |
| Mucosal lesions (eg, active peptic ulceration, bronchiectasis) |
| Acute stroke (within 24 hours) |
| Thrombocytopenia (platelets < 75 × 109/L) |
| Uncontrolled systolic hypertension (≥ 230/120 mmHg) |
| Untreated inherited bleeding disorders (eg, hemophilia or von Willebrand disease) |
| Active bleeding* |
| Lumbar puncture/epidural/spinal anesthesia within the previous 4 hours or expected within the next 12 hours* |
| Concurrent use of anticoagulants known to increase the risk of bleeding (eg, warfarin with INR > 2)* |
| Acquired bleeding disorders (eg, acute liver failure) |
| Mucosal lesions (eg, active peptic ulceration, bronchiectasis) |
| Acute stroke (within 24 hours) |
| Thrombocytopenia (platelets < 75 × 109/L) |
| Uncontrolled systolic hypertension (≥ 230/120 mmHg) |
| Untreated inherited bleeding disorders (eg, hemophilia or von Willebrand disease) |
Absolute contraindications.
The other major complication of heparin-based prophylactic anticoagulation is heparin-induced thrombocytopenia (HIT). HIT (specifically, we refer to immune-mediated, or type II HIT) is caused by antibodies against heparin platelet factor 4 complexes, which results in a thrombocytopenia that occurs typically 5 to 10 days after initiation of unfractionated or LMWH. Medical patients appear to be at lower risk of HIT than surgical patients, with reported rates of 0.8% for treatment both with UFH and LMWH.91,92 Venous or arterial thrombosis may occur in 30% to 70% of HIT cases.93 Although some guidelines recommend routine platelet count monitoring every 2 days in all patients receiving prophylactic anticoagulation, most patients are not monitored routinely.93 We recommend obtaining a platelet count if therapy continues for more than one week. In patients with a history of HIT (not active), now rehospitalized and at high risk for VTE, we would recommend the use of fondaparinux for thromboprophylaxis.94
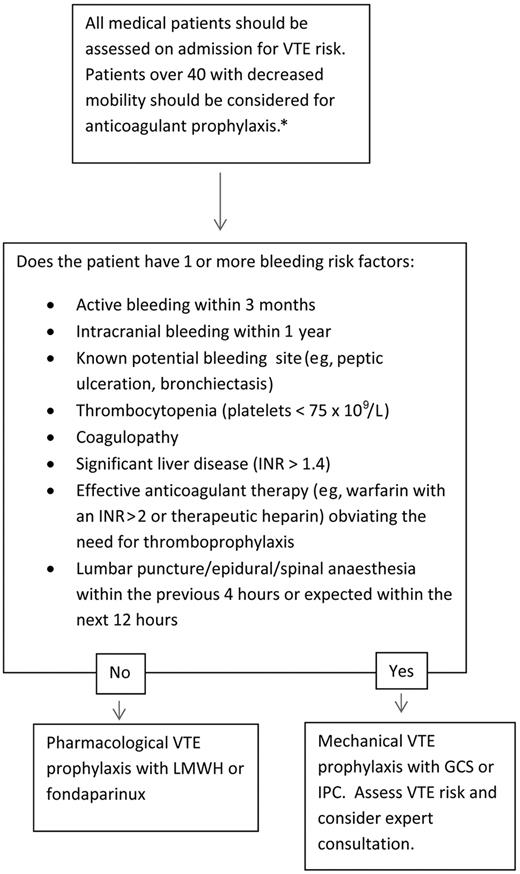
Our approach
Figure 1 presents our routine approach to VTE risk assessment for medical admissions. We favor an exclusion-based model, which is easy to implement routinely during medical admissions and minimizes the risk of underprescription of VTE prophylaxis. By focusing on exclusion criteria, our approach also aims to minimize prescription of anticoagulation in patients in whom it would pose the greatest risk (Table 2). A somewhat similar approach whereby VTE prophylaxis was included in a standard admission order set was found to increase VTE prophylaxis use generally, but most of all in those patients in whom it was a potential cause of harm.95 Our approach is designed to minimize this risk by highlighting assessment of bleeding risk during VTE risk assessment.
Initial VTE risk assessment. *Younger patients at risk with significant immobility should also be considered for thromboprophylaxis; those without significant immobility should not be considered for therapy.
Initial VTE risk assessment. *Younger patients at risk with significant immobility should also be considered for thromboprophylaxis; those without significant immobility should not be considered for therapy.
Perhaps a greater risk of exclusion-based models is the use of VTE prophylaxis in patients at low VTE risk. An exclusion-led model was evaluated in an audit of 497 hospitalized medical patients and was associated with an increase in the proportion of patients receiving a “correct” VTE prophylaxis decision from 49% to 75%.96 Despite the improvement, the overwhelming majority of errors that remained were from patients not receiving prophylaxis when indicated. Only a single case was found where prophylaxis was incorrectly prescribed, in a patient with diabetic ketoacidosis who did not qualify because of being younger than 40 years, a questionable error. In our experience, extremely few hospitalized medical patients lack at least a single risk factor for VTE.
We favor the use of an LMWH or fondaparinux over UFH, given the superior adverse effect profile. We reserve UFH for patients in whom LMWH is contraindicated because of renal impairment or other conditions. We recommend prophylaxis for a duration of 6 to 14 days, or for the duration of immobilization or hospitalization if the hospitalization and risk continue beyond 14 days. We routinely use mechanical prophylaxis for patients in whom anticoagulants are contraindicated, despite its unproven benefit in medical patients. We think that its use in general medical patients is weakly justified by evidence from other patient populations and by its general tolerability. However, contraindications are not infrequent (Table 3) and require consideration.89 We do not use mechanical prophylaxis in stroke patients, where they have been shown to increase complications without resulting in any significant decrease in VTE.80
Contraindications to mechanical VTE prophylaxis
| Suspected or proven peripheral arterial disease |
| Peripheral neuropathy or other causes of sensory impairment |
| Fragile skin, dermatitis, gangrene, or recent skin graft |
| Cardiac failure or severe leg edema |
| Allergy to material or inability to fit stocking |
| Suspected or proven peripheral arterial disease |
| Peripheral neuropathy or other causes of sensory impairment |
| Fragile skin, dermatitis, gangrene, or recent skin graft |
| Cardiac failure or severe leg edema |
| Allergy to material or inability to fit stocking |
Conclusion
Addressing the significant current deficiency in VTE prophylaxis among hospitalized medical patients should be a key priority for clinicians. Our approach attempts to reach a workable compromise between simplicity, safety, and the minimization of prophylaxis underprescription. Although complex patients at high risk of both bleeding and VTE will remain a management challenge, we hope our approach will assist in increasing VTE prophylaxis use in the large number of patients for whom it is clearly appropriate.
Case study 1
A 72-year-old woman is admitted to the medical ward with bilateral lower limb cellulitis, immobility, and deteriorating diabetic control. She has a history of type 2 diabetes treated with oral hypoglycemics. Treatment is begun with intravenous antibiotics. She has no recent history of bleeding. Investigation results are listed as follows: hemoglobin 12.9 g/dL; white cell count 13.5 × 109/L; platelets 215 × 109/L; glucose 9.9mM and +++ glycosuria; and urea and electrolytes, liver function studies, clotting studies, and fibrinogen levels within normal limits. What is her risk of VTE, and should she receive thromboprophylaxis?
This patient has multiple risk factors for VTE, including her advanced age, acute infectious disease, and immobility. Further, the presence of glycosuria puts her at risk for dehydration. Her Padua Prediction Risk Score is 5, suggesting an 11% risk of developing a VTE in the absence of prophylactic therapy.78 Conversely, she has no specific factors suggesting an increase in bleeding risk. She scores 1.5 on the IMPROVE Bleeding Risk Score, on account of her age, placing her risk of major bleeding at less than 1%.90 No contraindications to anticoagulant medication are present.
This case is a clear example of someone who would benefit from thromboprophylaxis. We would recommend the use of LMWH or fondaparinux for a period of 6 to 14 days, or longer if she remains hospitalized.
Case study 2
You are consulted for an opinion on a 29-year-old woman admitted to the psychiatric ward with severe depression. She has features of catatonia, with severely reduced mobility and decreased oral intake. There is no evidence of an organic cause and no other relevant medical history. You are asked whether VTE prophylaxis would be appropriate.
This case poses a challenge as the patient is of such a young age; thus, available clinical trial evidence does not directly apply. She does, however, have a significant risk factor for VTE, in that her mobility has been reduced for an unknown length of time. She may also be at risk of dehydration, further increasing VTE risk. However, she does not have any medical condition that has been investigated with regards to the effect of thromboprophylaxis. She does not appear to have any significant risk factors for bleeding. In this case, based on the current guidelines, we would not recommend the use of VTE prophylaxis with an LMWH or fondaparinux.
Case studies, however, have confirmed multiple cases of VTE, and often fatal PE, in psychiatric patients with decreased mobility.97,98 Some physicians think that the benefits of VTE prophylaxis significantly outweigh the bleeding risks in such cases. More research is needed on these types of atypical cases.
Authorship
Contribution: M.D. and A.T.C. wrote the manuscript and approved the final manuscript.
Conflict-of-interest disclosure: A.T.C. is a medical consultant and has received consultancy and clinical trial funding from pharmaceutical companies, including Astellas, AstraZeneca, Bayer, Boehringer-Ingelheim, BMS, Daiichi, GSK, Janssen (formerly Johnson & Johnson), Mitsubishi Pharma, Pfizer, Portola, Sanofi, Schering-Plough, and Takeda. He is an advisor to the United Kingdom Government Health Select Committee, the All-Party Working Group on Thrombosis, the Department of Health, and the NHS, on the prevention of VTE. He is also an advisor to Lifeblood: The Thrombosis Charity and is the founder of the European educational charity the Coalition to Prevent VTE. M.D. declares no competing financial interests.
Correspondence: Alexander T. Cohen, Vascular Medicine, Department of Surgery, King's College Hospital, London SE5 9RS, United Kingdom; e-mail: alexander.cohen@kcl.ac.uk.