Abstract
We recently defined a critical role for p53 in regulating the quiescence of adult hematopoietic stem cells (HSCs) and identified necdin as a candidate p53 target gene. Necdin is a growth-suppressing protein and the gene encoding it is one of several that are deleted in patients with Prader-Willi syndrome. To define the intrinsic role of necdin in adult hematopoiesis, in the present study, we transplanted necdin-null fetal liver cells into lethally irradiated recipients. We show that necdin-null adult HSCs are less quiescent and more proliferative than normal HSCs, demonstrating the similar role of necdin and p53 in promoting HSC quiescence during steady-state conditions. However, wild-type recipients repopulated with necdin-null hematopoietic stem/progenitor cells show enhanced sensitivity to irradiation and chemotherapy, with increased p53-dependent apoptosis, myelosuppression, and mortality. Necdin controls the HSC response to genotoxic stress via both cell-cycle–dependent and cell-cycle–independent mechanisms, with the latter occurring in a Gas2L3-dependent manner. We conclude that necdin functions as a molecular switch in adult hematopoiesis, acting in a p53-like manner to promote HSC quiescence in the steady state, but suppressing p53-dependent apoptosis in response to genotoxic stress.
Introduction
Hematopoietic stem cells (HSCs) can remain quiescent or can enter the cell cycle and either self-renew or differentiate.1 HSCs divide infrequently, and it has long been thought that the entire HSC pool turns over every few weeks and that HSCs regularly enter and exit the cell cycle.1,2 This paradigm has been challenged, because 2 functionally distinct HSC populations, dormant HSCs and activated HSCs, have been identified by independent groups of investigators.3,4
HSC quiescence is likely controlled by both HSC-intrinsic mechanisms and BM microenvironmental factors, with several genes and signaling pathways implicated in this process.5 Several regulators of the cell-cycle machinery have been shown to play critical regulatory roles in hematopoietic stem/progenitor cell (HSPC) proliferation, including p21, p57, p18, and the D-type cyclins and their catalytic partners Cdk4 and Cdk6.6–10 HSC cell-fate decisions are also regulated by several transcription factors (eg, Gfi-1, MEF/ELF4, Pbx-1, C-myc, and N-myc).11–14 Interestingly, recent studies indicate that tumor-suppressor genes, including PTEN, pRb, PML, APC, and Fbw7, may also play critical roles in maintaining HSCs in a quiescent state.15–19 In addition to HSC-intrinsic mechanisms, HSC function is regulated by ligand-receptor interactions, including angiopoietin and Tie-2, thrombopoietin and c-Mpl, SCF and c-Kit, and stromal-derived factor 1 (also known as Cxcl12) and Cxcr4.20–23
Recently, we defined a critical role for p53 in regulating HSC quiescence and identified necdin as a p53 target gene with a promoter that binds and is transactivated by p53.24,25 Necdin is a growth-suppressing protein first identified in postmitotic neurons,26 and the gene encoding necdin is one of several genes that are deleted in patients with Prader-Willi syndrome.27,28 Like the retinoblastoma protein, necdin interacts with multiple cell-cycle–promoting proteins, such as the simian virus 40 large T antigen, adenovirus E1A, and the transcription factor E2F1.29,30 Its functional effects are generally inhibitory on the cell-cycle progression that these proteins trigger.
We and others have shown that necdin is highly expressed in long-term reconstituting HSCs (LT-HSCs)24,31 and whereas its role in hematopoiesis is largely unknown, we have demonstrated that down-regulating necdin diminishes HSC quiescence and up-regulating it increases HSC quiescence.24 To more fully define the role of necdin in hematopoiesis, in the present study, we analyzed extensively the hematopoietic compartment of a strain of necdin-null mice that dies perinatally and has features resembling those seen in the human Prader-Willi syndrome.32 We found that although necdin is dispensable for fetal hematopoiesis, it regulates HSC quiescence and sensitivity to γ-irradiation and chemotherapy in adult BM cells. Despite being a p53 target, necdin appears to both mimic and antagonize p53 function. Therefore, whereas necdin functions like p53 to maintain HSC quiescence during the steady state, it opposes p53-dependent apoptosis under conditions of genotoxic stress.
Methods
Mice
The generation of necdin-null mice (C57BL/6, CD45.2) has been described previously.32 Wild-type C57BL/6 (CD45.2), B6.SJL (CD45.1) mice and p53-null mice (C57BL/6, CD45.2) were purchased from The Jackson Laboratory. Necdin-null mice were bred with p53 null mice to generate necdin/p53 double-null mice; however, no live necdin/p53 double-null pups were observed. Therefore, wild-type, necdin-null, p53-null, and necdin/p53 double-null fetal liver cells were isolated for transplantation. All mice were maintained in the Memorial Sloan-Kettering Cancer Center animal facility according to institutional animal care and use committee–approved protocols and kept in Thorensten units with filtered germ-free air.
Blood count
Peripheral blood was collected from tail veins and analyzed on an automated blood counter (HEMAVET HV950FS; Drew Scientific).
Fetal liver cell and donor repopulation assay
Fetal liver cells were isolated from embryonic day 14.5 embryos using techniques described previously. Fetal liver cells (1 × 106) from wild-type and necdin-null mice (CD45.2) were transplanted into lethally irradiated (9.5 Gy) B6.SJL mice (CD45.1). Sixteen weeks after transplantation, BM cells were harvested from reconstituted mice and analyzed. For the serial repopulation assays, 2 × 106 BM cells were transplanted into lethally irradiated B6.SJL mice (CD45.1). This procedure was repeated twice. For the competitive donor repopulation assays, 1 × 106 fetal liver cells (CD45.2) isolated from wild-type or necdin-null embryos were transplanted into lethally irradiated B6.SJL recipient mice (CD45.1) along with the same number (1 × 106) or 0.5 × 106 of competitor fetal liver cells (CD45.1).
Flow cytometry
Fetal liver or BM cells were stained with a lineage (Lin) cocktail of Abs from BD Biosciences (biotinylated anti–mouse Abs directed against CD3ϵ, CD45R/B220, Gr-1, and Ter119); Mac1, Sca-1, c-Kit, CD34, and FcγRII/III (BD Pharmingen); CD48 (eBiosciences); and/or CD150 (BioLegend) with various florescent conjugates; and streptavidin APC-Cy7 (BD Pharmingen). Stained cells were analyzed by flow cytometry using FACScan (BD Biosciences), FACSCalibur (BD Biosciences), MoFlo (Cytomation) or LSRII (BD Biosciences) flow cytometers. For peripheral blood analysis, RBCs were lysed and PBMCs were stained with anti-CD45.2 FITC, anti-CD45.1 PE (BD Pharmingen), and additional Abs. Nuclear staining of Ki67 was performed using a FITC-conjugated anti–human Ki67 Ab (BD Pharmingen), and fixation and permeabilization solutions from BD Biosciences. For the apoptosis assays, after staining for cell-surface markers, the cells were stained with anti–annexin V FITC or PE (BD Pharmingen) and analyzed with DAPI using MoFlo (Cytomation) or LSRII (BD Biosciences) flow cytometers.
Stem and progenitor cell assays
Clonogenic progenitors were determined in methylcellulose medium (MethoCult GF M3434; StemCell Technologies) using 2 × 104 fetal liver cells or BM mononuclear cells per well (6-well plate). Colonies were scored after 7 days of the initial culture, and all cells were collected and washed twice in PBS. Cells were then cultured at 2 × 104 per well in the same medium. Colony scoring and replating were repeated every 7 days for a total of at least 4 times or until no colonies were observed in the cultures.
For the long-term culture-initiating cell assay, 5 × 102 Lin−Sca1+Mac1+ fetal liver cells were cultured on MS5 stromal cells. After 4 weeks of weekly semi-replenishment of the media, cells were harvested and plated on methylcellulose medium (MethoCult GF M3434; StemCell Technologies). Clonogenic progenitors were determined after 10 days using 2 × 104 cells per well (6-well plate). Colonies were scored and expressed as number of the CFUs per 5 × 102 Lin−Sca1+Mac1+ cells.
BrdU incorporation assays
Mice received an intraperitoneal injection of 3 mg of bromodeoxyuridine (BrdU) and 1 mg/mL of BrdU was also added to their drinking water. Forty-eight hours later, BM cells were isolated and stained for cell-surface marker expression and nuclear staining of BrdU was then performed using the APC BrdU Flow Kit (BD Pharmingen) and fixation and permeabilization solutions. BrdU incorporation was detected in CD48−CD150+ Lin−Sca1+c-Kit+ (LSK) cells with an LSRII flow cycometer (BD Biosciences).
Homing and lodging assays
The homing and lodging ability of wild-type or necdin-null fetal liver cells were analyzed in irradiated and nonirradiated recipient mice, respectively. Wild-type or necdin-null fetal liver (5 × 106; CD45.2) cells were injected into lethally irradiated or nonirradiated recipient (CD45.1) mice. BM cells were harvested 18 hours after injection and donor-derived cells were evaluated by flow cytometry as CD45.2+ cells, simultaneously identifying Lin−Sca1+Mac1+ cells.
Chemotherapy and irradiation treatments
We administered single dose of 5-fluorouracil (5-FU; 200 mg/kg intraperitoneally) or weekly 5-FU (the initial dose was 125 mg/kg intraperitoneally and then 90 mg/kg intraperitoneally) or 6.5 or 8 Gy of irradiation to mice reconstituted with wild-type or necdin-null fetal liver cells 4 months after transplantation.
Gene-expression and pathways analysis
Gene-expression assays were performed as described previously,30 using RNA isolated from wild-type or necdin-null HSPCs (Lin−Sca1+c-Kit+) cells in both oligonucleotide arrays (Affymetrix) and quantitative PCR. The DNA sequence of all PCR primer pairs used is available on request. The Affymetrix data were analyzed using the Ingenuity Pathways Analysis program (Ingenuity Systems, www.ingenuity.com). Pathways that met the < 2-fold change cutoff and were associated with a canonical pathway in the Ingenuity Pathways Knowledge base were considered for the analysis. The significance of the association between the dataset and the identified canonical pathway was measured in 2 ways: (1) a ratio of the number of genes from the dataset that map to the pathway divided by the total number of genes from the dataset that map to the canonical pathway; and (2) Fisher exact test to calculate a P value determining the probability that the association between the genes in the dataset and the canonical pathway is explained by chance alone. All gene expression data have been deposited in the Gene Expression Omnibus public database under accession no. GSE39153.
Electroporation of siRNAs
LSK cells from BM cells were nucleofected using the Amaxa Mouse Macrophage Nucleofector Kit (Lonza) with program Y-01 and 100 pmol of siRNA. Cells were then cultured in serum-free medium (X-VIVO 15; Lonza) in the presence of cytokines (SCF, 100 ng/mL; thrombopoietin, 100 ng/mL; and Flt 3 ligand, 100 ng/mL). Twenty-four hours after nucleofection, the cells were analyzed by flow cytometry. siRNA pools were purchased from Dharmacon.
Retroviral transduction
cDNAs encoding Gas2L3 (Open Biosystems) were subcloned into the BglII and HpaI sites of the MigR1 retroviral vector. LSK cells from BM cells were infected by spinoculation for 3 days and used for the analyses.
Lentiviral-transduced fetal liver cell transplantation
Lentiviruses expressing shRNAs against Gas2L3 or a control shRNA cloned into pGIPZ vectors were purchased from Open Biosystems. Lentiviral vectors were produced by transfection of 293T cells. After cultured in X-VIVO 15 medium with 10 ng/mL of IL-3, 10 ng/mL of IL-6, and 100 ng/mL of SCF (PeproTech) for 24 hours, fetal liver cells from wild-type or necdin-null mice (CD45.2) were infected with high-titer lentiviral concentrated suspensions in the presence of 8 μg/mL polybrene (Sigma-Aldrich). Green fluorescent protein–positive cells were sorted using an LSRII flow cytometer (BD Biosciences) 72 hours later and used as the transduced cells. The B6.SJL (CD45.1) recipient mice were lethally irradiated with 9.5 Gy and the transduced fetal liver cells were transplanted into recipients with 5 × 105 helper cells (CD45.2). These mice were analyzed to assess adult hematopoiesis 10-12 weeks after transplantation after confirming complete donor repopulation.
Statistics
Statistical significance was assayed by the Student t test (for 2 groups) and 1-way ANOVA with the Tukey multiple comparison test as a post test (for more than 2 groups).
Results
Necdin is dispensable for fetal hematopoiesis
We demonstrated recently that necdin is a regulator of HSC quiescence using several in vitro stem cell assays.24 To further define the role of necdin in hematopoiesis, in the present study, we analyzed the hematopoietic compartment of necdin-null mice. Necdin is a maternally imprinted gene and thus is only expressed from the paternal allele. Whereas heterozygous mice inheriting the mutated maternal allele are indistinguishable from their wild-type littermates, mice carrying a paternally inherited deleted Ndn allele demonstrate early postnatal lethality.32 Therefore, we first investigated the role of necdin in fetal hematopoiesis, isolating fetal livers from embryonic day 14.5 embryos and examining their hematopoietic cell content.
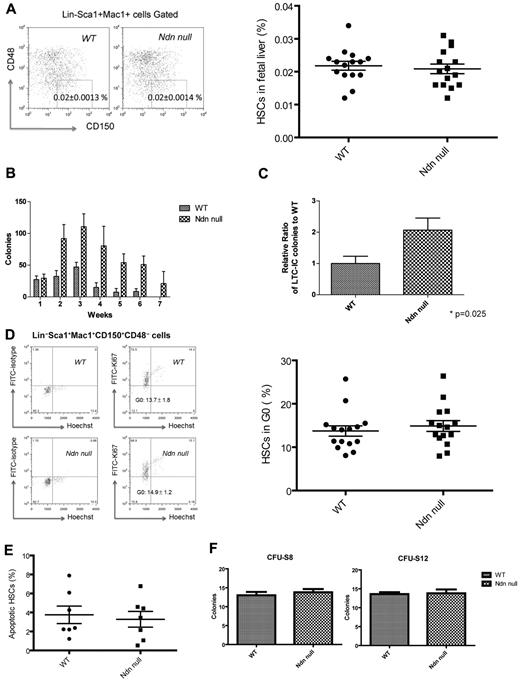
We found no change in the cellularity of necdin-null fetal livers compared with wild-type fetal livers (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and, using the SLAM family receptors CD150 and CD48 to characterize immunophenotypic HSCs,33 we found similar numbers of HSCs in the necdin-null and wild-type fetal livers (Figure 1A). Despite equal numbers, the necdin-null fetal liver HSCs (defined as Lin−Sca1+Mac1+CD48−CD150+ cells) showed increased serial replating in methylcellulose assays and maintained stemness in the stromal based, long-term culture-initiating cell assay better than wild-type fetal liver HSCs (Figure 1B-C). We then examined the cell-cycle status of necdin-null HSCs using the proliferation marker Ki67 and Hoechst 33342 DNA staining and found both wild-type and necdin-null fetal liver HSCs to be highly proliferative, with both showing approximately 15% quiescent cells (Figure 1D). Although necdin has been shown to inhibit p53-mediated apoptosis in U2OS cells,29 we also found normal numbers of apoptotic (annexin V+/DAPI−) HSCs in the necdin-null fetal livers at steady state (Figure 1E).
Necdin is dispensable for fetal hematopoiesis. (A) The frequency of immunophenotypic HSCs in necdin-null (Ndn-null) fetal livers (Lin−Sca-1+Mac1+CD48−CD150+ cells) quantified by flow cytometry was normal. The mean percentage (± SD) of Lin−Sca-1+Mac1+CD48−CD150+ cells in the fetal liver are shown (P = .64, n = 15). (B) Serial replating studies were performed using fetal liver cells. CFUs were quantified by methylcellulose culture using wild-type (WT) and necdin-null fetal liver cells. The methylcellulose cultures were serially replated weekly for 7 weeks. Mean values (± SD) are shown (n = 4). (C) Wild-type and necdin-null Lin−Sca1+Mac1+CD150+ cells (5 × 102) were cultured on MS5 stromal cells for 4 weeks and tested for colony formation in the long-term culture-initiating cell assay. Data shown are the mean relative number of colonies formed relative to wild-type colonies (± SD) from 2 independent studies (P = .025, n = 5). (D) Cell-cycle analysis of Lin−Sca-1+Mac1+CD48−CD150+ cells was performed by staining with Hoechst 33342 and Ki67 and analyzed by flow cytometry using an FITC mouse IgG1 Ab as the isotype control. Ki67− cells are defined as HSCs in G0 (left panels). Data shown are the mean values (± SD; P = .5, n = 15, right panel). (E) Apoptosis of wild-type and necdin-null fetal liver HSCs was assessed using DAPI and annexin V staining. Data shown are the mean percentage (± SD) of annexin V+/DAPI− Lin−Sca-1+Mac1+CD48−CD150+ cells (P = .71, n = 7). (F) Wild-type or necdin-null fetal liver cells (5 × 105) were transplanted into lethally irradiated recipients, and spleen colonies were scored on days 8 and 12 after transplantation. Numbers indicate average colony numbers (± SD). CFU-spleen day 8 (CFU-S8), P = .53, n = 5; CFU-spleen day 12 (CFU-S12), P = .86, n = 5.
Necdin is dispensable for fetal hematopoiesis. (A) The frequency of immunophenotypic HSCs in necdin-null (Ndn-null) fetal livers (Lin−Sca-1+Mac1+CD48−CD150+ cells) quantified by flow cytometry was normal. The mean percentage (± SD) of Lin−Sca-1+Mac1+CD48−CD150+ cells in the fetal liver are shown (P = .64, n = 15). (B) Serial replating studies were performed using fetal liver cells. CFUs were quantified by methylcellulose culture using wild-type (WT) and necdin-null fetal liver cells. The methylcellulose cultures were serially replated weekly for 7 weeks. Mean values (± SD) are shown (n = 4). (C) Wild-type and necdin-null Lin−Sca1+Mac1+CD150+ cells (5 × 102) were cultured on MS5 stromal cells for 4 weeks and tested for colony formation in the long-term culture-initiating cell assay. Data shown are the mean relative number of colonies formed relative to wild-type colonies (± SD) from 2 independent studies (P = .025, n = 5). (D) Cell-cycle analysis of Lin−Sca-1+Mac1+CD48−CD150+ cells was performed by staining with Hoechst 33342 and Ki67 and analyzed by flow cytometry using an FITC mouse IgG1 Ab as the isotype control. Ki67− cells are defined as HSCs in G0 (left panels). Data shown are the mean values (± SD; P = .5, n = 15, right panel). (E) Apoptosis of wild-type and necdin-null fetal liver HSCs was assessed using DAPI and annexin V staining. Data shown are the mean percentage (± SD) of annexin V+/DAPI− Lin−Sca-1+Mac1+CD48−CD150+ cells (P = .71, n = 7). (F) Wild-type or necdin-null fetal liver cells (5 × 105) were transplanted into lethally irradiated recipients, and spleen colonies were scored on days 8 and 12 after transplantation. Numbers indicate average colony numbers (± SD). CFU-spleen day 8 (CFU-S8), P = .53, n = 5; CFU-spleen day 12 (CFU-S12), P = .86, n = 5.
When we examined the differentiation capacity of the necdin-null HSCs, we found normal myeloid differentiation based on in vivo CFU-spleen assays (Figure 1F) and comparable frequencies of common myeloid progenitors, granulocyte monocyte progenitors, and megakaryocyte erythrocyte progenitors (supplemental Figure 1B). Therefore, although necdin loss affects fetal liver HSC self-renewal in vitro, it does not appear to affect in vivo HSC frequency or their differentiation, proliferation, or survival.
The role of necdin in adult hematopoiesis
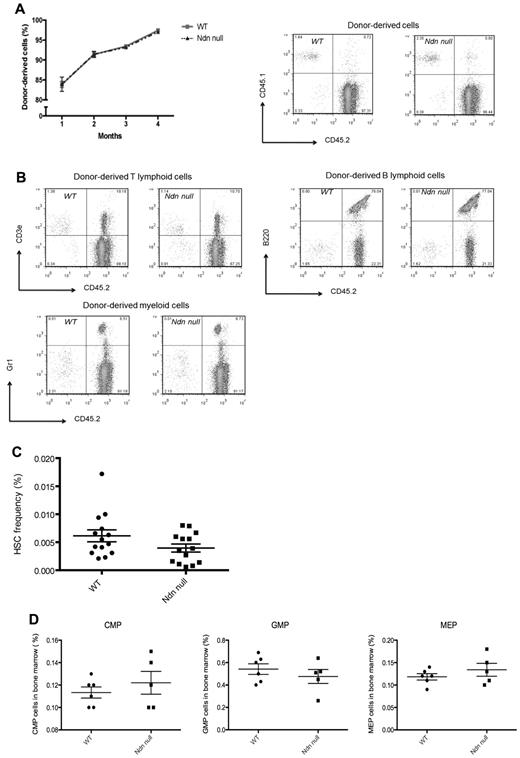
To define the role of necdin in adult hematopoiesis, we transplanted necdin-null fetal liver cells into lethally irradiated recipient mice. After long-term reconstitution, we examined adult HSC function in vivo, thereby using a model in which the BM microenvironment is composed of genetically normal cells. Although necdin-null fetal liver HSCs show increased serial replating capability in vitro and improved maintenance of “stemness” in long-term stromal-based cultures, the ability of necdin-null fetal liver HSCs to repopulate lethally irradiated recipient mice after fetal liver cell transplantation was normal (Figure 2A) based on the similar degree of long-term multilineage reconstitution and the normal frequency of donor-derived CD48−CD150+LSK cells, common myeloid progenitors, granulocyte monocyte progenitors, and megakaryocyte erythrocyte progenitors (Figure 2B-D). Therefore, wild-type mice repopulated with necdin-null fetal liver cells have normal, adult, steady-state (necdin-null) hematopoiesis.
The role of necdin in adult hematopoiesis. (A) Necdin-null (Ndn-null) fetal liver cells (CD45.2) normally repopulated lethally irradiated recipient mice (CD45.1). The frequency of donor-derived cells (CD45.2) in peripheral blood was measured monthly by flow cytometry at 4 time points. SDs are shown (left panel). Right panels show the data from flow cytometry 16 weeks after transplantation. (B) Recipient mice receiving necdin-null fetal liver cells (1 × 106) showed normal multilineage reconstituting activity, as assessed by the percentage of donor-derived myeloid cells (bottom left panel), B cells (top right panel), and T cells (top left panel) 16 weeks after transplantation using flow cytometry. (C) The frequency of HSCs (Lin−Sca-1+c-kit+CD48−CD150+ cells) in the BM of mice reconstituted with wild-type (WT) or necdin-null fetal liver cells was measured by flow cytometric analysis using SLAM cell-surface markers. Data shown are the mean percentage of HSCs (± SD; P = .1, n = 14). (D) Analysis of the common myeloid progenitor (CMP), granulocyte monocyte progenitor (GMP), and megakaryocyte erythrocyte progenitor (MEP) compartments showed comparable frequencies for those transplanted mice that received wild-type versus necdin-null fetal liver cells (P = .44, P = .41, P = .33, respectively, n = 6).
The role of necdin in adult hematopoiesis. (A) Necdin-null (Ndn-null) fetal liver cells (CD45.2) normally repopulated lethally irradiated recipient mice (CD45.1). The frequency of donor-derived cells (CD45.2) in peripheral blood was measured monthly by flow cytometry at 4 time points. SDs are shown (left panel). Right panels show the data from flow cytometry 16 weeks after transplantation. (B) Recipient mice receiving necdin-null fetal liver cells (1 × 106) showed normal multilineage reconstituting activity, as assessed by the percentage of donor-derived myeloid cells (bottom left panel), B cells (top right panel), and T cells (top left panel) 16 weeks after transplantation using flow cytometry. (C) The frequency of HSCs (Lin−Sca-1+c-kit+CD48−CD150+ cells) in the BM of mice reconstituted with wild-type (WT) or necdin-null fetal liver cells was measured by flow cytometric analysis using SLAM cell-surface markers. Data shown are the mean percentage of HSCs (± SD; P = .1, n = 14). (D) Analysis of the common myeloid progenitor (CMP), granulocyte monocyte progenitor (GMP), and megakaryocyte erythrocyte progenitor (MEP) compartments showed comparable frequencies for those transplanted mice that received wild-type versus necdin-null fetal liver cells (P = .44, P = .41, P = .33, respectively, n = 6).
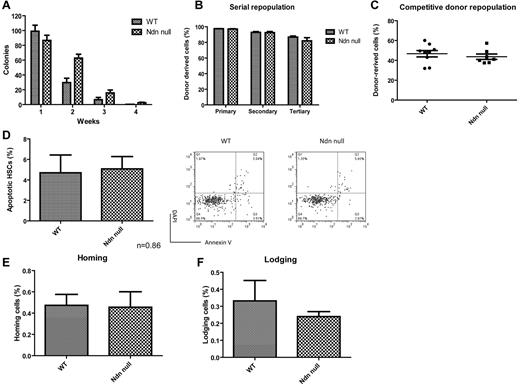
To further examine how the lack of necdin affects adult HSC behavior, we performed in vitro (serial replating) and in vivo (serial BM transplantation and competitive repopulation) functional assays. Adult primitive hematopoietic progenitor cells that lack necdin show increased serial replating in CFU assays (Figure 3A), which is consistent with our earlier findings.24
Loss of necdin has no effects on adult HSC self-renewal in vivo. (A) Serial replating studies. CFUs were quantified by methylcellulose culture using BM mononuclear cells from mice reconstituted with wild-type (WT) or necdin-null (Ndn null) fetal liver cells. The methylcellulose cultures were serially replated weekly for 4 weeks. Mean values (± SD) are shown (n = 4). (B) Donor repopulation after serial BM transplantation. Initially, we transplanted 1 × 106 fetal liver cells from wild-type or necdin-null mice (CD45.2) into lethally irradiated B6.SJL mice (CD45.1). Sixteen weeks after transplantation, we harvested 2 × 106 BM cells from mice reconstituted with wild-type and necdin-null fetal liver cells and transplanted the cells into lethally irradiated B6.SJL mice (CD45.1). The frequency of donor-derived cells (CD45.2) in the peripheral blood was measured 16 weeks after primary, secondary, and tertiary transplantation by flow cytometry. No differences were found between the groups (n = 10). (C) Lethally irradiated recipient mice (CD45.1) were transplanted with 1 × 106 wild-type or necdin-null fetal liver cells (CD45.2) plus 1 × 106 competitor fetal liver cells (CD45.1). The graph shows the mean percentage (± SD) of donor-derived (CD45.2) cells in the peripheral blood 16 weeks after transplantation (n = 7, P = .50). (D) BM cells from mice reconstituted with wild-type or necdin-null fetal liver cells were stained for HSC surface markers and assessed for apoptosis using DAPI and annexin V staining. Data shown are mean percentage of annexin V+/DAPI− HSCs (Lin−Sca1+Mac1+CD48−CD150+ cells) ± SD (P = .86, n = 4, left panel). The right panels show representative flow cytometry data: annexin V staining versus DAPI staining. (E-F) Fetal liver cell homing and lodging ability in irradiated and nonirradiated recipient mice was analyzed. Wild-type or necdin-null fetal liver cells (5 × 106; CD45.2) were injected into lethally irradiated (E) or nonirradiated (F) recipient mice (CD45.1). BM cells were harvested 18 hours after injection and CD45.2+ donor-derived cells were enumerated on the Lin−Sca1+Mac1+ population by flow cytometry. Data shown are mean percentages (± SD) of cells that homed (E) or lodged (F) within the Lin−Sca1+Mac1+ cells (E: P = .92, F: P = .48, n = 5).
Loss of necdin has no effects on adult HSC self-renewal in vivo. (A) Serial replating studies. CFUs were quantified by methylcellulose culture using BM mononuclear cells from mice reconstituted with wild-type (WT) or necdin-null (Ndn null) fetal liver cells. The methylcellulose cultures were serially replated weekly for 4 weeks. Mean values (± SD) are shown (n = 4). (B) Donor repopulation after serial BM transplantation. Initially, we transplanted 1 × 106 fetal liver cells from wild-type or necdin-null mice (CD45.2) into lethally irradiated B6.SJL mice (CD45.1). Sixteen weeks after transplantation, we harvested 2 × 106 BM cells from mice reconstituted with wild-type and necdin-null fetal liver cells and transplanted the cells into lethally irradiated B6.SJL mice (CD45.1). The frequency of donor-derived cells (CD45.2) in the peripheral blood was measured 16 weeks after primary, secondary, and tertiary transplantation by flow cytometry. No differences were found between the groups (n = 10). (C) Lethally irradiated recipient mice (CD45.1) were transplanted with 1 × 106 wild-type or necdin-null fetal liver cells (CD45.2) plus 1 × 106 competitor fetal liver cells (CD45.1). The graph shows the mean percentage (± SD) of donor-derived (CD45.2) cells in the peripheral blood 16 weeks after transplantation (n = 7, P = .50). (D) BM cells from mice reconstituted with wild-type or necdin-null fetal liver cells were stained for HSC surface markers and assessed for apoptosis using DAPI and annexin V staining. Data shown are mean percentage of annexin V+/DAPI− HSCs (Lin−Sca1+Mac1+CD48−CD150+ cells) ± SD (P = .86, n = 4, left panel). The right panels show representative flow cytometry data: annexin V staining versus DAPI staining. (E-F) Fetal liver cell homing and lodging ability in irradiated and nonirradiated recipient mice was analyzed. Wild-type or necdin-null fetal liver cells (5 × 106; CD45.2) were injected into lethally irradiated (E) or nonirradiated (F) recipient mice (CD45.1). BM cells were harvested 18 hours after injection and CD45.2+ donor-derived cells were enumerated on the Lin−Sca1+Mac1+ population by flow cytometry. Data shown are mean percentages (± SD) of cells that homed (E) or lodged (F) within the Lin−Sca1+Mac1+ cells (E: P = .92, F: P = .48, n = 5).
The absence of necdin preserves in vitro stemness within the stem or progenitor cell compartment. To more formally investigate the role of necdin in regulating adult HSC self-renewal, we performed serial transplantation assays using adult BM cells derived from wild-type or necdin-null fetal liver cells. We observed no difference in the ability of the necdin-null BM cells to repopulate lethally irradiated recipient mice in nonlimiting primary, secondary, or tertiary transplantation assays (Figure 3B). We also performed competitive repopulation assays, transplanting 1 × 106 fetal liver cells isolated from wild-type or necdin-null embryos into lethally irradiated B6.SJL recipient mice, along with the same number (1 × 106) of competitor fetal liver cells, and observed no differences in donor repopulation using wild-type or necdin-null cells (Figure 3C).
We also assayed apoptosis and the homing (or lodging) of necdin-null adult HSCs in the BM environment and found no difference in the percentage of apoptotic (annexin V+/DAPI−) HSCs in recipient mice repopulated with necdin-null or wild-type fetal liver cells (Figure 3D). We also saw no effect of lack of necdin on the homing or lodging of HSPCs to the BM of irradiated or nonirradiated hosts, respectively (Figure 3E-F). Therefore, necdin-null adult HSCs maintain normal self-renewal potential under the stress of transplantation.
Necdin maintains adult HSC quiescence
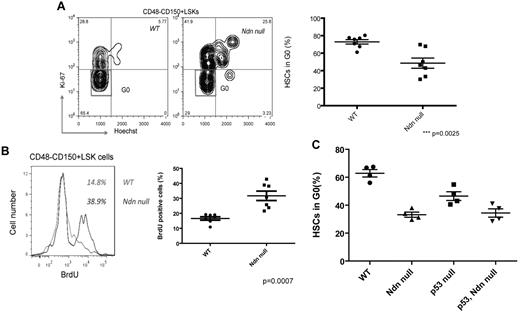
Down-regulating necdin expression in wild-type mouse BM HSPCs (using RNAi) led to decreased quiescence, suggesting that necdin may play an important role in regulating adult HSC quiescence.24 Therefore, we transplanted wild-type or necdin-null fetal liver cells into lethally irradiated recipient mice and examined the cell-cycle status of the donor-derived HSCs 16 weeks after transplantation. We stained necdin-null HSCs for the proliferation marker Ki67 and detected far fewer Ki67-negative HSCs and LSK cells than normal, indicating the presence of fewer quiescent HSCs; 72.9% of the wild-type CD48−CD150+ LSK cells were in G0 compared with 48.6% of necdin-null CD48−CD150+ LSK cells (P = .0025, Figure 4A and supplemental Figure 2A). Furthermore, to determine the proliferative rate of necdin-null HSCs in vivo, we administered BrdU to mice orally for 48 hours and isolated CD48−CD150+ LSK cells from the BM; 38.9% of necdin-null CD48−CD150+ LSK cells became BrdU+ compared with 14.8% of wild-type CD48−CD150+ LSK cells (P = .0007, Figure 4B), indicating that necdin promotes HSC quiescence and, in its absence, HSCs more easily enter the cell cycle.
Necdin maintains adult HSC quiescence. (A) Cell-cycle analysis of Lin−Sca-1+c-Kit+CD48−CD150+ cells (left panels) isolated from mice reconstituted with wild-type (WT) or necdin-null (Ndn null) fetal liver cells was performed by staining with Hoechst 33 342 and Ki67. Data shown are the mean values of G0 cells (± SD; P = .0025, n = 7). (B) The proliferation of Lin−Sca-1+c-Kit+CD48−CD150+ cells isolated from mice reconstituted with wild-type or necdin-null fetal liver cells was measured by in vivo BrdU incorporation over 48 hours. Greater proliferation of necdin-null Lin−Sca-1+c-Kit+CD48−CD150+ cells was observed (38.9% vs 14.8% for wild-type cells; P = .0007, n = 7). (C) Cell-cycle analysis of Lin−Sca-1+c-Kit+CD48−CD150+ cells isolated from the BM of mice reconstituted with wild-type, necdin-null, p53-null, or necdin/p53 double-null fetal liver cells was performed by staining with Hoechst 33342 and Ki67. Data shown are the mean values of G0 cells (± SD; P < .0001 by 1-way ANOVA, n = 4). Significant differences were observed between wild-type and necdin-null, wild-type and p53-null, wild-type and necdin/p53 double-null, necdin-null and p53-null, and p53-null and necdin/p53 double-null recipients.
Necdin maintains adult HSC quiescence. (A) Cell-cycle analysis of Lin−Sca-1+c-Kit+CD48−CD150+ cells (left panels) isolated from mice reconstituted with wild-type (WT) or necdin-null (Ndn null) fetal liver cells was performed by staining with Hoechst 33 342 and Ki67. Data shown are the mean values of G0 cells (± SD; P = .0025, n = 7). (B) The proliferation of Lin−Sca-1+c-Kit+CD48−CD150+ cells isolated from mice reconstituted with wild-type or necdin-null fetal liver cells was measured by in vivo BrdU incorporation over 48 hours. Greater proliferation of necdin-null Lin−Sca-1+c-Kit+CD48−CD150+ cells was observed (38.9% vs 14.8% for wild-type cells; P = .0007, n = 7). (C) Cell-cycle analysis of Lin−Sca-1+c-Kit+CD48−CD150+ cells isolated from the BM of mice reconstituted with wild-type, necdin-null, p53-null, or necdin/p53 double-null fetal liver cells was performed by staining with Hoechst 33342 and Ki67. Data shown are the mean values of G0 cells (± SD; P < .0001 by 1-way ANOVA, n = 4). Significant differences were observed between wild-type and necdin-null, wild-type and p53-null, wild-type and necdin/p53 double-null, necdin-null and p53-null, and p53-null and necdin/p53 double-null recipients.
Given the known effect of necdin on p53 function and the role of p53 in HSC self-renewal and quiescence, we bred necdin-null mice with p53-null mice and then isolated wild-type, necdin-null, p53-null, and necdin/p53 double-null fetal liver cells for transplantation into lethally irradiated recipients. We analyzed the cell-cycle status of the HSCs in these mice and found that necdin regulates HSC quiescence independently of p53, because the HSCs in mice reconstituted with either necdin-null or necdin/p53 double-null fetal liver cells showed similar decreases in quiescence (Figure 4C). Therefore, although necdin functions downstream of p53, its effects in quiescence are seen in the absence of p53, demonstrating its distinct role in HSC biology.
Necdin-null hematopoietic cells are highly sensitive to chemotherapy
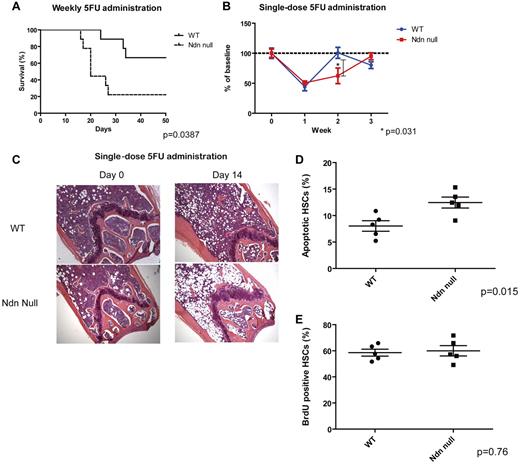
Given the known effects of quiescence on the cell's sensitivity to genotoxic stress, we hypothesized that the enhanced proliferative potential of necdin-null HSCs may sensitize the cells to chemotherapy. Indeed, using mice repopulated with necdin-null HSCs, we found a significantly enhanced sensitivity to weekly 5-FU administration (Figure 5A): only 20% of necdin-null mice survived 4 weekly 5-FU administrations, whereas 70% of the wild-type mice survived. We also followed peripheral blood counts after a single dose of 5-FU and found more prolonged myelosuppression in the mice reconstituted with necdin-null HSCs than wild-type HSCs (Figure 5B). Although the pretreatment BMs were similar, the BM of “necdin-null” mice showed a paucity of hematopoietic cells and the wild-type BM showed evidence of hematopoietic recovery on day 14 after 5-FU administration (Figure 5C). Consistent with these data, a single dose of 5-FU triggered greater apoptosis in the necdin-null HSCs (CD48−CD150+ LSK cells) than the normal HSCs (Figure 5D).
Necdin-null hematopoietic cells are highly sensitive to chemotherapy. (A) Survival after weekly 5-FU administration. 5-FU was administered intraperitoneally weekly (the initial dose was 125 mg/kg, with subsequent doses of 90 mg/kg) and the survival rates of mice repopulated with wild-type (WT) or necdin-null (Ndn null) fetal liver cells were measured. Results were analyzed with a log-rank nonparametric test and expressed as Kaplan-Meier survival curves (P = .0387, n = 10). (B) Hematopoietic reconstitution was monitored by serial peripheral blood count of mice injected with a single dose of 5-FU (200 mg/kg intraperitoneally). WBC counts are shown at each point after 5-FU administration as a percentage of the initial values for each group of mice (mean ± SD; n = 3 for each time point). (C) Gross morphology of femurs from untreated mice and mice 14 days after 5-FU administration is shown. Slides were stained with H&E. The slides were analyzed under a Zeiss Axioplan 2 Upright Wide-field Microscope (Carl Zeiss) equipped with a Zeiss AxiCam HRc Camera (Carl Zeiss; original magnification ×100 with a 10× objective). The images were acquired by a Volocity software (PerkinElmer). (D) HSCs isolated from the mice repopulated with wild-type or necdin-null fetal liver cells were assessed for apoptosis 60 hours after a dose of 5-FU (200 mg/kg intraperitoneally) using DAPI and annexin V staining. Data shown are mean percentage (± SD) of annexin V+/DAPI− Lin−Sca-1+c-Kit+CD48−CD150+ cells (P = .015, n = 5). (E) The proliferation of Lin−Sca-1+c-Kit+CD48−CD150+ cells isolated from mice reconstituted with wild-type or necdin-null fetal liver cells 4 days after 5-FU administration was measured by BrdU incorporation in vivo over 48 hours (P = .76, n = 5).
Necdin-null hematopoietic cells are highly sensitive to chemotherapy. (A) Survival after weekly 5-FU administration. 5-FU was administered intraperitoneally weekly (the initial dose was 125 mg/kg, with subsequent doses of 90 mg/kg) and the survival rates of mice repopulated with wild-type (WT) or necdin-null (Ndn null) fetal liver cells were measured. Results were analyzed with a log-rank nonparametric test and expressed as Kaplan-Meier survival curves (P = .0387, n = 10). (B) Hematopoietic reconstitution was monitored by serial peripheral blood count of mice injected with a single dose of 5-FU (200 mg/kg intraperitoneally). WBC counts are shown at each point after 5-FU administration as a percentage of the initial values for each group of mice (mean ± SD; n = 3 for each time point). (C) Gross morphology of femurs from untreated mice and mice 14 days after 5-FU administration is shown. Slides were stained with H&E. The slides were analyzed under a Zeiss Axioplan 2 Upright Wide-field Microscope (Carl Zeiss) equipped with a Zeiss AxiCam HRc Camera (Carl Zeiss; original magnification ×100 with a 10× objective). The images were acquired by a Volocity software (PerkinElmer). (D) HSCs isolated from the mice repopulated with wild-type or necdin-null fetal liver cells were assessed for apoptosis 60 hours after a dose of 5-FU (200 mg/kg intraperitoneally) using DAPI and annexin V staining. Data shown are mean percentage (± SD) of annexin V+/DAPI− Lin−Sca-1+c-Kit+CD48−CD150+ cells (P = .015, n = 5). (E) The proliferation of Lin−Sca-1+c-Kit+CD48−CD150+ cells isolated from mice reconstituted with wild-type or necdin-null fetal liver cells 4 days after 5-FU administration was measured by BrdU incorporation in vivo over 48 hours (P = .76, n = 5).
The delayed recovery after 5-FU administration occurred even though the necdin-null HSCs incorporated the same amount of BrdU as did wild-type HSCs 4 days after 5-FU administration (Figure 5E), which implies that the number of cycling cells in both the wild-type and necdin-null HSC compartment increases after 5-FU administration, with a greater increase in cycling wild-type HSCs (as these are more quiescent at steady state) or that the decreased quiescence of necdin-null HSPCs at steady state is rescued after 5-FU administration. Similarly, the necdin-null mice had the same numbers of HSCs (CD48−CD150+ LSK cells) as the wild-type mice 4 days after 5-FU administration (supplemental Figure 3). Therefore, it appears that the delayed recovery of the necdin-null mice was due to increased chemosensitivity rather than defective proliferation after 5-FU administration.
Necdin-null hematopoietic cells are highly sensitive to irradiation
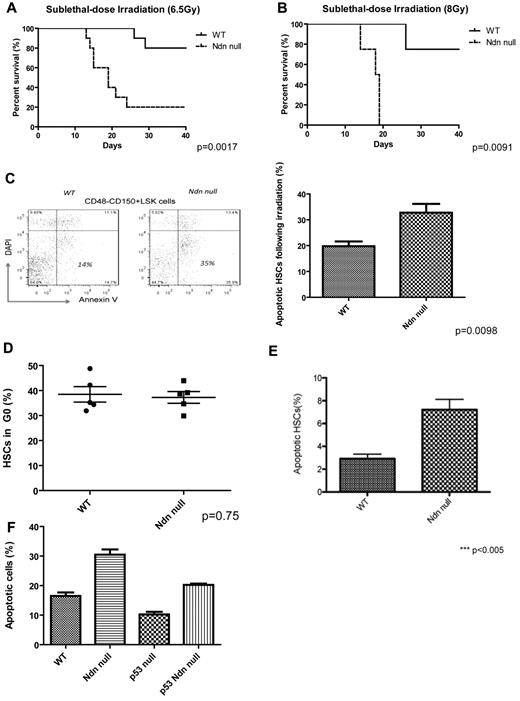
Mice reconstituted with necdin-null HSCs also show increased sensitivity to a single, sublethal dose of total body irradiation at both 6.5 and 8 Gy doses (Figure 6A-B). Using phosphorylation of histone H2AX (γ-H2AX) as an indicator of DNA damage,34 we observed no difference in the number of γ-H2AX+ wild-type or necdin-null HSCs after irradiation (6.5 Gy; supplemental Figure 4A). Nonetheless, we found 2-fold greater radiation-induced apoptosis of necdin-null HSCs (CD48−CD150+ LSK cells) than wild-type HSCs (Figure 6C), indicating their enhanced sensitivity to irradiation. To determine how much this sensitivity reflected decreased quiescence, we treated the mice with G-CSF (200 μg/kg daily for 5 days), which equalized the frequency of HSCs (CD48−CD150+ LSK cells) and quiescent HSCs in the wild-type and necdin-null BM compartments (Figure 6D and supplemental Figure 4B). However, when we irradiated these mice, we still found enhanced apoptosis in the necdin-null HSCs (CD48−CD150+ LSK cells) (Figure 6E). Therefore, necdin affects the response of HSCs to cytotoxic stress via both cell-cycle–dependent and cell-cycle–independent mechanisms.
Necdin-null hematopoietic cells are highly sensitive to irradiation. (A) Survival curves for mice reconstituted with wild-type (WT) or necdin-null (Ndn null) fetal liver cells, given total body irradiation (6.5 Gy), and monitored regularly for survival (P = .0017, n = 10). (B) Survival curves for mice reconstituted with wild-type or necdin-null fetal liver cells given TBI (8 Gy) and monitored regularly for survival (P = .0091, n = 8). (C) HSCs (Lin−Sca-1+c-Kit+CD48−CD150+ cells) from the BM of the mice repopulated with wild-type or necdin-null fetal liver cells were assessed for apoptosis 12 hours after a dose of total-body irradiation (6.5 Gy) using DAPI and annexin V staining (left panels). Data shown are the mean percentage (± SD) of the annexin V+/DAPI− population in Lin−Sca-1+c-Kit+CD48−CD150+ cells (P = .0098, n = 5, right panel). (D) Cell-cycle analysis of HSCs after G-CSF treatment (200 μg/kg daily). Lin−Sca-1+c-Kit+CD48−CD150+ cells isolated from mice reconstituted with wild-type or necdin-null fetal liver cells after 5 days of G-CSF treatment were analyzed by staining with Hoechst 33342 and Ki67. Data shown are the mean values of G0 cells (± SD; P = .75, n = 5). (E) Apoptosis of HSCs after G-CSF treatment (200 μg/kg daily). HSCs from the BM of the 5-day G-CSF–treated mice repopulated with wild-type or necdin-null fetal liver cells were assessed for apoptosis 12 hours after a dose of total body irradiation (6.5 Gy) using DAPI and annexin V. Data shown are the mean percentage (± SD) of the annexin V+/DAPI− population in Lin−Sca-1+c-Kit+CD48−CD150+ cells (P < .005, n = 4). (F) HSCs (Lin−Sca-1+c-Kit+CD48−CD150+) from the BM of mice repopulated with wild-type, necdin-null, p53-null, or necdin/p53 double-null fetal liver cells were assessed for apoptosis 12 hours after a dose of total body irradiation (6.5 Gy) using DAPI and annexin V staining. Data shown are the mean percentage ± SD of the annexin V+/DAPI− population within HSCs (P < .0001 by 1-way ANOVA, n = 4). Significant differences were observed between wild-type and necdin-null, wild-type and p53-null, necdin-null and p53-null, necdin-null and necdin/p53 double-null, and p53-null and necdin/p53 double-null recipients.
Necdin-null hematopoietic cells are highly sensitive to irradiation. (A) Survival curves for mice reconstituted with wild-type (WT) or necdin-null (Ndn null) fetal liver cells, given total body irradiation (6.5 Gy), and monitored regularly for survival (P = .0017, n = 10). (B) Survival curves for mice reconstituted with wild-type or necdin-null fetal liver cells given TBI (8 Gy) and monitored regularly for survival (P = .0091, n = 8). (C) HSCs (Lin−Sca-1+c-Kit+CD48−CD150+ cells) from the BM of the mice repopulated with wild-type or necdin-null fetal liver cells were assessed for apoptosis 12 hours after a dose of total-body irradiation (6.5 Gy) using DAPI and annexin V staining (left panels). Data shown are the mean percentage (± SD) of the annexin V+/DAPI− population in Lin−Sca-1+c-Kit+CD48−CD150+ cells (P = .0098, n = 5, right panel). (D) Cell-cycle analysis of HSCs after G-CSF treatment (200 μg/kg daily). Lin−Sca-1+c-Kit+CD48−CD150+ cells isolated from mice reconstituted with wild-type or necdin-null fetal liver cells after 5 days of G-CSF treatment were analyzed by staining with Hoechst 33342 and Ki67. Data shown are the mean values of G0 cells (± SD; P = .75, n = 5). (E) Apoptosis of HSCs after G-CSF treatment (200 μg/kg daily). HSCs from the BM of the 5-day G-CSF–treated mice repopulated with wild-type or necdin-null fetal liver cells were assessed for apoptosis 12 hours after a dose of total body irradiation (6.5 Gy) using DAPI and annexin V. Data shown are the mean percentage (± SD) of the annexin V+/DAPI− population in Lin−Sca-1+c-Kit+CD48−CD150+ cells (P < .005, n = 4). (F) HSCs (Lin−Sca-1+c-Kit+CD48−CD150+) from the BM of mice repopulated with wild-type, necdin-null, p53-null, or necdin/p53 double-null fetal liver cells were assessed for apoptosis 12 hours after a dose of total body irradiation (6.5 Gy) using DAPI and annexin V staining. Data shown are the mean percentage ± SD of the annexin V+/DAPI− population within HSCs (P < .0001 by 1-way ANOVA, n = 4). Significant differences were observed between wild-type and necdin-null, wild-type and p53-null, necdin-null and p53-null, necdin-null and necdin/p53 double-null, and p53-null and necdin/p53 double-null recipients.
The reported effects of necdin on p53 function appear to be cell context dependent; necdin has been shown to inhibit p53-induced apoptosis in U2OS cells but promote p53-dependent G1 arrest in SAOS-2 cells.29,30 We hypothesized that the enhanced apoptosis of necdin-null HSCs after genotoxic stress is p53 dependent. After we irradiated mice reconstituted with wild-type, necdin-null, p53-null, or necdin/p53 double-null fetal liver cells, we found more apoptosis in the necdin-null HSCs (CD48−CD150+ LSK cells), and less apoptosis in the p53 null HSCs, as expected. The necdin/p53 double-null HSCs showed an intermediate level of apoptosis, implying that some of the ability of necdin to inhibit radiation-induced HSC apoptosis is dependent on p53 (Figure 6F). This may reflect the necdin-independent ability of p53 to regulate HSC quiescence (Figures 4C and 6F).
Potential molecular mechanisms by which necdin regulates the response of HSCs to irradiation
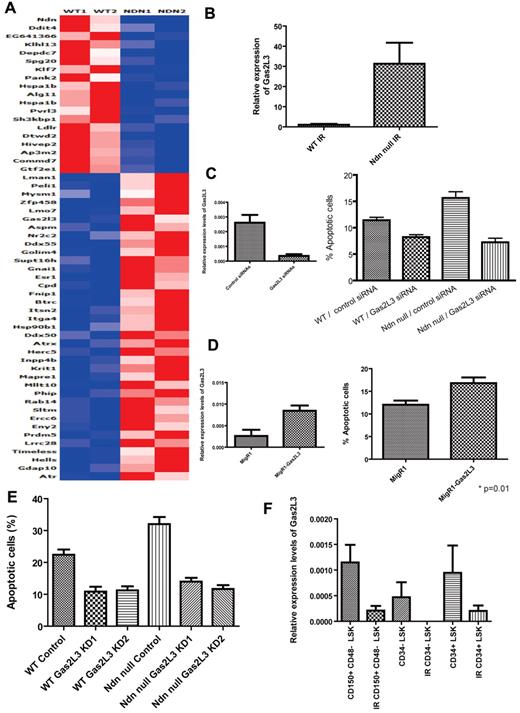
To investigate how necdin regulates the response of HSCs to genotoxic stress, we examined the expression of several apoptosis-related HSPC genes, including bcl-1, bcl-xL, and mcl-135,36 ; the expression of these genes was similar in both the wild-type and necdin-null HSPCs after irradiation, except for PUMA, a proapoptotic p53 target gene,37 which was actually lower in the irradiated necdin-null HSPCs (data not shown). Therefore, to identify necdin target genes that could account for the enhanced radiosensitivity of necdin-null HSCs, we performed gene-expression profiling (using microarray and quantitative PCR analyses) on LSK cells isolated from mice reconstituted with wild-type or necdin-null fetal liver cells after a sublethal dose of irradiation (6.5 Gy). We identified several deregulated signaling pathways in the necdin-null HSPCs, including the G2/M DNA damage and G1/S checkpoints (supplemental Figure 5). Of the putative necdin target genes differentially expressed in necdin-null versus wild-type cells (Figure 7A), one gene, Gas2L3, drew our attention.
Molecular mechanisms of necdin in regulating the response of HSCs to irradiation. (A) Transcript profiling of LSK cells from mice reconstituted with wild-type (WT) and necdin-null (Ndn null) fetal liver cells after a sublethal dose of irradiation (6.5 Gy) were analyzed by Affymetrix oligonucleotide array. Genes that are differentially expressed between wild-type and necdin-null HSPCs are shown. (B) The relative mRNA expression level of Gas2L3 in LSK cells isolated from mice reconstituted with wild-type or necdin-null fetal liver cells after a sublethal dose of irradiation (6.5 Gy) was evaluated by quantitative PCR and normalized to hypoxanthine-guanine phosphoribosyltransferase expression. Data shown are the mean ratio (± SD) of transcript levels relative to that of wild-type cells (n = 2). (C) Effect of down-regulating Gas2L3 expression on the HSPC response to irradiation. Wild-type or necdin-null LSK cells were nucleofected with control or Gas2L3-directed siRNAs. Twenty-four hours after nucleofection, the cells, which showed efficient Gas2L3 knockdown in LSK cells by quantitative PCR (left panel), were irradiated at 2 Gy and their apoptosis was measured by annexin V and DAPI staining. Data shown are mean values (± SD; P < .0001 by 1-way ANOVA, n = 6, right panel). Significant differences were observed between control/wild-type and Gas2L3 knockdown/wild-type, control/wild-type and control/necdin-null, control/wild-type and Gas2L3 knockdown/necdin-null, Gas2L3 knockdown/wild-type and control/necdin-null, and control/necdin-null and Gas2L3 knockdown/necdin-null. (D) Effect of Gas2L3 overexpression on the HSPC response to irradiation. Gas2L3 overexpression in LSK cells was confirmed by quantitative PCR (n = 2, left panel); the cells were then irradiated at 2 Gy and their apoptosis was measured by annexin V and DAPI staining. Data shown are mean values (± SD; P = .01, n = 5, right panel). (E) Green fluorescent protein–positive HSCs (Lin−Sca-1+c-Kit+CD48−CD150+) from the BM of the mice repopulated with wild-type control, wild-type Gas2L3 knocked-down 1 (KD1), wild-type Gas2L3 KD2, necdin-null control, necdin-null Gas2L3 KD1, or necdin-null Gas2L3 KD2 fetal liver cells were assessed for apoptosis 12 hours after a dose of total-body irradiation (6.5 Gy) using DAPI and annexin V staining. Data shown are the mean percentage ± SD of the annexin V+/DAPI− population within HSCs (P < .0001 by 1-way ANOVA). Significant differences were observed between wild-type control and wild-type Gas2L3 KD1, wild-type control and wild-type Gas2L3 KD2, wild-type control and necdin-null control, wild-type control and necdin-null Gas2L3 KD1, wild-type control and necdin-null Gas2L3 KD2, wild-type Gas2L3 KD1 and necdin-null control, wild-type Gas2L3 KD2 and necdin-null control, necdin-null control and necdin-null Gas2L3 KD1, and necdin-null control and necdin-null Gas2L3 KD2. (F) The relative mRNA expression levels of Gas2L3 in CD48−CD150+LSK cells, CD34−LSK cells, and CD34+LSK cells isolated from mice before and after a sublethal dose of irradiation (6.5 Gy) was evaluated by quantitative PCR and normalized to hypoxanthine-guanine phosphoribosyltransferase expression. Data shown are the mean ratio of transcript levels relative to the wild-type cells (± SD; n = 3).
Molecular mechanisms of necdin in regulating the response of HSCs to irradiation. (A) Transcript profiling of LSK cells from mice reconstituted with wild-type (WT) and necdin-null (Ndn null) fetal liver cells after a sublethal dose of irradiation (6.5 Gy) were analyzed by Affymetrix oligonucleotide array. Genes that are differentially expressed between wild-type and necdin-null HSPCs are shown. (B) The relative mRNA expression level of Gas2L3 in LSK cells isolated from mice reconstituted with wild-type or necdin-null fetal liver cells after a sublethal dose of irradiation (6.5 Gy) was evaluated by quantitative PCR and normalized to hypoxanthine-guanine phosphoribosyltransferase expression. Data shown are the mean ratio (± SD) of transcript levels relative to that of wild-type cells (n = 2). (C) Effect of down-regulating Gas2L3 expression on the HSPC response to irradiation. Wild-type or necdin-null LSK cells were nucleofected with control or Gas2L3-directed siRNAs. Twenty-four hours after nucleofection, the cells, which showed efficient Gas2L3 knockdown in LSK cells by quantitative PCR (left panel), were irradiated at 2 Gy and their apoptosis was measured by annexin V and DAPI staining. Data shown are mean values (± SD; P < .0001 by 1-way ANOVA, n = 6, right panel). Significant differences were observed between control/wild-type and Gas2L3 knockdown/wild-type, control/wild-type and control/necdin-null, control/wild-type and Gas2L3 knockdown/necdin-null, Gas2L3 knockdown/wild-type and control/necdin-null, and control/necdin-null and Gas2L3 knockdown/necdin-null. (D) Effect of Gas2L3 overexpression on the HSPC response to irradiation. Gas2L3 overexpression in LSK cells was confirmed by quantitative PCR (n = 2, left panel); the cells were then irradiated at 2 Gy and their apoptosis was measured by annexin V and DAPI staining. Data shown are mean values (± SD; P = .01, n = 5, right panel). (E) Green fluorescent protein–positive HSCs (Lin−Sca-1+c-Kit+CD48−CD150+) from the BM of the mice repopulated with wild-type control, wild-type Gas2L3 knocked-down 1 (KD1), wild-type Gas2L3 KD2, necdin-null control, necdin-null Gas2L3 KD1, or necdin-null Gas2L3 KD2 fetal liver cells were assessed for apoptosis 12 hours after a dose of total-body irradiation (6.5 Gy) using DAPI and annexin V staining. Data shown are the mean percentage ± SD of the annexin V+/DAPI− population within HSCs (P < .0001 by 1-way ANOVA). Significant differences were observed between wild-type control and wild-type Gas2L3 KD1, wild-type control and wild-type Gas2L3 KD2, wild-type control and necdin-null control, wild-type control and necdin-null Gas2L3 KD1, wild-type control and necdin-null Gas2L3 KD2, wild-type Gas2L3 KD1 and necdin-null control, wild-type Gas2L3 KD2 and necdin-null control, necdin-null control and necdin-null Gas2L3 KD1, and necdin-null control and necdin-null Gas2L3 KD2. (F) The relative mRNA expression levels of Gas2L3 in CD48−CD150+LSK cells, CD34−LSK cells, and CD34+LSK cells isolated from mice before and after a sublethal dose of irradiation (6.5 Gy) was evaluated by quantitative PCR and normalized to hypoxanthine-guanine phosphoribosyltransferase expression. Data shown are the mean ratio of transcript levels relative to the wild-type cells (± SD; n = 3).
Gas2L3 is a homolog of Gas2, a caspase-3 substrate that plays a role in regulating microfilament and cell-shape changes during apoptosis.38,39 Susceptibility to p53-dependent apoptosis has been shown to be correlated with increased levels of Gas2 protein, possibly because of enhanced p53 stability and transcriptional activity.38,40 Little is known about the biologic function of Gas2L3,41 so we first confirmed its radiation-induced up-regulation in necdin-null HSPCs using quantitative PCR analysis (Figure 7B). We hypothesized that up-regulation of Gas2L3 in irradiated necdin-null HSCs may account for their enhanced radiosensitivity and, indeed, found that the efficient knockdown of Gas2L3 expression decreased the radiation sensitivity of necdin-null LSK cells (Figure 7C). We also overexpressed Gas2L3 in wild-type LSK cells and found that it increased apoptosis (Figure 7D).
We further analyzed the in vivo radiation sensitivity of Gas2L3 knockdown HSCs. Lentiviruses expressing 2 shRNAs against Gas2L3 or a control shRNA were transduced into wild-type or necdin-null fetal liver cells. We verified that Gas2L3 expression levels were approximately 30% of normal in these transduced cells (supplemental Figure 6). Subsequently, the transduced fetal liver cells were sorted and transplanted into lethally irradiated recipients together with helper cells. Ten to 12 weeks after transplantation, we confirmed complete donor repopulation in these recipients and analyzed radiation-induced apoptosis in the green fluorescent protein–positive HSC (CD48−CD150+ LSK) population 12 hours after 6.5 Gy of total body irradiation (Figure 7E). Similar to the in vitro apoptosis assays, Gas2L3 knockdown HSCs showed less apoptosis after irradiation. Furthermore, knockdown of Gas2L3 in the necdin-null HSCs had the same effect on postirradiation apoptosis as in normal HSCs. Therefore, the enhanced up-regulation of Gas2L3 in irradiated necdin-null HSCs may account for their enhanced radiosensitivity.
Because increased Gas2L3 expression enhances the radiation sensitivity of HSPCs, this suggests that necdin regulates HSPC radiosensitivity by down-regulating Gas2L3 expression. In addition, whereas Gas2L3 is highly expressed in both CD48−CD150+ LSK and CD34+ LSK wild-type cells at steady state, Gas2L3 expression decreases after irradiation (Figure 7F), suggesting that cells may lower Gas2L3 expression to survive the effects of γ-irradiation.
Discussion
Having previously identified necdin as a potential regulator of HSC quiescence,24 in the present study, we used genetically modified mice that lack necdin expression to confirm that necdin controls HSC quiescence during the steady state. The effect of necdin on HSC quiescence is greater than the effect of p53 and, in fact, the effects of necdin on quiescence are not dependent on p53. This suggests that additional pathways that do not involve p53 or p21 converge on this critical property of HSCs. Despite this effect on quiescence, we did not see changes in the number or frequencies of HSCs and myeloid progenitors (Figure 2 and data not shown), suggesting that some compensation may have occurred to prevent the lack of necdin from affecting other HSC functions.
In the present study, we found profound cell-cycle–independent effects of necdin on the response of HSCs to genotoxic stress and identified distinct mediators of its effects on the cell cycle versus its effects on radiation- and chemotherapy-induced apoptosis. The absence of necdin does not appear to affect the frequency or differentiation potential of fetal liver HSCs. Although we did see an increase in self-renewal in vitro, necdin is clearly dispensable for fetal hematopoiesis, as was also shown recently by Kubota et al using a different necdin-null mouse that does not exhibit perinatal lethality.42 Given the perinatal lethality of our necdin-null mice, to investigate the role of necdin in adult hematopoiesis, we had to transplant necdin-null fetal liver cells into lethally irradiated wild-type recipient mice. Necdin-null fetal liver HSCs can normally repopulate lethally irradiated recipient mice in transplantation assays, which demonstrates that necdin is dispensable for the normal self-renewal of adult HSCs even under the stress of transplantation. By examining mice reconstituted with necdin-null fetal liver HSCs, we observed that, similar to p53, necdin serves to promote adult HSC quiescence in steady state. Although HSC quiescence aids in the preservation of self-renewal capacity,43 our analysis of necdin/p53 double-null HSCs suggests that the effects of necdin on HSC quiescence are mediated through a distinct pathway.
Changes in HSC quiescence are known to translate into reciprocal changes in chemoresistance and radioresistance,13,24 and, indeed, we found that the lack of necdin in HSCs sensitizes them to genotoxic stress (both 5-FU and sublethal doses of γ-irradiation). However, necdin has quiescence-independent effects that regulate the response of HSC to irradiation; whereas G-CSF stimulation normalizes the cell-cycle profile of necdin-null HSCs, these HSCs still show enhanced apoptosis after irradiation (Figure 6D-E). The ability of necdin to inhibit the p53-dependent radiation-induced apoptosis of LT-HSCs is reminiscent of the role of Slug, another p53 target gene that can antagonize the p53-mediated apoptosis of hematopoietic progenitor cells.37
Gas2L3 is a 683–amino acid protein with calponin homology and Gas2 homology domains, the function of which has been reported only recently: Gas2L3 is required for proper cytokinesis and maintenance of genomic stability.41 We show herein that changes in the level of Gas2L3 in HSCs play an important role in the regulation of apoptosis by necdin (Figure 7C-E). Therefore, it appears that necdin regulates HSC radiosensitivity in a Gas2L3-dependent manner. How the absence of necdin leads to enhanced Gas2L3 expression in irradiated but not steady-state HSCs is unclear. However, it is likely that changes in the expression of many genes or signaling pathways will explain the enhanced response of necdin-null HSCs to genotoxic stress.
The role of necdin in adult hematopoiesis that we have identified herein is different from that recently reported by Kubota et al.42 Those investigators found that necdin deficiency had no effect on HSC (defined as CD34−/low LSK cells) quiescence or self-renewal, that their necdin-null mice were resistant to weekly 5-FU treatment, and that necdin-null LSK cells were more proliferative after myelosuppression.42 For our studies, we primarily defined HSCs as CD48−CD150+ LSK cells, a purer LT-HSC population than LSK cells or CD34−/low LSK cells,44,45 for analyzing quiescence and the response to genotoxic stress. Therefore, our results can provide insight into the HSC intrinsic function of necdin. Furthermore, in our studies, the necdin-null HSCs reside in an otherwise normal host, eliminating any microenvironmental effects of the lack of necdin. Differences in the genetic background of the mice could also account for some of the differences observed. The necdin-null mice (Ndn tm1Ky) that Kubota et al studied were on an ICR background and backcrossed to the C57BL6 background for 5 generations. These differences are clearly important because the necdin-null mice that they studied showed no postnatal lethality, allowing BM cells from necdin-null mice to be analyzed. The mice that we have analyzed more closely mimic the human Prader-Willi syndrome, showing similar respiratory difficulties.46 Our present study found that the effect of necdin on HSC quiescence clearly demonstrates its stem cell intrinsic functions. This is relevant because inactivation of the retinoblastoma protein in the BM microenvironment results in profound myeloproliferation that is not intrinsic to the HSCs, but rather is the consequence of an retinoblastoma protein–dependent interaction between HSCs and the stem cell niche.47
Because necdin-null HSCs are less quiescent than wild-type HSCs and wild-type mice repopulated with necdin-null HSCs show enhanced sensitivity to chemotherapy and irradiation, targeting necdin may provide a therapeutic approach to eliminating quiescent leukemia-initiating cells.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the staff of the flow cytometry, mouse genotyping, molecular cytology, and genomics core facilities at the Memorial Sloan-Kettering Cancer Center for their support; the members of the Nimer laboratory for helpful comments; and Ms Erica Chuang for help in preparing the manuscript.
This work was funded by the National Institutes of Health (RO1 grant DK52208 to S.D.N.) and the Leukemia & Lymphoma Society (Specialized Center of Research [SCOR] grant to S.D.N.).
National Institutes of Health
Authorship
Contribution: T.A., Y.L, and S.D.N. designed the research; T.A., Y.L., S.D.G., N.B., D.N.-L., A.D., and S.M. performed the research; Y.A. and B.R. performed the bioinformatics analysis; R.W. contributed the animal models; T.A. and Y.L. analyzed the data; and T.A., Y.L., and S.D.N. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Stephen D. Nimer, Sylvester Comprehensive Cancer Center, Miller School of Medicine, University of Miami, 1550 NW 10th Ave, Fox 200, Miami, FL 33136; e-mail: snimer@med.miami.edu.
References
Author notes
T.A. and Y.L. contributed equally to this work.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal