Abstract
The atypical chemokine receptor CCX-CKR regulates bioavailability of CCL19, CCL21, and CCL25, homeostatic chemokines that play crucial roles in thymic lymphopoiesis. Deletion of CCX-CKR results in accelerated experimental autoimmunity induced by immunization. Here we show that CCX-CKR deletion also increases incidence of a spontaneous Sjögren's syndrome-like pathology, characterized by lymphocytic infiltrates in salivary glands and liver of CCX-CKR−/− mice, suggestive of a defect in self-tolerance when CCX-CKR is deleted. This prompted detailed examination of the thymus in CCX-CKR−/− mice. Negatively selected mature SP cells were less abundant in CCX-CKR−/− thymi, yet expansion of both DP and immature SP cells was apparent. Deletion of CCX-CKR also profoundly reduced proportions of DN3 thymocyte precursors and caused DN2 cells to accumulate within the medulla. These effects are likely driven by alterations in thymic stroma as CCX-CKR−/− mice have fewer cTECs per thymocyte, and cTECs express the highest level of CCX-CKR in the thymus. A profound decrease in CCL25 within the thymic cortex was observed in CCX-CKR−/− thymi, likely accounting for their defects in thymocyte distribution and frequency. These findings identify a novel role for CCX-CKR in regulating cTEC biology, which promotes optimal thymocyte development and selection important for self-tolerant adaptive immunity.
Key Points
The atypical chemokine receptor regulates T cell–development in the thymus and inhibits spontaneous autoimmunity.
Introduction
Chemokines direct leukocyte traffic during development, homeostasis, and during immune responses.1 However, chemokine regulation at the posttranslational level is poorly understood. A subfamily of atypical chemokine receptors has been identified that control chemokine levels and localization via high-affinity chemokine binding that is uncoupled from classic ligand-driven signal transduction cascades, resulting instead in chemokine sequestration, degradation, or transcytosis.2,3 The in vivo significance of these receptors has mainly been described for D6 and DARC, which regulate the biology of inflammatory chemokines after immune and inflammatory responses.3 Another of these atypical chemokine receptors, known as CCX-CKR (alternatively CCR11 or CCRL1), binds with high affinity to the homeostatic CCR7 ligands CCL19, CCL21, and the CCR9 ligand CCL25,4-6 chemokines that play a significant role during lymphocyte development and homeostasis.1,7 CCX-CKR has previously been shown to sequester and degrade CCR7-ligand chemokines in vitro and in vivo, thereby influencing adaptive immune responses.4,8 Furthermore, CCX-CKR deletion leads to early onset and increased disease in experimental autoimmune encephalomyelitis,8 a mouse model of multiple sclerosis, dependent on activation of T cells specific for self-antigen. This raises the possibility that T-cell selection and development are regulated by CCX-CKR.
Deletion of autoreactive T cells occurs in the thymus where BM-derived precursor cells progressively differentiate and are selected to form fully functional, self-MHC–restricted, generally non-autoreactive T cells that support effective adaptive immunity. Importantly, the 2 signaling chemokine receptors on which CCX-CKR impacts (CCR7 and CCR9) have well-described and significant roles in the thymus.9-13 These receptors are expressed on developing thymocytes at various stages of their maturation in the thymus and thymocytes are guided by CCX-CKR-ligand chemokines expressed by thymic epithelial cells (TECs) to distinct niches for optimal thymocyte differentiation and selection to proceed.9 Deletion of CCR7 impairs normal thymocyte development14-21 and leads to impaired negative selection that result in spontaneous autoimmunity.16,22,23 Specifically, CCR7 promotes seeding of the thymus by BM-derived progenitors,15,21 directs outward migration of double negative (DN) thymocyte precursors from the medulla to cortex,18 and directs inward migration of positively selected, single positive (SP) thymocytes from the cortex to medulla.14,16,17,19,20,24 CCR9 also plays a role in homing of BM-derived progenitors to the thymus and facilitates DN precursor migration outward from medulla to cortex.15,21,25 CCR7 and CCR9 expressing thymocytes and thymocyte precursors move in response to chemoattractant signals provided by CCL19, CCL21, and CCL25, expressed by TECs in distinct thymic compartments.9 CCX-CKR has also recently been shown to be expressed in the thymus by TECs in the thymic cortex (cTECs)26 and transgenic overexpression of CCX-CKR in the thymus influenced recruitment of BM-derived progenitors.27 In the present study, we investigated the role of CCX-CKR in regulation of lymphopoiesis in the context of thymocyte development. We report here that deletion of this receptor alters thymic architecture and T-cell development that is associated with spontaneous autoimmunity.
Methods
Mice
All experiments used aged-matched male mice 6-13 weeks of age unless stated otherwise. CCX-CKR−/− mice were generated and maintained as previously described.8 CCR7−/− mice were obtained from The Jackson Laboratory. Control wild-type (WT) C57BL/6 mice (age-matched) were either purchased from the University of Adelaide Laboratory Animal Services at 5-6 weeks of age and maintained in cages adjacent to CCX-CKR−/− and CCR7−/− mice or were bred alongside CCX-CKR−/− and CCR7−/− mice in the University of Adelaide Animal Housing Facility under specific pathogen-free conditions. Maintenance and use of these mice were carried out according to the guidelines set by the University of Adelaide Animal Ethics Committee.
Quantitative PCR
RNA preparation and quantitative PCR were performed as previously described.28 CCX-CKR primers are as follows: forward, AATGCTAGGTGCACTCCCATCT; and reverse, GCCGATTTCCAGCATCTGA.
Flow cytometry
Thymocytes were obtained from collagenase type IA (Sigma-Aldrich), treated thymi (30 minutes at 37°C), and then passed through a 70-μm nylon mesh with cold 1× PBS. Stromal cell isolation and antibody staining were performed as previously described.8,29 Antibodies used were anti-CD4–AlexaFluor-647 (RM4-5), anti–CD4-biotin (H129.19), anti-CD8α–PE-Cy7 (53-6.7), anti–CD8α-biotin (53-6.7), anti–CD11c-biotin (HL3), anti-CD25–FITC (7D4), anti–CD31-allophycocyanin (APC; MEC 13.3), CD44-APC-Cy7 (IM7), anti-CD45–APC-Cy7 (30F11), CD45R-peridinin chlorophyll protein-Cy5.5 (RA3–6B2), anti–Gr1-biotin (RB6-8C5), anti-Ly51–PE (6C3), streptavidin-peridinin chlorophyll protein-Cy5.5 purchased from BD Biosciences. Anti–CD3-biotin (145-2C11), anti-CD117–PE-Cy7 (2B8), and anti–Aire-488 (5H12-2) were purchased from eBioscience. Anti-CD326–APC (G8.8) and anti–I-A/I-E–Pacific Blue (M5/114.15.2) were purchased from BioLegend. UEA-1–FITC was purchased from Vector Laboratories. Anti-CCR7 (4B12, hybridoma) and anti-CCR9 (242503) were purchased from R&D Systems. Annexin V–FITC was purchased from MBL international. BrdU-powder was purchased from Sigma-Aldrich. Anti-BrdU–FITC was purchased from BD Biosciences. Cells were acquired on a FACSCanto or LSR II flow cytometer running FACSDiva Version 5.0.3 (BD Biosciences) and analyzed using FlowJo Version 7.6.5 software (TreeStar).
Histology
Tissues were snap-frozen in Tissue-Tek OCT embedding medium (Sakura Finetek) using liquid nitrogen and stored at −80°C. Blocks were sectioned on a Shandon cryostat (Thermo Scientific). Sections were collected on Polysine slides (Menzel-Glaser), fixed in 60% acetone/40% methanol (volume/volume) at room temperature for 10 minutes and then rehydrated in 1× PBS for 2 minutes, stained with hematoxylin and eosin followed by mounting coverslips with DePeX (Merck). Thymus hematoxylin and eosin images were acquired on a Nanozoomer (Hamamatsu) and viewed using NDP.view Version 1.1.6 (Hamamatsu). Submandibular gland/liver hematoxylin and eosin images were acquired on an Axiophot light microscope with 2.5× lens (Carl Zeiss) and a CC-12 camera (Olympus) with AnalySIS LS Research Version 2.5 (Olympus). Area analysis was performed using ImageJ Version 1.42q software (National Institutes of Health [NIH]).
Immunofluorescence
Sections were fixed in cold 100% acetone for 10 minutes and air dried before storing O/N at −20°C. Incubations were performed in a humid chamber at room temperature and washes involved 3 changes, for 2 minutes, through 1× TBS at room temperature. Sections were rehydrated in 1× TBS, blocked with 2% normal mouse serum and 2% normal goat serum in TBS for 30 minutes. Directly conjugated, purified, or biotinylated primary antibodies were added to sections for 1 hour. Slides were washed and primary antibodies detected with either anti–rat AlexaFluor-488, anti–rat AlexaFluor-647, or streptavadin–AlexaFluor-546 (purchased from Invitrogen) incubated on sections for 45 minutes at room temperature followed by washing. Slides were air-dried, and coverslips mounted using Vectashield (Vector Laboratories). Antibodies used were anti-CD4–purified (RM4-5), anti–CD8-biotin (53-6.7), anti–CD25-biotin (7D4), anti–CD44–FITC (IM7) purchased from BD Biosciences. Anti-CCR7–purified (4B12, hybridoma), anti-CCR9 (242503), anti–CCL21-biotin (BAF457), and anti–CCL25-biotin (BAF481) were purchased from R&D Systems. Images were acquired using a Leica SP5 spectral scanning confocal microscope at room temperature with a 10×/0.25 lens and LAS AF software and analyzed using ImageJ Version 1.42q (NIH; http://rsbweb.nih.gov/ij/) and the colocalization plugin (http://rsbweb.nih.gov/ij/plugins/colocalization.html). Equal thresholding values for colocalization analysis were applied. Contrast adjustments were applied to whole images equally. Pixel intensity analysis was performed using a selection 450 μm × 125 μm centered over the corticomedullary junction (CMJ) and the plot profile feature. Apoptotic cells were detected with the in situ cell death detection kit, TMR red (Roche Diagnostics) according to the manufacturer's instructions.
ELISA
Thymi were weighed, frozen in liquid N2, and stored at −80°C. ELISA protocol was followed as previously described.8 Capture antibodies used were anti-CCL19 (AF880), anti-CCL21 (AF457), and anti-CCL25 (MAB4811). Detection antibodies used were anti-CCL19 (BAF880), anti-CCL21 (BAF457), and anti-CCL25 (BAF481) purchased from R&D Systems and streptavidin-HRP (Rockland). The reaction was developed using TMB substrate (eBioscience), stopped with 1M orthophosphoric acid, and read at 450 nm on an GE Healthcare Biotrak II plate reader. The concentration of chemokine in the thymus homogenate was calculated from the standard curve using Graphpad Prism Version 5.0.
Statistics
Statistical analysis was performed using Graphpad Prism Version 5.0. Datasets were first subjected to an F test to compare SD variances and then subjected to the appropriate statistical analysis as described in the figure legends.
Results
Deletion of CCX-CKR results in spontaneous autoimmunity
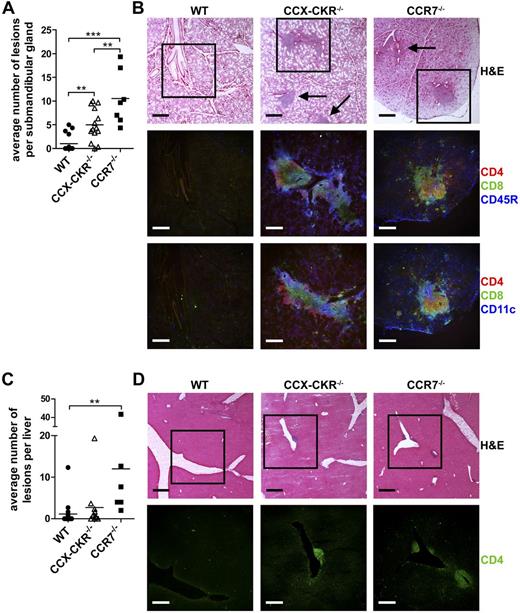
Altered thymocyte development in the absence of CCR7, the signaling receptor for 2 key CCX-CKR ligands CCL19 and CCL21, has previously been reported to lead to increased spontaneous autoimmunity,16,22,23 and CCX-CKR−/− mice develop accelerated experimental autoimmunity.8 These findings prompted us to examine whether CCX-CKR plays a role in the development of central tolerance. As a measure of this, we examined peripheral organs from aged (8-10 months old) WT, CCX-CKR−/−, and CCR7−/− mice looking for signs of spontaneous autoimmune inflammation (Table 1). The sublingual gland and pancreas did not contain any lesions in any of the strains studied. However, inflammatory lesions containing segregated CD4+, CD8+, CD11c+, and CD45R+ cells were apparent in the submandibular glands of both CCX-CKR−/− and CCR7−/− mice (Figure 1A-B). No obvious difference was apparent between CCX-CKR−/− and CCR7−/− lesions. The incidence of liver lesions in CCX-CKR−/− and CCR7−/− mice was also elevated compared with WT (Table 1), whereas CCR7−/− mice had increased numbers of lesions compared with WT mice (Figure 1C). Liver lesions were composed almost exclusively of CD4+ cells (Figure 1D). Lesions were also apparent in the kidney of CCX-CKR−/− mice, although the incidence of these was not significantly elevated (Table 1).
Frequency of lymphocytic infiltrate in peripheral organs of aged WT, CCX-CKR−/−, and CCR7−/− mice
| Genotype . | Submandibular gland . | Liver . | Kidney . |
|---|---|---|---|
| WT | 4/16 (25%) | 3/16 (18.8%) | 1/16 (6.3%) |
| CCX-CKR−/− | 13/14 (92.9%)* | 7/12 (58.3%)† | 4/12 (33.3%) |
| CCR7−/− | 7/7 (100%)‡ | 6/6 (100%)§ | 0/6 (0%) |
| Genotype . | Submandibular gland . | Liver . | Kidney . |
|---|---|---|---|
| WT | 4/16 (25%) | 3/16 (18.8%) | 1/16 (6.3%) |
| CCX-CKR−/− | 13/14 (92.9%)* | 7/12 (58.3%)† | 4/12 (33.3%) |
| CCR7−/− | 7/7 (100%)‡ | 6/6 (100%)§ | 0/6 (0%) |
WT and CCX-CKR−/− mice are 8-10 months of age, and CCR7−/− mice are 6.5-11 months of age.
P = .0002, compared with WT (2-tailed Fisher exact test).
P = .0497, compared with WT (2-tailed Fisher exact test).
P = .0013, compared with WT (2-tailed Fisher exact test).
P = .0011, compared with WT (2-tailed Fisher exact test).
Deletion of CCX-CKR results in enhanced spontaneous autoimmunity characterized by lymphocytic infiltrate in 8- to 10-month-old mice. (A) Quantitation of the average number of lesions identified in 3 sections per gland cut ∼ 100 μm apart, with the second section being the approximate middle of the tissue. WT, n = 16; CCX-CKR−/−, n = 14; and CCR7−/−, n = 7. Bars represent means. (B) Serial sections of submandibular glands were stained with hematoxylin and eosin (H&E) to identify lesions (indicated with arrows). Boxed region indicates areas shown below stained with anti-CD4, anti-CD8, anti-CD11c, and anti-CD45R to identify infiltrating cell populations. (C) Same as panel A, but in the liver. (D) Serial sections of the liver stained with H&E to identify lesions. Boxed region represents areas shown below stained with anti-CD4. H&E scale bar represents 400 μm. IF scale bar represents 250 μm. (A,C) One-way ANOVA with Bonferroni posttest. *P < .05. **P < .01. ***P < .001.
Deletion of CCX-CKR results in enhanced spontaneous autoimmunity characterized by lymphocytic infiltrate in 8- to 10-month-old mice. (A) Quantitation of the average number of lesions identified in 3 sections per gland cut ∼ 100 μm apart, with the second section being the approximate middle of the tissue. WT, n = 16; CCX-CKR−/−, n = 14; and CCR7−/−, n = 7. Bars represent means. (B) Serial sections of submandibular glands were stained with hematoxylin and eosin (H&E) to identify lesions (indicated with arrows). Boxed region indicates areas shown below stained with anti-CD4, anti-CD8, anti-CD11c, and anti-CD45R to identify infiltrating cell populations. (C) Same as panel A, but in the liver. (D) Serial sections of the liver stained with H&E to identify lesions. Boxed region represents areas shown below stained with anti-CD4. H&E scale bar represents 400 μm. IF scale bar represents 250 μm. (A,C) One-way ANOVA with Bonferroni posttest. *P < .05. **P < .01. ***P < .001.
Thus, CCX-CKR−/− mice develop spontaneous autoimmune disease resembling Sjögran syndrome, characterized by lymphocytic lesions in the submandibular gland and liver.
CCX-CKR is expressed by cortical thymic epithelial cells and regulates cortical and medullary size
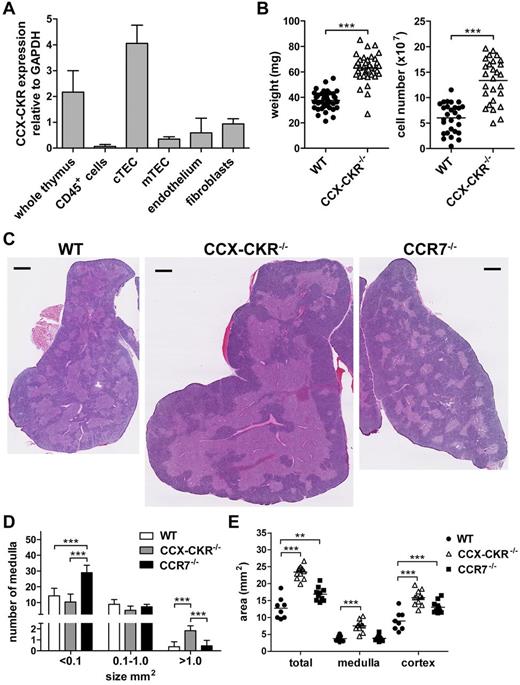
The occurrence of spontaneous autoimmunity resulting from deletion of CCX-CKR implies that this receptor may play a role in central tolerance of T cells in the thymus. CCX-CKR has been reported to be expressed by cTECs but not by thymocytes.26,27 We verified this by quantitative PCR using FACS-purified (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) CD45+ cells, cTECs, mTECs, endothelial cells, and fibroblasts (Figure 2A). cTECs were identified as the major source of CCX-CKR mRNA in the thymus, although some expression was also detected in mTECs, endothelial cells, and fibroblasts (Figure 2A). cTECs play an important role in thymocyte differentiation and selection and are an important source of CCX-CKR-ligand chemokines in the thymus. Therefore, we examined the effect of CCX-CKR deletion in the thymus of adult mice, as a previous study had reported normal thymocyte development in newly weaned CCX-CKR−/− mice.27 In 8- to 12-week-old CCX-CKR−/− mice, an ∼ 1.6-fold increase in thymic weight and an ∼ 2.2-fold increase in the total number of cells recoverable from CCX-CKR−/− thymi compared with WT were observed (Figure 2B). Structurally, CCX-CKR−/− thymi differed from WT by containing more numerous, larger (> 1.0 mm2), continually fused medullary regions surrounded by a thicker cortex compared with WT and CCR7−/− (Figure 2C-D). Thymi from CCR7−/− mice, kept in identical conditions, displayed the contrasting phenotype and contained more numerous, smaller (< 0.1 mm2), segregated medullary areas compared with WT (as previously reported18-20 ) and CCX-CKR−/− (Figure 2C-D). Analysis of the 2D area of thymic sections showed that CCX-CKR−/− thymi had a 2-fold increase in total area resulting from increased size of both medulla and cortex (Figure 2E).
CCX-CKR is expressed by cTECs and increased size, cell number, and aberrant thymic architecture in CCX-CKR−/− mice. (A) Thymocytes and thymic stromal cells from WT mice were sorted by flow cytometry and subjected to quantitative PCR analysis to identify CCX-CKR transcript. Data are 3 biologic replicates from a single experiment. (B) Thymus weight and total viable cell counts. Pooled thymi weights from 5 independent experiments: WT, n = 39; and CCX-CKR−/−, n = 38. Pooled cell counts from 4 independent experiments: WT, n = 28; and CCX-CKR−/−, n = 27. Two-tailed unpaired t test with Welch correction: ***P < .0001. (C) Thymus sections from WT (left), CCX-CKR−/− (middle), and CCR7−/− (right) mice stained with hematoxylin and eosin. Representative images from 12 WT, 11 CCX-CKR−/−, and 10 CCR7−/− mice. Scale bar represents 0.5 mm. (D) Quantitation of the medulla size range ± SD: WT, n = 8; CCX-CKR−/−, n = 11; and CCR7−/−, n = 10. (E) Quantitation of the total, medullary, and cortical area of WT, CCX-CKR−/−, and CCR7−/− thymic lobes. Sections were cut in the approximate middle of the thymus, and 2D area was determined: WT, n = 8; CCX-CKR−/−, n = 11; and CCR7−/−, n = 10. (B,E) Bars represent mean values. (D-E) One-way ANOVA with Bonferroni posttest: **P < .01, ***P < .001.
CCX-CKR is expressed by cTECs and increased size, cell number, and aberrant thymic architecture in CCX-CKR−/− mice. (A) Thymocytes and thymic stromal cells from WT mice were sorted by flow cytometry and subjected to quantitative PCR analysis to identify CCX-CKR transcript. Data are 3 biologic replicates from a single experiment. (B) Thymus weight and total viable cell counts. Pooled thymi weights from 5 independent experiments: WT, n = 39; and CCX-CKR−/−, n = 38. Pooled cell counts from 4 independent experiments: WT, n = 28; and CCX-CKR−/−, n = 27. Two-tailed unpaired t test with Welch correction: ***P < .0001. (C) Thymus sections from WT (left), CCX-CKR−/− (middle), and CCR7−/− (right) mice stained with hematoxylin and eosin. Representative images from 12 WT, 11 CCX-CKR−/−, and 10 CCR7−/− mice. Scale bar represents 0.5 mm. (D) Quantitation of the medulla size range ± SD: WT, n = 8; CCX-CKR−/−, n = 11; and CCR7−/−, n = 10. (E) Quantitation of the total, medullary, and cortical area of WT, CCX-CKR−/−, and CCR7−/− thymic lobes. Sections were cut in the approximate middle of the thymus, and 2D area was determined: WT, n = 8; CCX-CKR−/−, n = 11; and CCR7−/−, n = 10. (B,E) Bars represent mean values. (D-E) One-way ANOVA with Bonferroni posttest: **P < .01, ***P < .001.
Collectively, these data indicate that CCX-CKR plays an important role in the adult thymus and influences the cellularity and size of thymic cortical and medullary regions. Further, the overall effect of deletion of CCX-CKR is quite distinct from the deletion of the active CCL19/21 receptor, CCR7.
Thymocyte development is altered in the absence of CCX-CKR
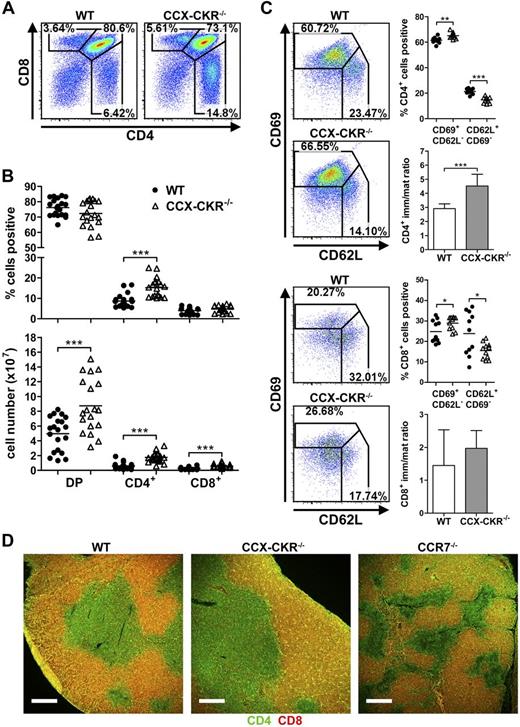
These changes in thymic architecture likely result from or influence thymocyte development as spatial and temporal niche occupation will be altered. Therefore, the effect of loss of CCX-CKR on the 3 major thymocyte populations (DP, CD4 SP, and CD8 SP cells) was examined. CCX-CKR−/− thymi contained significantly increased proportions of CD4 SP cells (Figure 3A-B), whereas all 3 DP, CD4 SP, and CD8 SP populations were increased numerically compared with WT thymi (Figure 3B). Proportions of immature (CD69+CD62L−, positively selected) and mature (CD62L+CD69−, negatively selected) CD4 and CD8 SP cells were increased and decreased, respectively, in CCX-CKR−/− thymi compared with WT (Figure 3C), indicating that postpositive selection differentiation is impaired. For CCX-CKR−/− CD4 SP cells, this was reflected in an increased ratio of immature to mature CD4 SP cells compared with WT (Figure 3C). The maturation defect in CD8 SP cells from CCX-CKR−/− thymi did not lead to an altered immature to mature ratio or increased proportions of CD8 SP cells (Figure 3B-C). As mature SP cells are exported from the CMJ/medulla,30 we determined whether SP cells were capable of reaching the medulla when CCX-CKR was absent. No differences in CD4 or CD8 cell localization were apparent between WT and CCX-CKR−/− thymi (Figure 3D). CCR7−/− thymi had a reduction in the prevalence of CD8 SP in the medulla, consistent with previous reports.14,16,17,19,20 We then determined whether thymic export was impaired in the absence of CCX-CKR, which may have led to the accumulation of SP cells. To do this, we performed in vitro thymocyte egress assays similar to those previously described.31,32 Egress of immature CD4 SP thymocytes from CCX-CKR−/− thymi was increased compared with WT (supplemental Figure 2A). In vivo egress of thymocytes was also assessed by enumerating recent thymic emigrants in the spleen. Increased numbers of CD4+ recent thymic emigrants were present in the spleen of CCX-CKR−/− mice compared with WT (supplemental Figure 2B-C), supporting the contention that export is enhanced in CCX-CKR−/− thymi, despite these cells apparently not undergoing optimal maturation. Furthermore, we detected increased proportions and numbers of CD3+CD4+CD8+ cells in the spleens of CCX-CKR−/− mice compared with WT mice (supplemental Figure 2D-E), providing further evidence that thymocytes are more readily exported from CCX-CKR−/− thymi without optimal T-cell selection occurring.
Altered thymocyte development in CCX-CKR−/− mice. (A) Representative dot plots showing CD4 and CD8 expression on thymocytes to assess proportions of DP (CD4+CD8+) and SP (CD4+CD8− or CD8+CD4−). (B) Quantitation of proportions (top) and total number (bottom) of DP and SP cells. Representative dot plots and data pooled from 4 independent experiments: WT, n = 20; and CCX-CKR−/−, n = 19. Two-tailed unpaired t test: ***P < .001. (C) Analysis of CD62L and CD69 expression to identify immature and mature CD4 SP (top) and CD8 SP (bottom) and the ratio of immature to mature CD4 SP (top) and CD8 SP (bottom). Representative dot plots and data pooled from 2 independent experiments; n = 11 per group. Two-tailed unpaired t test (immature and mature CD4 SP proportions: **P = .0013, ***P < .0001; and immature CD8 SP proportions: *P = .0425. Two-tailed unpaired t test with Welch correction (CD4 immature/mature ratio): ***P < .0001; mature CD8 SP proportions: *P = .0308. (D) Analysis of DP and SP cell localization in thymus sections stained with anti-CD4 (green) and anti-CD8 (red) of WT (left), CCX-CKR−/− (middle), and CCR7−/− (right). DP cells appear yellow. Representative images from one of 3 independent experiments: WT, n = 7; CCX-CKR−/−, n = 9; and CCR7−/−, n = 6. Scale bar represents 250 μm. (A,C) Numbers adjacent to gates indicate percent positive. (B-C) Bars represent means.
Altered thymocyte development in CCX-CKR−/− mice. (A) Representative dot plots showing CD4 and CD8 expression on thymocytes to assess proportions of DP (CD4+CD8+) and SP (CD4+CD8− or CD8+CD4−). (B) Quantitation of proportions (top) and total number (bottom) of DP and SP cells. Representative dot plots and data pooled from 4 independent experiments: WT, n = 20; and CCX-CKR−/−, n = 19. Two-tailed unpaired t test: ***P < .001. (C) Analysis of CD62L and CD69 expression to identify immature and mature CD4 SP (top) and CD8 SP (bottom) and the ratio of immature to mature CD4 SP (top) and CD8 SP (bottom). Representative dot plots and data pooled from 2 independent experiments; n = 11 per group. Two-tailed unpaired t test (immature and mature CD4 SP proportions: **P = .0013, ***P < .0001; and immature CD8 SP proportions: *P = .0425. Two-tailed unpaired t test with Welch correction (CD4 immature/mature ratio): ***P < .0001; mature CD8 SP proportions: *P = .0308. (D) Analysis of DP and SP cell localization in thymus sections stained with anti-CD4 (green) and anti-CD8 (red) of WT (left), CCX-CKR−/− (middle), and CCR7−/− (right). DP cells appear yellow. Representative images from one of 3 independent experiments: WT, n = 7; CCX-CKR−/−, n = 9; and CCR7−/−, n = 6. Scale bar represents 250 μm. (A,C) Numbers adjacent to gates indicate percent positive. (B-C) Bars represent means.
Thus, deletion of CCX-CKR leads to accumulation of DP and SP cells in the thymus, reduced transition of immature SP to mature SP cells, and increased export of sub-optimally differentiated and selected thymocytes.
DN3 thymocyte precursors are reduced in CCX-CKR–deficient mice
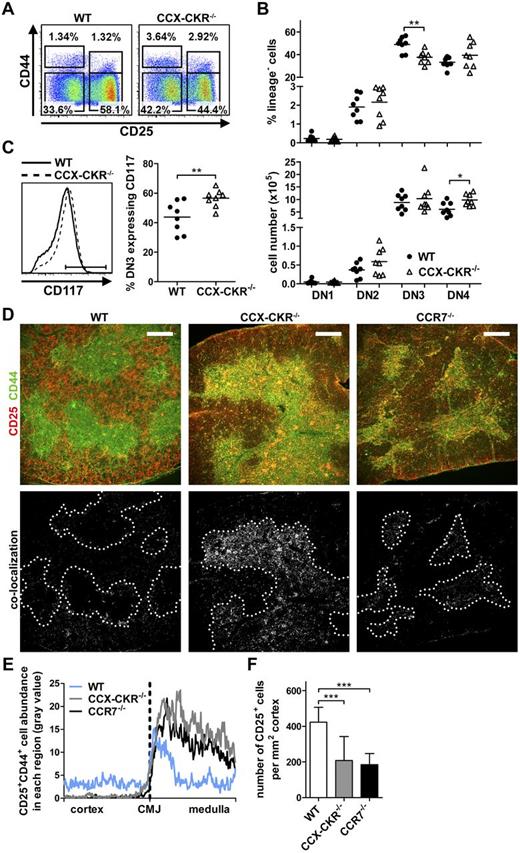
Given that thymocyte development was clearly affected by CCX-CKR deletion, whether altered development of precursor populations contributed to this was examined. Analysis of DN1-DN4 populations revealed no changes in DN1 or DN2 proportions in CCX-CKR−/− mice compared with WT, yet a significant reduction in the proportion, but not overall number, of DN3 cells and a significant increase in the number of DN4 cells in CCX-CKR−/− thymi was apparent (Figure 4A-B). Further, an increased proportion of CCX-CKR−/− DN3 cells expressed CD117, suggesting that these cells are of a less mature state than in WT (Figure 4C). Immunofluorescence (IF) analysis of precursors in WT and CCX-CKR−/− thymi revealed that, as expected, WT thymi contained CD44+CD25− cells in the medulla (representing DN1 cells and mature SP) and CD25+CD44+ DN2 cells at the CMJ. Distribution of these subsets was strikingly altered in CCX-CKR−/− thymi where CD25+CD44+ DN2 cells accumulate in both the CMJ and medulla (Figure 4D). These are most likely DN2 cells as triple staining of thymic sections showed that the accumulated CD25+ cells do not express CD4 or CD8 (supplemental Figure 3). CCR7−/− mice exhibited accumulation of CD25+CD44+ cells at the CMJ as has been previously reported18 (Figure 4D). CD25+CD44+ colocalization, plotted as a function of distance from the CMJ, quantitatively measured the accumulation of CD25+CD44+ cells in the CMJ/medulla of CCX-CKR−/− thymi (Figure 4E). As DN2 cells mature to DN3, they lose CD44 expression and are distributed throughout the cortex. This was evident in WT thymi; however, CCX-CKR−/− thymi clearly contained fewer CD25+CD44− cells in the cortex (Figure 4D). Enumeration of CD25+ cells in the cortex revealed a significant reduction in both CCX-CKR−/− and CCR7−/− thymi compared with WT (Figure 4F).
Reduction of the DN3 precursor population in CCX-CKR−/− mice. (A) Representative dot plots of DN subsets showing expression of CD25 and CD44 expression within the lineage negative compartment (defined as CD3−CD4−CD8−CD11c−CD45R−Gr1−). DN populations were further classified by CD117 expression, DN1 CD117+, DN2 CD117+, DN3 CD117−/lo, and DN4 CD117−. Numbers adjacent to gates indicate percent positive in each. (B) Quantitation of proportions (top) and total number (bottom) of DN subsets. Data are pooled from 3 independent experiments: WT, n = 8; and CCX-CKR−/−, n = 8. Two-tailed unpaired t test: *P = .011, **P = .0022. (C) Representative histogram of CD117 expression on DN3 cells (left) and quantitation of CD117 expression (right) from mice used in panel B. Two-tailed unpaired t test: **P = .0095. (D) Analysis of DN cell localization in thymus sections stained with anti-CD25 (red) and anti-CD44 cells (green) of WT (top left), CCX-CKR−/− (top middle), and CCR7−/− (top right). CD25+CD44+ cells appear yellow. Images below show colocalization of CD25+CD44+ cells (white) with CMJ indicated by dotted line. Representative images from 2 independent experiments: WT, n = 9; CCX-CKR−/−, n = 10; and CCR7−/−, n = 9. Scale bar represents 250 μm. (E) Quantitation of CD25+CD44+ cell distribution in thymus sections. (F) Enumeration of CD25+ cells in the cortex of thymic sections ± SD. One-way ANOVA with Bonferroni posttest: ***P < .0001. Two to 4 fields of view per thymus from those used in panel D were analyzed in panels E and F. (B-C) Bars represents mean values.
Reduction of the DN3 precursor population in CCX-CKR−/− mice. (A) Representative dot plots of DN subsets showing expression of CD25 and CD44 expression within the lineage negative compartment (defined as CD3−CD4−CD8−CD11c−CD45R−Gr1−). DN populations were further classified by CD117 expression, DN1 CD117+, DN2 CD117+, DN3 CD117−/lo, and DN4 CD117−. Numbers adjacent to gates indicate percent positive in each. (B) Quantitation of proportions (top) and total number (bottom) of DN subsets. Data are pooled from 3 independent experiments: WT, n = 8; and CCX-CKR−/−, n = 8. Two-tailed unpaired t test: *P = .011, **P = .0022. (C) Representative histogram of CD117 expression on DN3 cells (left) and quantitation of CD117 expression (right) from mice used in panel B. Two-tailed unpaired t test: **P = .0095. (D) Analysis of DN cell localization in thymus sections stained with anti-CD25 (red) and anti-CD44 cells (green) of WT (top left), CCX-CKR−/− (top middle), and CCR7−/− (top right). CD25+CD44+ cells appear yellow. Images below show colocalization of CD25+CD44+ cells (white) with CMJ indicated by dotted line. Representative images from 2 independent experiments: WT, n = 9; CCX-CKR−/−, n = 10; and CCR7−/−, n = 9. Scale bar represents 250 μm. (E) Quantitation of CD25+CD44+ cell distribution in thymus sections. (F) Enumeration of CD25+ cells in the cortex of thymic sections ± SD. One-way ANOVA with Bonferroni posttest: ***P < .0001. Two to 4 fields of view per thymus from those used in panel D were analyzed in panels E and F. (B-C) Bars represents mean values.
Together, these data show that deletion of CCX-CKR leads to accumulation of DN2 cells in the thymic CMJ/medulla, with a concurrent reduction in abundance of cortical DN3 cells. Therefore, CCX-CKR facilitates development of DN cells by regulating migration of DN2 and DN3 cells to the outer cortex where stromal signals stimulate further thymocyte differentiation. These defects may make an important contribution to the downstream changes in thymocyte development and impaired T-cell selection.
Thymic chemokines are dysregulated in the absence of CCX-CKR
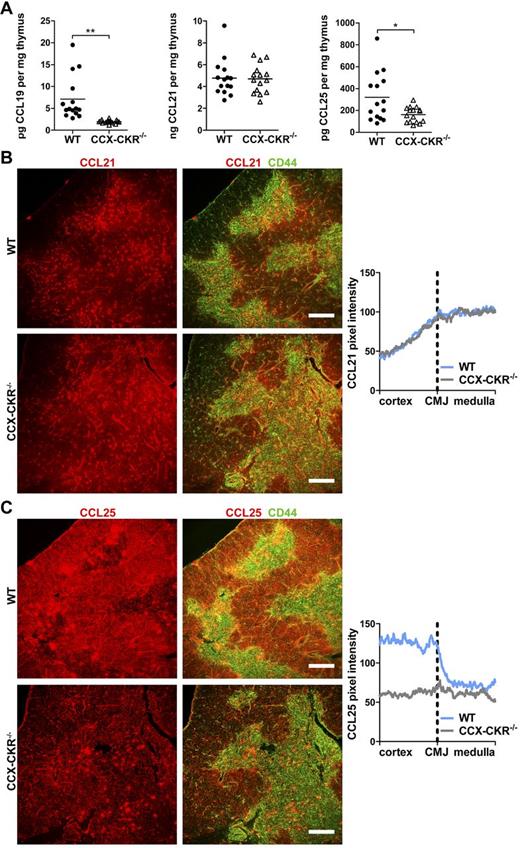
The measured defects in thymocyte development and distribution in the absence of CCX-CKR could conceivably result from alterations in thymocyte proliferation, apoptosis, or changes to thymocyte chemokine receptor expression. However, thymocytes from CCX-CKR−/− thymi recorded similar results to WT with respect to proliferation, apoptosis, and chemokine receptor expression (supplemental Figures 4 and 5). To determine why progenitor distribution in CCX-CKR−/− thymi was altered, we next analyzed chemokines that instruct cells to migrate between microenvironmental niches. In the thymus, this primarily involves CCL19, CCL21, and CCL25.9-13 ELISA analysis revealed a significant reduction in CCL19 and CCL25 levels in CCX-CKR−/− compared with WT thymi, normalized per milligram tissue, whereas no difference in the level of CCL21 between WT and CCX-CKR−/− thymi was observed (Figure 5A). CCL21 was primarily located in the medulla with diffuse staining in the adjacent cortex in both WT and CCX-CKR−/− thymi (Figure 5B). CCL21 pixel intensity analysis in thymic sections over an area composing the cortex, CMJ, and medulla allowed appreciable visualization of CCL21 distribution. These data show low levels in the cortex that rose steadily when approaching the medulla where CCL21 was highly abundant. However, differences between WT and CCX-CKR−/− thymi were not apparent with respect to CCL21 distribution (Figure 5B). In contrast, strikingly reduced cortical CCL25 was apparent in CCX-CKR−/− compared with WT thymi (Figure 5C). Quantitation of CCL25 distribution in WT thymi showed high levels in the cortex dropping sharply at the CMJ. Notably, CCX-CKR−/− thymi do not exhibit an appreciable change in CCL25 intensity comparing the cortex to medulla (Figure 5C). CCL19 was not detectable by IF of the thymus in our hands.
Decreased levels of chemokines and disruption to CCL25 distribution in CCX-CKR−/− thymi. (A) Levels of CCL19, CCL21, and CCL25 were measured by ELISA in thymus homogenates. Data are pooled from 3 independent experiments; n = 15 or 16. Two-tailed unpaired t test with Welch correction: *P = .0184, **P = .0014. Bars represent means. (B) Analysis of chemokine distribution in thymus sections stained with anti-CD44 cells (green) to indicate the medulla and anti-CCL21 (red). Visualization of CCL21 distribution (right) by plotting CCL21 pixel intensity versus distance from the CMJ. Representative IF images from 2 independent experiments: WT, n = 6; and CCX-CKR−/−, n = 6. (C) Same as panel B, but stained with anti-CCL25 (red). Representative IF images from 2 independent experiments: WT, n = 7; and CCX-CKR−/−, n = 9. CCL21 and CCL25 distribution data pooled from analysis of the corresponding sections with 2 fields of view per thymus. Scale bar represents 250 μm.
Decreased levels of chemokines and disruption to CCL25 distribution in CCX-CKR−/− thymi. (A) Levels of CCL19, CCL21, and CCL25 were measured by ELISA in thymus homogenates. Data are pooled from 3 independent experiments; n = 15 or 16. Two-tailed unpaired t test with Welch correction: *P = .0184, **P = .0014. Bars represent means. (B) Analysis of chemokine distribution in thymus sections stained with anti-CD44 cells (green) to indicate the medulla and anti-CCL21 (red). Visualization of CCL21 distribution (right) by plotting CCL21 pixel intensity versus distance from the CMJ. Representative IF images from 2 independent experiments: WT, n = 6; and CCX-CKR−/−, n = 6. (C) Same as panel B, but stained with anti-CCL25 (red). Representative IF images from 2 independent experiments: WT, n = 7; and CCX-CKR−/−, n = 9. CCL21 and CCL25 distribution data pooled from analysis of the corresponding sections with 2 fields of view per thymus. Scale bar represents 250 μm.
Thus, loss of CCX-CKR abolishes the typical CCL25 distribution likely responsible for guiding CCR9+ DN2 and DN3 cells to the outer cortex where normal thymocyte differentiation proceeds. As CCX-CKR has been demonstrated to be a chemokine scavenger,4,8 we expected that levels of CCL19, CCL21, and CCL25 would be increased in CCX-CKR−/− thymi. Although our findings to the contrary may suggest an alternative function of CCX-CKR in the thymus, the selective loss of cortical CCL25 prompted us to examine whether deletion of CCX-CKR impacted on cTECs, which are responsible for CCL25 production.
cTECs fail to expand in CCX-CKR−/− thymi
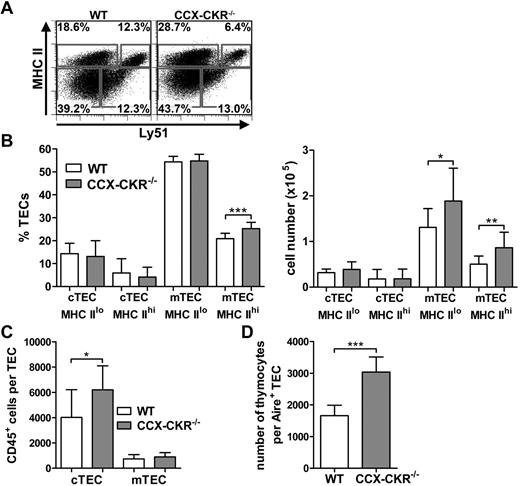
Thymocytes receive survival, differentiation, and migration signals from TECs during their time in the thymus. cTECs are the major source of CCL25, whereas mTECs produce CCL19 and CCL21.9 Having established that CCX-CKR is expressed principally by cTECs and that CCX-CKR deletion leads to reduced levels of CCL19 and CCL25 per milligram of tissue and an absence of cortical CCL25, we compared the proportions and numbers of TECs in WT and CCX-CKR−/− thymi. Analysis revealed that, although mTECs are present in proportions in accordance with the larger thymi in CCX-CKR−/− mice, cTECs are present in similar numbers to WT thymi (Figure 6A-B). Because of the larger DP and SP compartment, more thymocytes are programmed per cTEC in CCX-CKR−/− thymi compared with WT thymi (Figure 6C). Aire expression by TECs facilitates their expression of peripheral self- antigens and their ability to negatively select thymocytes that have a high affinity TCR for these self-antigens. CCX-CKR−/− thymi displayed a significant increase in the number of thymocytes per Aire+ TEC (Figure 6D).
Reduced cTEC to thymocyte ratio in CCX-CKR−/− thymi. (A) Representative dot plots of MHC II and Ly51 expression on CD45−EpCAM+ cells to delineate TEC populations: cTEClo, MHC IIloLy51+; cTEChi, MHC IIhiLy51+; mTEClo, MHC IIloLy51−; and mTEChi, MHC IIhiLy51−. Numbers adjacent to gates indicate percent positive in each. (B) Quantitation of TEC proportions (left) and numbers (right). Data pooled from 2 independent experiments ± SD: WT, n = 12; and CCX-CKR−/− = 12. Two-tailed unpaired t test (% mTEC MHC IIhi and number of mTEC MHC IIlo): *P = .0249, ***P = .0004. Two-tailed unpaired t test with Welch correction (number mTEC MHC IIhi): **P = .0048. (C) Quantitation of the number of CD45+ cells per cTEC and mTEC ± SD from the data in panel B. Two-tailed unpaired t test: *P = .0166. (D) Quantitation of the number of thymocytes per Aire+ TEC (right). Data from a single experiment ± SD; n = 5. Two-tailed unpaired t test: ***P = .0007.
Reduced cTEC to thymocyte ratio in CCX-CKR−/− thymi. (A) Representative dot plots of MHC II and Ly51 expression on CD45−EpCAM+ cells to delineate TEC populations: cTEClo, MHC IIloLy51+; cTEChi, MHC IIhiLy51+; mTEClo, MHC IIloLy51−; and mTEChi, MHC IIhiLy51−. Numbers adjacent to gates indicate percent positive in each. (B) Quantitation of TEC proportions (left) and numbers (right). Data pooled from 2 independent experiments ± SD: WT, n = 12; and CCX-CKR−/− = 12. Two-tailed unpaired t test (% mTEC MHC IIhi and number of mTEC MHC IIlo): *P = .0249, ***P = .0004. Two-tailed unpaired t test with Welch correction (number mTEC MHC IIhi): **P = .0048. (C) Quantitation of the number of CD45+ cells per cTEC and mTEC ± SD from the data in panel B. Two-tailed unpaired t test: *P = .0166. (D) Quantitation of the number of thymocytes per Aire+ TEC (right). Data from a single experiment ± SD; n = 5. Two-tailed unpaired t test: ***P = .0007.
Together, these data show that the increased cellularity of the CCX-CKR−/− thymus is not accompanied with increased proportions of cTECs in the absence of CCX-CKR. Therefore, as the DP and SP compartment is increased in CCX-CKR−/− mice, this may lead to fewer interactions between thymocytes and cTECs and/or Aire+ TECs, which may reduce the efficiency of thymocyte differentiation, impair negative selection, and provide an explanation for the increase in spontaneous autoimmune disease that occurs in the absence of CCX-CKR.
Discussion
The data presented in this manuscript provide novel insights into the biologic function of the atypical chemokine receptor CCX-CKR, demonstrating increased spontaneous autoimmunity in the submandibular glands and liver of aged mice, indicative of compromised self-tolerance. We show an important role for CCX-CKR in the regulation of chemokine distribution and thymic function. In this study, we confirm that CCX-CKR is expressed by cTECs, and we show that deletion of CCX-CKR alters the ratio of cTECs to thymocytes, which is associated with abnormal thymocyte differentiation and enlarged thymic architecture. Specifically, DP and SP cells are over-represented in CCX-CKR−/− thymi and DN2 cells accumulate in the medulla with a concurrent reduction in cortical DN3 cells in CCX-CKR−/− thymi. These migration defects are associated with profoundly reduced cortical CCL25 distribution in CCX-CKR−/− thymi.
Previous work in our laboratory has shown that CCX-CKR−/− mice develop earlier onset and a higher cumulative disease score in experimental autoimmune encephalomyelitis,8 indicating these mice are more susceptible to a triggered autoimmune disease. To investigate whether CCX-CKR−/− mice were more prone to spontaneous autoimmunity, we examined aged mice, which are known to have decreased thymic output and increased risk of spontaneous autoimmunity.33 The observed autoimmunity in the absence of CCX-CKR suggests that self-reactive thymocytes are more likely to escape negative selection. Further evidence that negative selection is compromised in CCX-CKR−/− thymi is provided by the increased abundance of CD3+CD4+CD8+ cells in the spleen, although whether these are DP cells that have escaped from the thymus or whether they are of extra-thymic origin as has previously been reported34 is unclear. Interestingly, nTregs are present in similar numbers in the lymph node and spleen of CCX-CKR−/− mice (data not shown) yet are not sufficient to limit the observed autoimmunity. The lymphocytic infiltrate observed in peripheral organs of CCX-CKR−/− mice is similar to that seen in CCR7−/− mice,16,22 demonstrating that self-reactive cells are not dependent on CCR7 to migrate to these organs. Migration signals may instead be initiated by CXCR3 and CXCR5 signaling.35-37 The infiltrates are similar in structure to ectopic lymphoid aggregates with distinct compartmentalization of cell subsets and closely resemble infiltrates seen in mouse models of Sjögren syndrome.38
Previous studies into the roles of chemokines in thymocyte differentiation have focused on the impact of chemokine receptor expression by thymocytes. In those studies, deletion of the active chemokine signaling receptors, CCR7 and CCR9, have highlighted the role they and their ligands, CCL19/21 and CCL25, respectively, play in T-cell development within the thymus. In contrast, our approach was fundamentally different in that we investigated the effect of deleting a novel chemokine regulatory receptor, CCX-CKR, which is expressed in the cortex by cTECs with minimal expression by other stromal cells and thymocytes. This approach left CCR7 and CCR9 intact but altered the levels and distribution of the signaling chemokines, CCL19 and CCL25. This has provided novel insights into the biologic function of this receptor and has allowed an assessment of the importance of CCX-CKR expression by cTECs and the downstream implications for thymocyte development. Our data provide interesting similarities and differences between the effect of deletion of CCX-CKR and CCR7 on T-cell development, thymic architecture, and function.
Deficiency of CCX-CKR leads to an accumulation of DN2 cells in the CMJ/medulla, similar to the phenotype of CCR7−/− mice,18 although some differences are apparent. For instance, the accumulation of DN2 cells in the CMJ/medulla is much more striking in CCX-CKR−/− thymi compared with CCR7−/− thymi, perhaps indicating that dysregulation (reduction in expression) of CCL19 and CCL25 has a cumulative negative effect, by impacting both CCR7 and CCR9, on the outward migration of DN2 cells. Our data suggest that DN2 cells will accumulate at the CMJ/medulla when both CCR7 and CCR9 are disrupted, although this is yet to be investigated in the CCR7/CCR9−/− mouse.
DN3 cells are proportionately reduced in CCX-CKR−/− thymi and less abundant in the cortex, similar to the observations made in CCR7-, CCR9-, and CCR7/CCR9-deficient thymi.15,18,25 These data suggest that activity of CCR7, CCR9, and their associated ligands is important for optimal differentiation of DN3 cells. Despite the abnormal development of DN1-DN3 populations in the chemokine receptor- and CCX-CKR–deficient mice, the DP population is able to recover to near normal abundance.15,18,20,21,39 Indeed, in CCX-CKR−/−, thymi, DP, and SP cells are expanded significantly compared with WT, correlating with the larger CCX-CKR−/− thymus, which potentially provides additional niches for DP and SP cells to develop. These data contrast with those obtained in CCR7−/−, CCR7/CCR9−/− thymi,15,18,20 and 1 report of CCR9−/− thymi,15 where a reduction in SP was observed. No differences in localization of DP and SP cells were noted in CCX-CKR−/− thymi, indicating that CCX-CKR does not influence the medullary migration of SP cells. This highlights another difference compared with CCR7−/−, which show reduced SP cell abundance in the medulla.14,16,17,19,20
The results of a study by Heinzel et al showed that DP and CD8 SP cell proportions, DP and SP cell localization and proliferation were similar in the thymi of WT and CCX-CKR−/− mice,27 findings in keeping with observations in the present study. However 3 main differences between our observations and those of Heinzel et al27 are apparent: (1) we identified CCX-CKR−/− thymi as being significantly larger than WT counterparts; (2) accumulation of DN2 cells in the medulla; and (3) reduced proportions and impaired homing of DN3 cells to the subcapsular zone in CCX-CKR−/− mice. Because of these differences between the 2 studies, we also conducted a number of duplicate experiments in CCX-CKR−/− mice at the University of Glasgow to control for environmental influences, with the same outcome (data not shown). Furthermore, analysis of CCR7−/− mice kept in identical conditions confirmed the previously reported phenotype, which differed from both WT and CCX-CKR−/−, further indicating our data are not an artifact of environmental conditions. Of note, the study performed by Heinzel et al27 examined the thymi of 1-week-old and 3- to 5-week-old CCX-CKR−/− mice compared with our 6- to 13-week-old mice. To rule out age as a contributing factor to the differences between the studies, we repeated the experiments in 3.5-week-old mice and replicated the phenotype observed in the present study (data not shown), implying that chemokine dysregulation and subsequent developmental abnormalities are also present in younger mice.
A surprising finding of the present study is that the relative levels of CCL21 in the thymus were unchanged and reduced levels of CCL19 and CCL25 were apparent in CCX-CKR−/− thymi despite our previous observations indicating that CCX-CKR negatively regulates the bioavailability of these chemokines in vivo.8 This is probably because of altered chemokine production in the CCX-CKR−/− thymus, which also have alterations in the frequencies of TECs, the main source of chemokine production in the thymus. Therefore, we also analyzed chemokine levels in the thymus on a per TEC basis (supplemental Figure 6). Whereas CCL19 levels are reduced per TEC, CCL21 levels were increased per TEC, suggesting differential production of these chemokines in CCX-CKR−/− thymi. Further, CCL25 production per TEC was not altered, but the increased cortical area of CCX-CKR−/− thymi likely contributes to the reduction in cortical CCL25 observed by IF. Thus, our observations suggest either that CCX-CKR does not act as a chemokine scavenger in the thymus and has an alternative function or that the scavenging effects are manifest much earlier during thymus development. With respect to the first possibility, cTEC-expressed CCX-CKR may be required for optimal cTEC development through an as yet unidentified CCX-CKR signaling pathway as the cTEC compartment was not increased in CCX-CKR−/− thymi, in contrast to mTECs, thymocytes, and dendritic cells (dendritic cell data not shown). However, against this possibility, no link between CCX-CKR and growth pathways has yet been identified. With respect to the second possibility, scavenging of chemokines by CCX-CKR in the fetal thymus may be required for subsequent normal cTEC growth and differentiation. Although the molecular basis for this is unclear, the results of previous studies have shown that altered thymocyte development can impact stromal cells via reciprocal crosstalk between stromal cells and thymocytes.40,41 Scavenging of chemokines by CCX-CKR may be required in the fetal thymus to establish appropriate migration signals that facilitate cTEC/thymocyte interactions. Therefore, in CCX-CKR−/− mice, cTEC growth and expansion may be disturbed as a result of defects at an early developmental stage relating to chemokine scavenging.
The differential effect of CCX-CKR−/− on the number of cTEC and mTEC raises 2 important issues. The first is that, despite larger thymi in CCX-CKR−/− mice, cTECs were not present in proportionately increased numbers. Similarly, although mTECs were increased, there was a reduction in potential interactions between thymocytes and Aire+ cells. This reduction in the threshold of interactions necessary between the developing thymocytes and TECs42 is insufficient numerically or functionally to enable establishment of normal self-tolerance with symptoms reminiscent of Sjögren syndrome developing. The other key point is the apparent dislocation of cTEC and mTEC expansion. This raises the question of their mechanisms of maintenance and developmental relationship from common or distinct stem/progenitor cells. A common TEC stem cell in the fetal thymus has been proposed to give rise to both cTECs and mTECs,43 whereas in the adult no such cell has been defined. Both mTECs and cTECs, however, can support their own respective self-renewal26,44 in the normal adult, maintaining a constant proportional ratio with cortical and medullary thymocytes. Removing CCX-CKR appears to functionality disrupt this, affecting primarily cTECs, supporting a model where cTEC and mTEC production and maintenance are independent. Regardless of the mechanism involved, it is probable that the relative reduction in the number of cTECs in the CCX-CKR−/− thymi plays a central role in the observed dysregulation of thymocyte development and that this leads to the spontaneous autoimmune phenotype observed. However, future studies incorporating cTEC-specific CCX-CKR deletion or transplantation of CCX-CKR–deficient thymi into immunocompromised mice will be required for definitive assessment of the functional significance of CCX-CKR in cTECs.
This study demonstrates, for the first time, that CCX-CKR expression by cTECs is important for thymic homeostasis. Deletion of CCX-CKR, a CCL19/21/25 regulatory receptor, results in a larger thymus with relatively fewer cTECs and is associated with severe reductions in cortical CCL25 distribution and accumulation of DN2 cells in the medulla. The downstream effects materialize in reduced proportions of DN3 cells and significantly reduced numbers of cortical DN3 cells. DP and SP cells are increased significantly in number, accounting for the increase in thymus mass and are likely responsible for the abnormal architecture of CCX-CKR−/− thymi. Aberrant thymocyte development in the absence of CCX-CKR culminates in increased prevalence of spontaneous autoimmune-like disease, characterized by lymphocytic infiltration of peripheral organs. Therefore, we demonstrate that expression of the atypical chemokine receptor CCX-CKR is required for normal thymocyte development and contributes to the control of spontaneous autoimmune disease.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Dale Godfrey (Department of Microbiology & Immunology, University of Melbourne) for advice on flow cytometric quantification of thymocyte subsets.
M.D.B. and S.R.M. were supported by the National Health and Medical Research Council and the Australian Research Council. I.C. was supported by MS Research Australia. N.S., M.V.H., and R.L.B. were supported by NH&MRC and Australian Stem Cell Center. D.L.A. and R.J.B.N. were supported by a Medical Research Council Program Grant.
Authorship
Contribution: M.D.B. designed and performed experiments, prepared figures, and wrote the manuscript; I.C. designed experiments and wrote the manuscript; N.S. and M.V.H. performed TEC analysis and quantitative PCR; N.S. contributed to manuscript preparation; D.L.A. and R.J.B.N. designed and performed the supporting study at the University of Glasgow; R.J.B.N. contributed to the manuscript preparation; H.K. contributed reagents; R.L.B. provided reagents and contributed to experimental design and manuscript preparation; and S.R.M designed experiments and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Iain Comerford, Chemokine Biology Laboratory, School of Molecular and Biomedical Science, Molecular Life Sciences Building, University of Adelaide, North Terrace Campus, Rm 5.11, 5005 South Australia, Australia; e-mail: iain.comerford@adelaide.edu.au; and Shaun R. McColl, Chemokine Biology Laboratory, School of Molecular and Biomedical Science, Molecular Life Sciences Building, University of Adelaide, North Terrace Campus, Rm 5.11, 5005 South Australia, Australia; e-mail: shaun.mccoll@adelaide.edu.au. Address requests for CCX-CKR−/− mice to Robert J. B. Nibbs, Institute of Infection, Immunity and Inflammation, University of Glasgow, Glasgow G11 6NT, United Kingdom; e-mail: robert.nibbs@glasgow.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal