Abstract
CD59 deficiency is a common finding in RBCs and WBCs in patients with chronic hemolysis suffering from paroxysmal nocturnal hemoglobinuria in which the acquired mutation in the PIGA gene leads to membrane loss of glycosylphosphatidylinositol-anchored membrane proteins, including CD59. The objective of the present study was to elucidate the molecular basis of childhood familial chronic Coombs-negative hemolysis and relapsing polyneuropathy presenting as chronic inflammatory demyelinating polyradiculoneuropathy in infants of North-African Jewish origin from 4 unrelated families. A founder mutation was searched for using homozygosity mapping followed by exome sequencing. The expression of CD59, CD55, and CD14 was examined in blood cells by flow cytometry followed by Western blot of the CD59 protein. A homozygous missense mutation, p.Cys89Tyr in CD59, was identified in all patients. The mutation segregated with the disease in the families and had a carrier rate of 1:66 among Jewish subjects of North-African origin. The mutated protein was present in the patients' cells in reduced amounts and was undetectable on the membrane surface. Based on the results of the present study, we conclude that the Cys89Tyr mutation in CD59 is associated with a failure of proper localization of the CD59 protein in the cell surface. This mutation is manifested clinically in infancy by chronic hemolysis and relapsing peripheral demyelinating disease.
Key Points
A novel clinical syndrome is reported which is triggered by common febrile episodes in infancy and presents with Coombs' neg hemolysis and demyelineating polyneuropathy.
A gene mutation in CD59 leading to loss of expression of CD59 on the cell surface is presented as the genetic basis for the disease.
Introduction
CD59 deficiency is a common finding in adult patients with paroxysmal nocturnal hemoglobinuria (PNH). In this condition, there is a clonal expansion of hematopoietic stem cell which acquired a mutation in the PIGA gene. PIGA encodes a glycosylphosphatidylinositol (GPI) biosynthesis protein, and erythrocytes deficient in GPI-anchored membrane proteins, including CD59, undergo a complement-mediated hemolysis.1-3
Chronic immune-mediated neuropathies are rare in infancy and early childhood. The most common disease of these entities is chronic inflammatory demyelinating polyradiculoneuropathy (CIDP), an acquired immune-mediated disorder characterized by progressive or relapsing weakness and sensory symptoms and signs. The estimated prevalence of CIDP is 1-9/100 000 among adults and 0.48/100 000 in children, with a male preponderance at all ages. CIDP in childhood typically manifests as lower limb weakness and/or ataxia; few affected children present with sensory symptoms, but sensory findings are common. Subacute onset and relapses are more common in children than in adults and the course may be monophasic, relapsing-remitting, slowly progressive, or stepwise progressive. A history of previous intercurrent infection can be elicited in approximately half of patients and immunization may lead to relapse. An indication for the inflammatory nature of the disease is the increased level of cerebrospinal fluid protein and the major neuroimaging findings including thickened, hyperintense nerve roots, trunks, or plexi. Demyelination is the ultimate result of CIDP, with slowing of nerve conduction in various segments culminating in conduction blocks. Although children with CIDP have a favorable response to immunomodulation, the overall prognosis is variable; many patients improve and even recover, but a subset remains with significant neurologic deficits that may lead to death.4-11
Although childhood CIDP is a distinctive entity, there are clinical variants in which CIDP research criteria cannot be met but which do respond to immune modulating therapy. In the present study, we detected CD59 deficiency in 5 infants from 4 unrelated families of North-African Jewish origin with chronic hemolysis accompanied by sensory-motor, demyelinating, or axonal peripheral polyneuropathy. The children experienced a relapsing progressive course with improvement in upper limb muscle strength after immune modulation, mimicking CIDP.
Methods
Case reports
The subjects of this study were 5 patients, 3 male and 2 female, 1-4.5 years of age and members of 4 unrelated families. All parents were of North-African Jewish extraction. In only 1 family were the parents aware of being consanguineous. The pedigrees for all identified patients are presented in Figure 1 and detailed clinical symptoms and findings are presented in Table 1. The disease onset was at 3-7 months and was usually preceded by a minor infectious, mostly viral illness. The major symptoms were symmetric muscle weakness accompanied by hypotonia and absent tendon reflexes involving the legs more than the arms. The first episode lasted from days to weeks and treatment with IV Igs and pulse or oral corticosteroids was associated with regain of muscle strength in the upper extremities. In the following months the course was relapsing-remitting, with acute or subacute aggravation of weakness after intercurrent infections. Between episodes, upper limb strength and function were partially regained in a proximal to distal gradient, but there was a progressive muscle wasting involving the feet and the hands and permanent areflexia accompanied by persistent flaccid paralysis of the lower limbs. Few of the exacerbations were accompanied by respiratory insufficiency requiring artificial ventilation. The CNS was not involved and the patients' cognitive level was age appropriate. During a typical episode, the C-reactive protein level was high and blood hemoglobin level decreased to 6-10 Gr% with marked reticulocytosis, negative Coombs test, increased lactate dehydrogenase level (peak level, 1306 IU/L; < 260 IU/L in healthy controls) and low haptoglobin, consistent with acute hemolysis. Blood smear revealed polychromasia. The hemolysis was characterized by chronic course with paroxysmal episodes. RBC transfusion was occasionally required. Patient 2888 had a single episode of acute renal failure, hemoglobinuria, thrombocytopenia, and intravascular hemolysis during a plasmapheresis session. These symptoms, consistent with a hemolytic-uremic–like syndrome, resolved spontaneously with complete normalization of renal function. Cerebrospinal fluid protein level was increased in all patients and nerve-conduction studies revealed demyelination of sensory and motor nerves with axonal damage in 2 of 3 patients. Magnetic resonance imaging of the spine performed in 3 patients revealed root enhancement in 2 patients. Ongoing corticosteroid, IV Ig, rituximab, and cyclosporine therapy did not prevent recurrences but did affect relapse length and severity. Plasmapheresis seemed to be clinically beneficial in 2 patients, but may have been related to the development of a hemolytic-uremic–like syndrome. At the time of this writing, 4 patients remain alive, the oldest 5 years of age, and 1 patient has died of acute respiratory failure at 3.5 years.
Clinical, hematologic, biochemical, electrophysiologic, and radiologic findings of the patients
| Patient no. . | 2888 . | 5078 . | 5119 . | 2962 . | 3316 . |
|---|---|---|---|---|---|
| Age of onset, mo | 3.5 | 7 | 3 | 3 | 4 |
| Family origin | Libyan Jewish | Libyan Jewish | Libyan/Moroccan Jewish | Moroccan/Egyptian Jewish | Libyan Jewish; parents first cousins |
| Family history | Affected sibling | Affected sibling | Negative | Negative | Negative |
| Hemoglobin, g/dL | 6.0-11 | 7.8-12 | 8.0-11.8 | 7.8-11.8 | 7.7-11.5 |
| WBCs, × 109/L | 5500 | 7200 | 5600 | 5400 | 7500 |
| Platelets, ×109/L | 405.0 | 370.0 | 560.0 | 380.0 | 373.0 |
| Reticulocyte count, % | 5.4 | 5.7 | 5.85 | 4.5 | 4.95 |
| LDH, U/L (normal, 240-480) | 1030 | 885 | 920 | 1192 | 770 |
| Haptoglobin, mg/dL (normal, 30-200) | NA | 4.7 | 3.23 | 5.3 | 4.1 |
| Total bilirubin, mol/L (normal, 0-17) | 6.0 | 5.8 | 6.1 | 6.2 | 4.2 |
| Direct Coombs test | |||||
| Other systemic manifestations | Hemolytic-uremic syndrome–like disease during plasmapheresis | No | No | Communication difficulties | No |
| Nerve conduction studies | Sensory motor demyelination with secondary axonal damage | Normal sensory conduction motor demyelination with secondary axonal damage | Sensory motor demyelinating with secondary axonal damage | Sensory/motor axonal neuropathy | Sensory motor demyelination with secondary axonal damage |
| Cerebrospinal fluid protein, control < 40 mg/100 mL | 140 | 68 | 113 | 8240 | 70 |
| Spine magnetic resonance findings | NA | NA | Roots enhancement | Roots enhancement | Normal imaging |
| Nerve biopsy | EM: thinning of myelin in several fibers (demyelinization ); no inflammatory infiltrates | NA | Mild reduction in fiber number; axonal degeneration in a few fibers | Demyelination with some axonal loss | NA |
| Previous therapies | CS, HIG, PE, cyclosporine, rituximab | CS, HIG | CS, HIG | CS, HIG | CS, HIG |
| Response to therapy | Partially positive in upper limbs; loss of effect with time | Partially positive in upper limbs. | Partially positive in upper limbs | Partially positive in upper limbs | Partially positive in upper limbs |
| Current age, mo | Died at 3.5 y of age | 18 | 13 | 54 | 55 |
| Functional level | Flaccid paralysis of lower limbs; rolls over; sits with support; moderate weakness in hands | Flaccid paralysis of lower limbs; sits with support; severe paralysis of hands with slight movement of palms and right shoulder | Walks with orthoses; bilateral drop foot; mild weakness in hands | Stands on knees; stands with orthoses; mild to moderate weakness in hands |
| Patient no. . | 2888 . | 5078 . | 5119 . | 2962 . | 3316 . |
|---|---|---|---|---|---|
| Age of onset, mo | 3.5 | 7 | 3 | 3 | 4 |
| Family origin | Libyan Jewish | Libyan Jewish | Libyan/Moroccan Jewish | Moroccan/Egyptian Jewish | Libyan Jewish; parents first cousins |
| Family history | Affected sibling | Affected sibling | Negative | Negative | Negative |
| Hemoglobin, g/dL | 6.0-11 | 7.8-12 | 8.0-11.8 | 7.8-11.8 | 7.7-11.5 |
| WBCs, × 109/L | 5500 | 7200 | 5600 | 5400 | 7500 |
| Platelets, ×109/L | 405.0 | 370.0 | 560.0 | 380.0 | 373.0 |
| Reticulocyte count, % | 5.4 | 5.7 | 5.85 | 4.5 | 4.95 |
| LDH, U/L (normal, 240-480) | 1030 | 885 | 920 | 1192 | 770 |
| Haptoglobin, mg/dL (normal, 30-200) | NA | 4.7 | 3.23 | 5.3 | 4.1 |
| Total bilirubin, mol/L (normal, 0-17) | 6.0 | 5.8 | 6.1 | 6.2 | 4.2 |
| Direct Coombs test | |||||
| Other systemic manifestations | Hemolytic-uremic syndrome–like disease during plasmapheresis | No | No | Communication difficulties | No |
| Nerve conduction studies | Sensory motor demyelination with secondary axonal damage | Normal sensory conduction motor demyelination with secondary axonal damage | Sensory motor demyelinating with secondary axonal damage | Sensory/motor axonal neuropathy | Sensory motor demyelination with secondary axonal damage |
| Cerebrospinal fluid protein, control < 40 mg/100 mL | 140 | 68 | 113 | 8240 | 70 |
| Spine magnetic resonance findings | NA | NA | Roots enhancement | Roots enhancement | Normal imaging |
| Nerve biopsy | EM: thinning of myelin in several fibers (demyelinization ); no inflammatory infiltrates | NA | Mild reduction in fiber number; axonal degeneration in a few fibers | Demyelination with some axonal loss | NA |
| Previous therapies | CS, HIG, PE, cyclosporine, rituximab | CS, HIG | CS, HIG | CS, HIG | CS, HIG |
| Response to therapy | Partially positive in upper limbs; loss of effect with time | Partially positive in upper limbs. | Partially positive in upper limbs | Partially positive in upper limbs | Partially positive in upper limbs |
| Current age, mo | Died at 3.5 y of age | 18 | 13 | 54 | 55 |
| Functional level | Flaccid paralysis of lower limbs; rolls over; sits with support; moderate weakness in hands | Flaccid paralysis of lower limbs; sits with support; severe paralysis of hands with slight movement of palms and right shoulder | Walks with orthoses; bilateral drop foot; mild weakness in hands | Stands on knees; stands with orthoses; mild to moderate weakness in hands |
LDH indicates lactate dehydrogenase; CS, corticosteroids; HIG, human Ig; PE, plasmapheresis; and NA, not available.
Linkage analysis
Homozygous regions were searched for in the DNA samples of patients 5078 and 2888 using Affymetrix GeneChip Human Mapping 250K Nsp Array as described previously.4 The homozygous regions in the patients' samples were identified by manual inspection and were compared with those identified in the DNA sample of their healthy sister, subject 5079. This study was approved by the Hadassah Medical Center and was conducted in accordance with the Declaration of Helsinki.
Whole-exome sequencing and bioinformatics analysis
The DNA sample of patient 2888 was enriched with the SureSelect Human All Exon v.2 Kit that targeted 44 Mb (Agilent Technologies). Sequencing was carried out on a HiSeq2000 (Illumina) as 100-bp paired-end runs. Image analysis and base calling were performed with the Genome Analyzer Pipeline Version 1.5 with default parameters. Read alignment and variant calling were performed with the October 2012 release of DNAnexus software using the default parameters with the human genome assembly hg19 (GRCh37) as a reference.
CD59, CD55, and CD14 expression in blood cells
Materials.
The cell culture medium consisted of RPMI 1640 (Invitrogen-Gibco) supplemented with 1% l-glutamine and 1% penicillin/streptomycin (Biologic Industries). Mouse anti–human CD59-PE was from Invitrogen and mouse anti–human CD55-FITC and CD14 were from BioLegend. The gel-agglutination PNH test was from Bio-Rad.
Cell isolation and culturing.
Blood mononuclear cells (MNCs) were isolated from fresh blood obtained from patients, patient's parents, and healthy donors who signed an informed consent. Blood was collected into lithium-heparin vacuum tubes. RBCs were sedimented by adding 6% hetastarch in 0.9% NaCl solution (STEMCELL Technologies) and kept at 25°C for up to 25-30 minutes. The leukocyte-rich upper layer of the suspension was then collected and centrifuged on a density gradient with Ficoll-Paque (Amersham Biosciences). At the end of centrifugation, plasma, MNCs, and neutrophils were separated. Residual erythrocytes were removed by hypotonic lysis. Cells were > 95% purified as determined by morphologic analysis and were > 99% viable as determined by Trypan blue dye (Beit-Haemek) exclusion. Cells were collected, washed, and analyzed by flow cytometry for CD59, CD55, and CD14.
Generation of lymphoblast cell lines.
MNCs were isolated from patient and control donor peripheral blood as described in the previous paragraph. Cells were then transformed with EBV (ATCC) for 1 hour, washed and cultured in RPMI 1640 medium, and supplemented with 20% heat-inactivated FBS, 2mM l-glutamine, and 1% penicillin/streptomycin antibiotics. Cells were then monitored daily for viability with Trypan blue staining and media were replaced once a week. On reaching a stable cell-division rate, cells were passaged 2-3 times a week.
Western blotting.
Transformed lymphoblasts from patients and healthy controls were lysed using RIPA lysis buffer (150mM NaCl, 50mM Tris, pH 8.0, 0.1% SDS, 0.5% sodium deoxycholate, and 1% Triton X-100; Sigma-Aldrich). Total cell lysates were treated with Laemmli sample buffer, boiled to 85°C degrees for 10 minutes, and analyzed with SDS-PAGE 15% separating gel (1.5 Tris-HCl, pH 8.3, 40% acrylamide/bisacrylamide 1:37.5; Sigma-Aldrich). Proteins were then transferred to a nitrocellulose membrane (Millipore). Membrane was blocked using 20% skim milk solution (BD Biosciences). CD59 was traced using monoclonal mouse anti–human CD59 (Serotec) and anti–mouse IgG horseradish peroxidase (Promega), followed by an enhanced chemiluminescence reaction (Biologic Industries). Membranes were exposed to UV irradiation and photographed using an LAS3000 imaging system (Fuji).
Immunohistochemical staining for CD59.
CD59 was traced using monoclonal mouse anti–human CD59 (Serotec, concentration 1:1000) on paraffin sections of a sural nerve biopsy from patient 2888.
Results
Identification of the CYS89TYR mutation in the CD59 gene
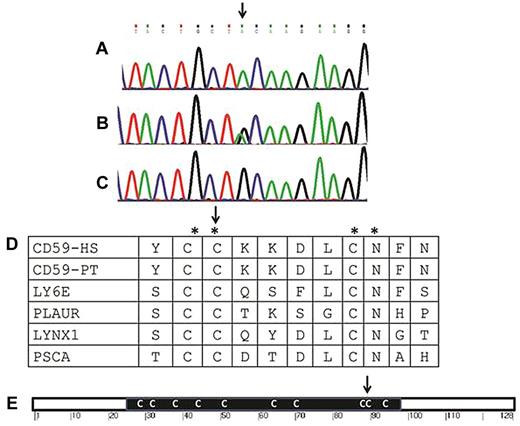
The search for homozygous regions in the DNA samples of the siblings patients 5078 and 2888 and the exclusion of those regions shared by their healthy sister, subject 5079, disclosed only 2 homozygous regions larger than 2 Mb: chr1:68.16-81.66 and chr9:93.44-98.75 (numbering according to HG19). Because the regions contained 91 protein-coding genes and none of them appeared as an immediate candidate, we opted for whole-exome sequencing. Using this analysis, a total of 19 564 single-nucleotide polymorphisms (SNPs) and indels were detected within the targeted coding sequence. We removed those covered less than 8 times; those present in dbSNP129 or in the inhouse database; and heterozygous, synonymous, and chromosome X variants. Thirty-nine changes survived this filtering process, but only one of them, chr11:33731793 C → T segregated with the disease state, being present within a genomic region where both patients, but not their healthy sister, shared a common haplotype. This region on chr11:32.29-34.38 Mb (rs6484562-rs2611134) escaped our initial homozygosity search because of its marginal size and because 7 of the encompassed 176 SNPs were heterozygous. The chr11:33731793 C → T variant corresponds to c.266 G → A in exon 3 of CD59 and results in the amino acid substitution p.Cys89Tyr (Figure 2). We genotyped the other 3 CIDP infants (patients 5119, 2962, and 3316) and identified homozygosity for the mutation in all of them. Using DNA SNP chip analysis in these 3 patients, we detected homozygous regions and identical haplotype around the mutation site, ranging from chr11:24.80-40.32 Mb in patient 3316 to chr11:32.30-33.49 Mb in patient 5119, suggesting that the Cys89Tyr mutation in CD59 is a founder mutation in Jewish subjects of North-African ancestry. Sequence determination of exon 3 of CD59 in 197 anonymous Jewish subjects of North-African origin identified 3 carriers, indicating a carrier rate of 1:66 in this community. The mutation was not found in the presently 5379 exomes from healthy subjects available through the Exome Variant Server of the National Heart, Lung, and Blood Institute Exome Sequencing Project (http://evs.gs.washington.edu/EVS/).
Genomic and protein sequence around the mutation. (A-C) Sequence of exon 3 of CD59 around the Cys89Tyr mutation (arrow) in a patient (A), a carrier (B), and a healthy control (C). (D) Amino acid conservation around the mutation site (CD59-HS, human CD59). The region is not only conserved among the Eutheria (eg, in the CD59 protein of Pan troglodytes, CD59-PT), but also among other LY-6 domain–containing proteins in humans, including the human lymphocyte antigen 6E (LY6E), the human urokinase plasminogen activator surface receptor (PLAUR), the ly-6/neurotoxin-like protein 1 (LYNX1), and the prostate stem cell antigen (PSCA). The conserved residues are marked by an asterisk and the mutated codon by an arrow. (E) Schematic representation of the CD59 protein. The black rectangle stands for the LU domain with its multiple Cys residues (C). The site of the mutation (Cys89) of our patients is shown (arrow).
Genomic and protein sequence around the mutation. (A-C) Sequence of exon 3 of CD59 around the Cys89Tyr mutation (arrow) in a patient (A), a carrier (B), and a healthy control (C). (D) Amino acid conservation around the mutation site (CD59-HS, human CD59). The region is not only conserved among the Eutheria (eg, in the CD59 protein of Pan troglodytes, CD59-PT), but also among other LY-6 domain–containing proteins in humans, including the human lymphocyte antigen 6E (LY6E), the human urokinase plasminogen activator surface receptor (PLAUR), the ly-6/neurotoxin-like protein 1 (LYNX1), and the prostate stem cell antigen (PSCA). The conserved residues are marked by an asterisk and the mutated codon by an arrow. (E) Schematic representation of the CD59 protein. The black rectangle stands for the LU domain with its multiple Cys residues (C). The site of the mutation (Cys89) of our patients is shown (arrow).
Characterization of the mutant CD59 protein
To elucidate the effect of the Cys89Tyr mutation on the expression of CD59 protein, we examined RBCs from 3 of the 4 living patients. This analysis revealed a lack of expression of the CD59 protein on the RBC membrane of the patients compared with the normal expression of CD55 (Figure 3). Similarly, CD59 was not expressed on monocytes and lymphocytes from the patients, but the expression of CD55 (Figure 3) and CD14 (not shown) was normal. The parents who carried the mutation had normal expression of the CD59 protein on their RBC membrane. Sural nerve biopsy showed no expression of CD59 in patient 2888 (Figure 4), and Western blot analysis disclosed a reduced amount of the CD59 protein in all the subjects, suggesting that the protein is synthesized but fails to reach the membrane (Figure 5). Because the mutation involves a residue that participates in the formation of a disulfide bond, it is possible that the tertiary structure of the protein is affected, leading to abnormal processing of the precursor protein or disrupting the attachment of the protein to the GPI anchor.
The expression of CD59 and CD55 proteins in RBCs and MNCs. RBCs and MNCs from patients (represented in the figure by patient 5119), carriers of the Cys89Tyr mutation, and a normal control were stained with anti–CD59-FITC and anti–CD-55-PE. Top panel shows RBCs costained with anti–CD59-PE and anti–CD-55-FITC. The percentages of CD-59+ cells are indicated in the right top quadrant together with mean fluorescence (MF). Bottom panel shows MNCs (left) of patient 5119 and RBCs of patients 2962 and 5078 (middle and right) stained for anti–CD59-PE.
The expression of CD59 and CD55 proteins in RBCs and MNCs. RBCs and MNCs from patients (represented in the figure by patient 5119), carriers of the Cys89Tyr mutation, and a normal control were stained with anti–CD59-FITC and anti–CD-55-PE. Top panel shows RBCs costained with anti–CD59-PE and anti–CD-55-FITC. The percentages of CD-59+ cells are indicated in the right top quadrant together with mean fluorescence (MF). Bottom panel shows MNCs (left) of patient 5119 and RBCs of patients 2962 and 5078 (middle and right) stained for anti–CD59-PE.
Results of immunohistochemical staining for CD59. Shown are results of immunohistochemical staining (concentration 1:1000) on paraffin sections of a sural nerve biopsy (original magnification, 60×) from a control subject (A) and from patient 2888 (B). There is endothelial (cytoplasmic-luminal pattern) staining of epineurial blood vessels (A; black arrow) in the control subject, whereas is a complete lack of vascular staining in the patient (B; black arrow).
Results of immunohistochemical staining for CD59. Shown are results of immunohistochemical staining (concentration 1:1000) on paraffin sections of a sural nerve biopsy (original magnification, 60×) from a control subject (A) and from patient 2888 (B). There is endothelial (cytoplasmic-luminal pattern) staining of epineurial blood vessels (A; black arrow) in the control subject, whereas is a complete lack of vascular staining in the patient (B; black arrow).
Western blot of the CD59 protein. CD59 was detected by monoclonal mouse anti–human CD59 (Serotec) and anti–mouse IgG horseradish peroxidase (Promega) followed by an enhanced chemiluminescence reaction (Biological Industries) in lymphoblasts. Four patients and a normal control are presented. Actin, as a housekeeping gene, is shown at the bottom.
Western blot of the CD59 protein. CD59 was detected by monoclonal mouse anti–human CD59 (Serotec) and anti–mouse IgG horseradish peroxidase (Promega) followed by an enhanced chemiluminescence reaction (Biological Industries) in lymphoblasts. Four patients and a normal control are presented. Actin, as a housekeeping gene, is shown at the bottom.
Discussion
CD59 encodes a 128–amino acid GPI-anchored cell surface glycoprotein. Its major functional determinant is the Cys-rich LU (LY-6) domain (Figure 2E) where the conserved Cys89 residue resides, generating an intramolecular disulfide bond. Cys residues in 2 other Ly6 proteins, GPIHBP1 and SLURP-1, are also critical for protein function and missense mutations involving conserved Cys residues of the Ly6 domain in these proteins were shown to drastically affect protein function.13,14 The CD59 protein inhibits the final step of membrane attack complex (MAC) formation. MAC is a C5b-9 complex that is generated after activation of the complement system. It is formed by the sequential binding of C5b to C6, C7, C8, and multiple C9 molecules. The insertion of C8 and C9 into lipid bilayers causes cell lysis. Host cells are protected from MAC-mediated injury by CD59, which binds to C8 and C9 and prevents pore formation.15 Therefore, CD59, also called protectin, protects cells from complement-mediated lysis, as its name implies. The results of the functional analysis in the present study indicated that the Cys89Tyr mutation does not interfere with CD59 biosynthesis but likely perturbs its transport to its site of action, the membrane surface.
CD59 deficiency is a common finding in adult patients with PNH. In this condition, there is a clonal expansion of hematopoietic stem cells that acquired a mutation in the PIGA gene. PIGA encodes a GPI biosynthesis protein and erythrocytes deficient of GPI-anchored membrane proteins, including CD59, undergo a complement-mediated hemolysis. Interestingly, it was shown previously that CD59 deficiency is the main factor leading to susceptibility of PNH erythrocytes to lysis16 and that, although CD55 and CD59 act in concert to control susceptibility to acidified serum lysis, CD59 is the most important.17,18 As expected, in the present study, our 4 subjects had normal expression of CD55; however, consistent with the lack of CD59 on their RBCs, all of them had evidence of chronic and paroxysmal Coombs-negative hemolysis manifested by lower hemoglobin, high lactate dehydrogenase, and low haptoglobin (Table 1). CD59-deficient mice have been shown to suffer from mild intravascular hemolysis.19
Primary CD59 deficiency in humans has been reported only once, and that patient suffered from hemolytic anemia and recurrent cerebral infarction.20 The disease onset was in adolescence and peripheral nervous system involvement was not reported at 22 years of age. The primary molecular defect in the patient was reported to be homozygosity for 2 separate single nucleotide deletions in the gene, resulting in a frameshift mutation initiated at codon 34.21 Activation of MAC has been suggested to mediate demyelination in both the central and peripheral nervous systems, and deposits of MAC were observed in areas of demyelination in multiple sclerosis, Guillain-Barré syndrome, and CIDP.22-24 Furthermore, an inability to form MAC, as seen in C6-deficient rats, was associated with attenuation of demyelination and axonal injury in the Ab-mediated demyelinating form of experimental autoimmune encephalomyelitis model. Deficiency of CD59 in mice rendered all mutant animals susceptible to experimental autoimmune encephalomyelitis, compared with only 12%-42% of wild-type animals. The inflammatory disease and axonal loss were more severe in the mutant animals and demyelination was present only in the mutants, leaving the animals severely paralyzed. MAC deposits, which are abundant in C9, were found on perivascular tissue elements in the areas of demyelination.25 These findings suggest that CD59 deficiency is associated with demyelination via MAC activation. Alternatively, the accumulation of unprocessed CD59 in neural or Schwan cells causing endoplasmic reticulum stress might be part of the pathogenesis, and it might also be possible that the mutation for the neurologic phenotype is an unknown mutation cosegregating with the CD59 mutation.
We conclude that the Cys89Tyr mutation in CD59 is associated with a failure of the proper localization of CD59 protein to the cell surface. This is clinically manifested in infancy by chronic hemolysis and relapsing peripheral demyelinating disease. In view of the data obtained in the CD59-deficient animals and in the presented patients, it is plausible that MAC is a major driver of myelin and axonal damage in early onset CIDP and possibly other demyelinating diseases and treatment with eculizumab or other complement inhibitors should be considered.
Gene discovery is an essential starting point both for understanding the genetic mechanisms underlying diseases and for providing clues to therapeutic approaches. The results of the present study underscore the usefulness of linkage analysis followed by exome sequencing for the elucidation of the underlying molecular basis in rare monogenic disorders.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Noa Cohen, Rachel Dahan, Mali Romano, and Michal Meimon for excellent technical assistance.
Authorship
Contribution: Y.N. and B.B.-Z. provided clinical identification of patients and wrote the clinical section of the manuscript; A.T. performed the research and analyzed and described the data; R.S., Y.A., Z.S., A.F.-V., S.A., and M.R. provided clinical identification of patients and wrote the data section of the manuscript; A.T.-S., S.Z., and A.S. performed the research and analyzed the data; H.G. and Y.F. performed the pathology staining and interpretation; D.M. provided the clinical evaluations, performed the research, analyzed the data, wrote the research section of manuscript, and supervised the writing; and O.E. designed the genetics approach, performed the research, analyzed the data, wrote the research section of manuscript, and supervised the writing.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dror Mevorach, MD, Rheumatology Research Center, Department of Medicine, Hadassah-Hebrew University Medical Center, Jerusalem, 91120 Israel; e-mail: mevorach@hadassah.org.il; or Orly Elpeleg, MD, Monique and Jacques Roboh Department of Genetic Research, Hadassah-Hebrew University Medical Center, Jerusalem, 91120 Israel; e-mail: elpeleg@hadassah.org.il.
References
Author notes
Y.N. and B.B.-Z. contributed equally to this work.
D.M. and O.E. contributed equally to this work.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal