Abstract
Natural regulatory T cells (Tregs) participate in responses to various chronic infections including HIV. HIV infection is associated with a progressive CD4 lymphopenia and defective HIV-specific CD8 responses known to play a key role in the control of viral replication. Persistent immune activation is a hallmark of HIV infection and is involved in disease progression independent of viral load. The consequences of Treg expansion, observed in HIV infection, could be either beneficial, by suppressing generalized T-cell activation, or detrimental, by weakening HIV-specific responses and thus contributing to viral persistence. The resulting balance between Tregs contrasting outcomes might have critical implications in pathogenesis. Topics covered in this review include HIV-induced alterations of Tregs, Treg cell dynamics in blood and tissues, Treg-suppressive function, and the relationship between Tregs and immune activation. This review also provides a focus on the role of CD39+ Tregs and other regulatory cell subsets. All these issues will be explored in different situations including acute and chronic infection, antiretroviral treatment-mediated viral control, and spontaneous viral control. Results must be interpreted with regard to both the Treg definition used in context and to the setting of the disease in an attempt to draw clearer conclusions from the apparently conflicting results.
Introduction
Human immunodeficiency virus (HIV) infection is associated with a progressive depletion of CD4 T lymphocytes and defective HIV-specific T-cell responses. Persistent immune activation plays a central role in driving CD4 T-cell depletion and progression to AIDS.1,2 HIV-specific CD8 and CD4 T-cell responses play a crucial role, from the time of primary infection, in the control of early viremia and in the maintenance of viral set point.3-5 CD4+ natural regulatory T cells (nTregs) express high levels of CD25 (α chain of IL-2 receptor) and the nuclear transcription factor forkhead box P3 (FoxP3).6-8 In addition, nTregs were reported to down-modulate the membrane expression of the α chain of IL-7 receptor, CD1279 and to express markers associated with regulatory function such as CTLA-4 and the glucocorticoid-induced TNFR-related (GITR) protein.10-12 Natural Tregs are able to suppress antigen-specific T-cell responses against a variety of pathogens and may also control inappropriate or exaggerated immune activation, thus limiting immune-mediated tissue damages but allowing pathogen persistence.13 Hence, nTregs may influence the outcome of various infections including hepatitis C virus, herpes simplex virus, Helicobacter pylori and also Leishmania.14
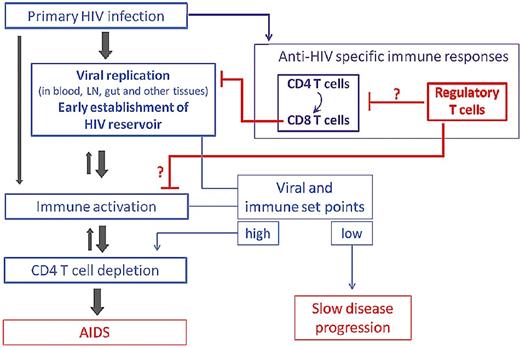
Natural Tregs are detected in peripheral blood and in mucosal and lymphoid tissues of HIV-infected patients and may contribute to immune deficiency and/or, conversely, be beneficial through a decrease in immune activation, depending on the stage of the disease (ie, level of viral replication and of immune activation in primary vs chronic infection; Figure 1). Although most studies focused on nTregs, other regulatory cell subsets may also play a role in the pathogenesis of HIV infection. The present review will focus mainly on the interrelationship between HIV, natural Tregs, and the host immune response in humans.
Potential roles for Tregs in the natural history of HIV infection. Tregs may contribute to immune deficiency by inhibiting HIV-specific T-cell responses involved in the control of HIV replication. Conversely, Tregs might be beneficial through a decrease in immune activation, known to independently predict disease progression.
Potential roles for Tregs in the natural history of HIV infection. Tregs may contribute to immune deficiency by inhibiting HIV-specific T-cell responses involved in the control of HIV replication. Conversely, Tregs might be beneficial through a decrease in immune activation, known to independently predict disease progression.
HIV-induced alterations of Treg cells
Susceptibility of Tregs to HIV infection
CD4 T cells expressing the major CCR5 coreceptor are the main HIV target cells. Peripheral Tregs were found to express similar CCR5 levels compared with bulk CD4 T cells.15 Moreover, following TCR activation, naive CD45RA+ Tregs up-regulate membrane expression of the HIV coreceptors CCR5 and CXCR4.16 Thus, Tregs are susceptible to HIV infection in vitro. Whether nTregs are more susceptible to HIV than conventional CD4 T cells has been debated. Oswald-Richter et al first suggested that Tregs might be the preferential target cells for HIV rather than memory CD4 T cells.17 Naive CD45RA+ Tregs might be more infected by HIV compared with naive CD4+ T cells but they remained less infected than memory CD4 T cells.16 Subsequent data reported on lower expression of HIV-DNA in CD4+CD127low T cells compared with conventional CD4+CD127hi T cells.18 Tregs were found to be more susceptible to R5 viruses than effector T cells while the opposite was observed using X4 strains. Finally, in vivo studies demonstrated that circulating CD25+ Tregs are not preferentially infected by HIV compared with effector T cells.19 In addition, viral rebound and immune activation following antiretroviral treatment interruption resulted in Treg expansion rather than a preferential depletion of Tregs.20
Interestingly, the transcription factor FoxP3, critical for Treg development and function, was shown to modulate the HIV-1 promoter's transcription activity. Although FoxP3 was shown to enhance HIV transcription in one study performed with the Jurkat T-cell line,21 foxp3 transfection was found to inhibit HIV transcription in primary human CD4 T cells.22,23 HIV transcription down-regulation could be dependent on NFκB-binding sites within the HIV enhancer22 or be mediated by the decrease of NFAT2 binding to the HIV promoter.23 Thus, permissiveness to HIV replication in Tregs might be reduced, partly as a result of FoxP3-mediated inhibition of HIV-1 transcription.
Induction/expansion of Tregs by HIV
Most studies have reported a relative increase in Treg frequency among CD4 T cells regardless of phenotype used (see next section). Treg expansion could solely be the consequence of exaggerated immune activation, but other mechanisms have also been proposed to explain this relative increase in Treg frequency. Naturally arising regulatory T cells are continuously produced in the normal thymus. An increase in the proportion of CD4+CD25+FoxP3+ cells in HIV-infected thymus may suggest enhanced Treg cell generation.24 Increased Treg frequency could also result from increased survival of Tregs. Thus, human Tregs were reported to be less sensitive to TCR restimulation in vitro compared with CD4+CD25neg T cells, suggesting a relative resistance to activation-induced cell death.25 In addition, Nilsson et al showed that CD4 T cells exposed to inactivated HIV up-regulated FoxP3 mRNA levels and increased their surface expression of CD25, CTLA-4, and GITR regulatory cell markers.26 Interactions between HIV gp120 envelop glycoprotein and CD4 were found to be crucial in HIV-driven Treg expansion. In this model, CD4-gp120 interaction resulted in inhibition of Treg apoptosis leading to increased survival of pre-existing Tregs, an effect that could be mediated by up-regulation of the antiapoptotic protein Bcl-2.26,27 Interestingly, binding of CD4 to gp-120 was used as an approach to modulate Treg function in vivo preventing lethal xenogeneic graft-versus-host disease in NOD-Scid mice.28
In addition to a direct effect of HIV, alterations of Tregs could also be mediated through dendritic cells (DCs). DCs exposed to the HIV-1 Tat protein were reported to induce up-regulation of CTLA-4 expression on autologous Treg cells.29 DCs generated from monocytes of HIV-infected individuals were more prone to induce Tregs than DCs from healthy donors.30 Conversion to Tregs was also shown to be induced by lymph node DCs from untreated HIV-infected patients.31 Furthermore, HIV-exposed human plasmacytoid DCs (pDCs) induced the differentiation of naive CD4 T cells into Tregs with suppressive function. This effect occurred following binding of HIV to CD4 and triggering of TLR7 by HIV genomic RNA, and was dependent on the pDC expression of indoleamine 2,3-dioxygenase (IDO).32 Hence, HIV-driven Treg expansion may occur as a consequence of T-cell activation and may result from enhanced Treg generation in the thymus, enhanced Treg survival, and/or Treg conversion following contact with DCs.
Treg cell dynamics in HIV-infected patients
Treg frequency changes in peripheral blood
According to the peripheral CD4 T-cell depletion associated with progressive HIV infection, absolute numbers of peripheral CD4 Tregs and proportions of Tregs among total CD3+ T cells were mainly found to be decreased in HIV-infected patients compared with healthy donors. Thus, results of Treg numbers or frequencies among total T cells could be solely interpreted as a direct consequence of changes in CD4 T-cell counts. In contrast, most studies that assessed the frequency of Tregs among CD4 T cells found an expansion of peripheral Tregs. We decided to focus on Treg proportion in different clinical situations including chronic or acute untreated HIV infection and spontaneous or antiretroviral therapy (ART)–mediated HIV viral control.
Some conflicting results pertaining to the dynamics of Treg cells might be because of the lack of consensus phenotypic markers that define Treg cells, especially in HIV infection where CD25 might be up-regulated on conventional CD4 T cells as a result of generalized immune activation. In addition, FoxP3 may be transiently up-regulated in activated, conventional CD4 T cells. Glycoprotein-A repetitions predominant (GARP) was suggested as a marker that could effectively discriminate between CD25+ Tregs and IL-17–producing CD25+ cells or T cells that transiently express FoxP3 following activation in HIV infection.33 However, a combination of markers, including CD25, CD127, FoxP3 protein, or alternatively FoxP3 mRNA, was consistently used in studies assessing Tregs in HIV-infected patients.
Acute and chronic untreated infection.
Little data on Tregs in acute HIV infection is available. Treg proportions among CD4 T cells were reported to be either lower34 or higher35 compared with healthy controls. In contrast, an increase in Treg frequencies was consistently reported in untreated patients with chronic infection compared with healthy donors when Tregs were assessed by flow cytometry. This seemed to hold true regardless of the phenotype analyzed, and such phenotypes included CD4+FoxP3+,36,37 CD4+CD25+/hi,38,39 CD4+CD25+/hiFoxP3+,35,40-42 CD4+CD25+CD127low,43,44 or CD4+CD25+CD127lowFoxP3+.15,45
Although not consistently found,46 most studies showed an inverse relationship between Treg frequency and CD4 cell counts.15,35,37,39,40,43-45,47 Treg frequency and plasma viral load were either not related39,40,48 or positively correlated.15,35,37,43-45 Increase in Treg frequency was also observed in lymphopenic HIV-2–infected patients who display low-to-undetectable viremia.49 Thus, Treg expansion seems to be more directly related to the degree of CD4 depletion than to viremia.
Contradictory to various studies showing an expansion of Tregs in infected patients, other reports suggested decreased levels of Tregs.50-52 Interestingly, in the latter studies, Treg levels were measured by quantitative RT-PCR of FoxP3 mRNA in PBMCs,51 purified T cells,50 or CD4 T cells.52 There is no obvious explanation for this paradox, but decreased FoxP3 mRNA could: (1) result from a lower level of FoxP3 transcripts at a per-cell basis, as suggested17 ; (2) mirror the peripheral CD4 T-cell depletion when FoxP3 is measured in unfractionated PBMCs or T cells; or (3) result from the expansion of inducible Tregs (iTregs) that do not express FoxP3 as a result of antigen persistence.53
In this review, excluding in tissues, we will focus on Treg frequencies that have been measured by flow cytometry, which is considered a more relevant method for determining cell-subset frequencies.
Impact of antiretroviral therapy.
Few longitudinal studies involving a low number of patients have investigated the effect of ART on Treg levels. Most studies suggest that treatment is able to significantly decrease or even normalize Treg frequency at levels similar to that of healthy donors, at least in patients with successful ART.15,36,37,42,54 Moreover, in most cross-sectional studies, peripheral Treg frequency was reported to be lower in ART-treated patients compared with untreated, chronically infected patients.15,40,41,44 Interestingly, Treg frequency remained higher in patients who did not respond to treatment, that is, immunologic55 or virologic40 nonresponders, compared with full responders.
Hence, successful ART might reduce Treg expansion associated with HIV infection. Accordingly, in patients undergoing structured treatment interruption, an increase in Treg frequency has been reported following ART interruption.20
Tregs in HIV controllers and long-term nonprogressors.
Some HIV-infected patients, known as long-term non progressors. (LTNPs), remain asymptomatic for many years and maintain high CD4 cell counts without ART. In addition, a small proportion (< 0.5%) of HIV patients spontaneously control viral replication and are called “HIV or elite controllers.”56 The assessment of Treg frequencies in these particular patient populations may help address the potential harmful or beneficial role of Tregs in HIV infection.
In contrast to progressors, HIV controllers, and LTNPs have been shown to exhibit Treg levels similar15,35,40,52,57 or even lower than healthy donors.44,57 These data allow us to conclude that high levels of Tregs are, at least, not required for disease nonprogression. Low levels of Tregs could account for the high specific anti-HIV CD8 T-cell responses described in most HIV controllers, but also for the relatively high immune activation levels compared with ART-treated patients.44 In addition to the levels of Tregs, their functional activity (discussed in “Treg cell function”) may also be involved in the overall in vivo effect of regulatory T cells in this particular group of patients.
Tregs in patients receiving immune-based therapy.
Recombinant IL-2 therapy has been shown to improve CD4 counts beyond that observed with antiretroviral therapy alone. However, recent phase 3 trials revealed that IL-2–treated patients did not experience a better clinical outcome.58 We demonstrated that long-term IL-2 therapy leads to the expansion of naive CD25loFoxP3+ and activated CD25hiFoxP3hi nTregs. Expanded Tregs suppressed proliferation of effector T cells in vitro, a result that may explain why these patients did not experience a clinical benefit despite sustained increases in CD4 counts.59
In contrast to IL-2, recombinant IL-7 therapy was found to preferentially expand non-Treg cells resulting in a relative decrease of Treg within the total CD4 T-cell population.60 The role of Treg should also be considered in therapeutic vaccine trials for HIV infection since Treg may prevent the vaccine-induced anti-HIV-1–specific polyfunctional responses.61
Dynamics of Tregs in tissues
Treg cells have been reported to accumulate in lymphoid tissues and mucosa of HIV-infected patients.41,50,62 One may hypothesize that Tregs relocate to sites where HIV replication occurs preferentially. In addition, Tregs were shown to increase membrane expression of the homing receptors CD62L and integrin α4β7 following HIV-1 binding in vitro, an observation that could explain the accumulation of Tregs in peripheral lymph nodes and lymphoid mucosal tissues in vivo.27 Hence, higher Treg frequencies are found in lymph nodes compared with blood.15 Although of great interest, given that tissue samples are not easy to recover, only scarce studies have been performed to assess Tregs in tissues of HIV-infected patients.
Phenotype of tissue Tregs.
FoxP3 and/or CD25 are usually used to identify tissue Tregs by flow cytometry or immunohistochemistry. FoxP3 expression levels were found to be higher in rectal and lymph node (LN) Tregs compared with peripheral Tregs in patients and in healthy controls.15,57,62 Expression of CTLA-4 was similar in Tregs from LN and blood (∼ 40%) whereas expression of GITR was higher in LN Tregs (∼ 50%) than in peripheral Tregs.62 In lamina propria of duodenal mucosa, most FoxP3+ cells expressed CD25, CTLA-4, and GITR.41
Chronic untreated infection.
Most studies reported highly elevated Treg levels in patients compared with healthy donors in different tissues including duodenal and rectal mucosa,41,57 thymus,24 and tonsils,26 regardless of the way Tregs were defined. In contrast to the blood, even when assessing by means of FoxP3 mRNA, higher Treg levels were found in untreated patients.26,50 In tonsils from progressor patients, FoxP3 mRNA positively correlated with plasma viral load.50
As shown for peripheral blood, LTNPs and HIV controllers were reported to have low levels of Tregs in tissues similar to healthy controls.57 Thus, tonsilar tissues from LTNPs showed lower mRNA levels of FoxP3, IDO, and TGF-β than progressors. Moreover, a high perforin/Treg ratio was associated with nonprogressive disease.26 Rectal Treg frequency negatively correlated with rectal CD4 count and was positively related to plasma viral load and rectal T-cell activation (PD-1 and CD38-expressing CD4+ and CD8+ cells).57
Effect of ART.
As shown in the periphery, tissue Treg levels were found to be lower in treated compared with untreated patients.41,50 Accordingly, in a longitudinal study, ART resulted in normalization of mucosal Treg frequency.41
Thus, there is now clear convergent evidence that numbers of Tregs are decreasing over time but at a lower rate than the whole CD4 T-cell population, meaning that Treg frequency increases. Treg expansion was observed both in the periphery and, to a greater extent, in mucosal and lymphoid tissues in HIV-infected patients with progressive disease. In contrast, HIV controllers and LTNPs exhibit lower Treg levels. In this regard, Treg may be considered nonprotective in HIV infection.
Control of immune activation
High levels of immune activation occur early in primary HIV infection and may be related to activation of innate and adaptative immune responses, microbial translocation, activation by HIV viral proteins, and reactivation of other viruses (eg, cytomegalovirus, hepatitis viruses; reviewed in Appay and Sauce63 ). One of the main functions of Tregs during infections is to control collateral tissue damage associated with an inflammatory response.64 Several studies addressed the question of the ability of Tregs to control exaggerated immune activation/inflammation in the context of HIV infection at different stages of the disease.
Few studies had suggested a negative relationship between immune activation and Treg levels.47,65 However, correlations were performed using absolute counts and/or frequency of CD4+CD25hiCD62Lhi among total CD3+ T cells, both of which depend on CD4 cell counts known to be negatively related to systemic immune activation, especially in untreated patients. Further studies performed in untreated chronically infected patients showed a positive relationship between Treg frequencies and T-cell activation15,35,40,43,66,67 regardless of activation markers analyzed (ie, CD38 and/or HLA-DR expressed on CD8 and/or CD4 T cells). Furthermore, rectal Treg frequency was also found to positively correlate with rectal T-cell activation in untreated patients.57
In patients successfully treated with ART, CD4 and CD8 T-cell activation dramatically decreased with viral suppression. However, the level of immune activation remains higher compared with healthy controls. In HIV controllers, peripheral T-cell activation is lower than in patients with progressive disease.68 However, the level of T-cell activation is higher in HIV controllers compared with ART-treated patients, an observation that could be related to low Treg frequency, as discussed in the previous section.44 In the situation of viral control under ART, Treg frequency was found to negatively correlate with residual immune activation. Interestingly, the negative relationship between Treg frequency and CD8 T-cell activation was lost after treatment interruption (when immune activation increased as a result of viral rebound).20 Another aspect to consider in high-level systemic immune activation is primary HIV infection. Although we first reported on a negative relationship between Tregs and immune activation in acutely infected patients,48 in a further study using a more stringent Treg definition, including CD127 and FoxP3, we did not find any such correlation.69
Microbial translocation is suggested to be one of the leading causes of generalized immune activation.70 Th17 cells contribute to host defenses against microbial agents and to the maintenance of the integrity of the mucosal barrier. Thus, loss of Th17 cells may promote microbial translocation and sustained inflammation.71,72 Th17 cells are closely related to Tregs; these 2 subsets are flexible and share reciprocal developmental pathways.73 Preliminary data in humans suggest that the inversion of the Th17/Treg balance is associated with a chronic inflammatory state in progressive HIV disease.74
Taken together, data suggest that Tregs, although capable of controlling low residual T-cell activation in ART-treated patients, are not sufficient, in terms of numbers and/or activity, to dampen the generalized immune activation associated with high levels of HIV replication.
HIV-exposed seronegative individuals
HIV-exposed seronegative individuals (HESN) are one of the best models of resistance to HIV infection. Card et al studied the level of T-cell activation in HIV-exposed seronegative Kenyan commercial sex workers.75 They found higher Treg frequencies and lower CD69 expression on peripheral T cells compared with HIV-seronegative controls. Tregs might thus play a role in reducing T-cell activation in HIV-resistant women, thereby decreasing the pool of susceptible CD4 T cells. This is consistent with data in HIV-exposed uninfected infants or neonates76 showing that in utero CD4+CD25+ Tregs may contribute to a lack of vertical transmission by reducing T-cell activation.
Immune reconstitution inflammatory syndrome
Immune reconstitution inflammatory syndrome (IRIS) is a potentially severe clinical complication that can occur in patients starting ART with advanced HIV disease, and which is characterized by an exaggerated inflammatory reaction in the context of an underlying diagnosed or undiagnosed opportunistic infection. The immunopathogenesis of IRIS remains unclear. One may hypothesize that IRIS represents a dysregulated CD4 and/or NK-cell response to antigens of opportunistic pathogens and that altered levels and/or function of Tregs could result in the exacerbated inflammation observed in IRIS patients.
No decrease in Treg frequency was observed.77 One study even reported a Treg expansion but these cells secreted low IL-10 and seemed to be poorly suppressive.78 The impairment of regulatory function might be because of high levels of IL-7. Alternatively, Treg dysfunction could result from increased PD-1 expression since Tregs from IRIS patients, alike other CD4 T-cell subsets, are highly activated and express high levels of Ki67 and PD-1.77
Treg cell function
Tregs can exert their suppressive function through cell-cell contact or by releasing soluble suppressive factors.79 Several studies suggest the presence of HIV-specific Tregs that produce immunosuppressive cytokines. Indeed, following stimulation with HIV p24 protein, nTregs from patients were shown to express TGF-β and IL-10.38 The presence of Tregs responding to p24 stimulation by IL-10 production from the time of primary HIV infection48 suggests that nTreg cells expand as a direct consequence of HIV infection. Angin et al recently reported the first direct evidence of the presence of p24 Gag-specific Tregs in some chronically infected untreated patients using HLA-class II tetramers.80 In tissues from untreated patients, Treg cells up-regulate functional markers of Treg activity including TGF-β and IDO.50
In vitro suppressive assays
Tregs from ART-treated patients are able to suppress HIV-specific proliferative CD4 responses through a mechanism independent of IL-10 or TGF-β secretion.38 Further studies confirmed this finding showing potent Treg activity in patients with chronic or acute infection.48,62 Tregs were also shown to inhibit HIV-specific cytokine production by T cells. HIV-1 binding to CD4 on Tregs enhanced their survival and increased their suppressor activities, namely their ability to inhibit proliferation and IL-2 production in autologous conventional CD4 T cells.27 Suppression of IL-2 and IFN-γ production was also cell-contact dependent and TGF-β and IL-10 independent.81 However, in the presence of antigen-presenting cells, the suppression by Tregs of HIV-specific CD4 T-cell responses can also be mediated through IL-10 production by monocytes or DCs.30,82 Depletion of the Treg-containing CD25+ T-cell population in PBMCs greatly enhanced TNF-α and IFN-γ responses of CD4 and CD8 T cells stimulated with HIV and also HCMV antigens.47,83 Furthermore, Treg depletion was also shown to enhance HIV-specific CD8 cytotoxic activity.62
Treg-mediated suppression of proliferative responses was found to be similar in untreated chronically HIV-infected patients and in HIV controllers.35,52,57 In addition, CD4+CD25+ T cells from patients who stopped ART retain a suppressive activity.20 In tissues, LN Treg cells maintain a suppressive activity by exerting a potent inhibition of HIV-specific cytotoxic CD8 T-cell responses throughout the course of disease.62 Thus, taken together, data suggest that Treg function appears to be preserved in HIV infection.
Ex vivo HIV-specific T-cell responses
Most studies in untreated patients found no correlation between Treg frequency and ex vivo HIV-specific responses.47,84,85 Indeed, data from INF-γ-ELISpot performed with PBMCs stimulated with overlapping peptides spanning the entire genome, from both controllers and progessors, indicated that Treg frequency did not correlate with the breadth or magnitude of HIV-specific T-cell responses.84 In contrast, in patients with ART-mediated viral suppression, the proportion of Tregs negatively correlated with anti-HIV CD8 T-cell responses.20
As already discussed about control of activation, the efficiency of Treg cells to suppress HIV-specific T-cell responses could depend on the intensity of these responses in relation to the stage of the disease and the level of viral replication.
In vivo interventional studies in SIV-infected nonhuman primates
Most published studies on Tregs in patients with HIV infection are only correlative lacking causality and giving little evidence on the definite role of Tregs in vivo. To gain insight into the role of Tregs during HIV/SIV infection, blocking Treg function in vivo is feasible in nonhuman primate (NHP) models. Several approaches can be used to either block Treg function or to deplete Treg cells. The administration of anti-CTLA-4 blocking antibody in chronically SIV-infected ART-treated macaques was not found to be detrimental and had rather beneficial virologic effects.86 However, in untreated acute SIV infection, CTLA-4 blockade did not restore virus-specific immune responses and increased viral replication and CD4 T-cell loss, particularly at mucosal sites.87 Indeed, CTLA-4 blockade may provide more target cells for the virus by decreasing the threshold for T-cell activation. Moreover, further CTLA-4 blockade during subsequent ART treatment reduced the responsiveness to a suboptimal ART regimen. Specific immune responses induced by a therapeutic T-cell vaccine were unchanged, suggesting a limited contribution of Tregs to the suppression of immune responses in vivo. However, a limitation of such studies is that CTLA-4 expression is not limited to Tregs but is also found in effector T cells.88 Another approach used the IL-2/diphtheria toxin fusion protein (Ontak) in chronically SIVagm-infected African green monkeys (AGMs) to deplete CD25+ T cells.89 Ontak treatment in those AGMs resulted in higher Ki-67 and HLA-DR expression in T cells as well as an increased viral load and subsequent depletion of intestinal CD4 T cells at the end of treatment. This study suggested a beneficial role of Tregs but drawing conclusions was difficult because of the inability of Ontak treatment to decrease the absolute number of Tregs defined by CD25 or FoxP3 expression.89
T-cell sensitivity to Treg-mediated suppression
Besides studies reporting a preserved function of Tregs, other studies suggested an increased suppressive activity of Tregs from patients with progressive disease.55,90 Most studies, however, were not able to discriminate the actual Treg potency from the sensitivity of effector cells to Treg suppression. To address this question, Treg activity was tested in both autologous and allogeneic systems in which Tregs from patients and healthy donors were cocultured with effector cells from a unique donor and vice versa. The high Treg-mediated suppression observed in an autologous system did not persist when Tregs from patients were cocultured with effector CD4 T cells from healthy donors.90,91 This suggests that patients' cells are more sensitive to Treg-mediated suppression.
HLA alleles B*27 and B*57 are known to be over represented in cohorts of HIV controllers.92 In an innovative study, Elahi et al provided a potential explanation for why HLA-B*27 and HLA-B*57 allele groups are associated with delayed HIV-1 disease progression.93 They show that HIV-specific proliferative responses of CD8 T cells, restricted by these protective HLA alleles, are not suppressed by Tregs, whereas, within the same individual, T cells restricted by control HLAs are highly suppressed ex vivo. Two mechanisms were proposed to explain this differential sensitivity of HIV-specific T cells to Tregs. Natural Tregs mediate suppression in part through Gal-9 interactions with Tim-3 inhibitory receptor expressed on effector cells.94 Interestingly, CD8 T cells restricted by protective alleles exhibit no/low up-regulation of Tim-3 following stimulation with their cognate epitopes, compared with other CD8 T cells. Moreover, blocking interactions between Gal-9 and Tim-3 prevented Treg-mediated suppression of proliferating CD8 T cells restricted by any alleles other than B*27 and B*57. An additional mechanism has been proposed in which HLA-B*27- and HLA-B*57–restricted effector cells evade suppression by directly killing Tregs via a Granzyme B–dependent pathway.
The low sensitivity of HLA-B*27- and HLA-B*57–restricted effector cells to Treg-mediated suppression, together with the low frequency of peripheral Tregs, might contribute to the strong anti-HIV CD8 T-cell responses detected in most HIV controllers.
Role of CD39
CD39, which is expressed by a subset of FoxP3+ regulatory T cells,95 is an ectoenzyme (nucleoside triphosphate diphosphohydrolase 1 [NTPDase 1]) that converts ATP into AMP, leading, in concert with the AMPase CD73, to the release of adenosine. Extracellular ATP provides a proinflammatory stimulus while adenosine suppresses T-cell responses by directly binding to the adenosine 2a receptor (A2aR). CD39 Tregs could thus dampen the generalized immune activation while having implications in T-cell dysfunction via CD73 coexpression. The most consistent and striking feature of Tregs in HIV infection is their high expression of CD39, which is not, or only partially, normalized under ART.15,45,96
CD39 and disease progression
In humans, CD39 expression is mainly restricted to Tregs. Both CD39+ Treg frequency and CD39 density on Tregs are elevated in HIV-infected patients and correlate negatively with CD4 cell count and positively with viral load and T-cell activation.15,45,96 In addition, CD8 and CD4 T cells from untreated patients express a higher density of A2a receptor and are more susceptible to adenosine suppressive effects.45 A genetic case-control association study demonstrated that a genetic variant of cd39, linked to a lower cd39 gene expression, was associated with a slower progression to AIDS.45
In functional studies, CD25hiCD39+ CD4 T cells, unlike CD25hiCD39neg T cells, were identified as a cell population able to suppress Staphylococcal Enterotoxin B (SEB)–induced T-cell proliferation.15 Moreover, CD39 blocking mAbs abolished the Treg-mediated inhibition of gag-stimulated CD8 T-cell cytokine production.45 Thus, it can be hypothesized that CD39+ Tregs would be the most potent Treg subset inhibiting HIV-specific T-cell responses, which could account for their association with disease progression.
CD39 Tregs and control of HIV replication
It has been suggested that Tregs are able to reduce HIV replication in CD4 T cells.97 The underlying mechanism for the control of HIV replication in conventional activated T cells was shown to be dependent on cAMP. Indeed, the blockade of CD39 activity, the decrease of cAMP levels in Tregs, and the inhibition of protein kinase A in T cells, were all found to abolish the Treg-mediated suppression of HIV replication.97 Thus, CD39+ Tregs might be involved in the control of HIV infection of target cells, suggesting that they may also play a beneficial role, especially within the first days following infection, before dissemination to the draining lymph node, when few effector cells are activated.
Other regulatory cell subsets
Myeloid-derived suppressor cells (MDSCs), which have been directly involved in the suppression of immune responses in cancer,98 were recently reported to induce natural Tregs in HIV infection. Indeed, higher Treg levels were detected when PBMCs from patients were cultured in the presence of MDSCs.99 High MDSC levels were shown to be associated with chronic progressive HIV disease. MDSC frequency decreased following ART initiation.
In contrast to natural CD4 Tregs, FoxP3-expressing CD8+CD25+ Tregs are rare and their proportion among CD8 T cells does not seem to increase in HIV-infected patients100 (and L.W., unpublished data, 2012). Although potentially involved in the pathogenesis of HIV infection, other FoxP3-negative regulatory T-cell subsets such as IL-10–producing type 1 regulatory T (Tr1) cells or TGF-β–producing Th3 cells were not investigated.
We reported that double-negative (DN) CD4negCD8neg T cells strongly negatively correlate with T-cell activation in patients with early primary HIV infection. In addition, the proportion of DN T cells at baseline was predictive of the immune set point (ie, plateau of T-cell activation in post-primary infection). High proportions of stimulated DN T cells produce the immunosuppressive cytokines TGF-β and/or IL-10 in acutely infected patients. Thus, DN T cells may be beneficial by controlling immune activation in acute infection through the production of TGF-β/IL-1069 but this remains to be confirmed in larger cohorts.
Conclusion
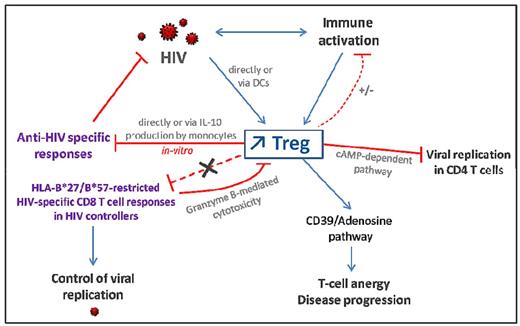
The increased frequency of peripheral and mucosal Tregs, which seems to be a characteristic feature of untreated HIV infection, triggers various effects that are either beneficial or detrimental (Figures 2 and 3). The influence of natural Treg cells on disease outcome depends on the equilibrium between a balanced Treg to effector T-cell response and immune activation.
Consequences of Treg expansion in HIV-infected patients. HIV infection leads to increased Treg frequencies directly through gp120/CD4 interaction and, indirectly, via alteration of DCs or as a consequence of generalized immune activation. Tregs can inhibit HIV-specific T-cell responses directly via a cell-cell contact dependent mechanism or through induction of IL-10 production by monocytes. However, HIV-specific CD8 T cells restricted by protective HLA alleles (in HIV controllers) are resistant to Treg-mediated suppression. Tregs may dampen low level of residual immune activation in ART-treated patients but are not efficient in primary and chronic untreated HIV-infected patients exhibiting high levels of immune activation. CD39 expression on Tregs increases during HIV infection and is associated with disease progression. Indeed, CD39 activity leads to elevated adenosine levels which activates the A2aR pathway involved in T-cell anergy and unresponsiveness. On the other hand, CD39+ Tregs might decrease viral replication in activated CD4 T cells through a cAMP-dependent mechanism.
Consequences of Treg expansion in HIV-infected patients. HIV infection leads to increased Treg frequencies directly through gp120/CD4 interaction and, indirectly, via alteration of DCs or as a consequence of generalized immune activation. Tregs can inhibit HIV-specific T-cell responses directly via a cell-cell contact dependent mechanism or through induction of IL-10 production by monocytes. However, HIV-specific CD8 T cells restricted by protective HLA alleles (in HIV controllers) are resistant to Treg-mediated suppression. Tregs may dampen low level of residual immune activation in ART-treated patients but are not efficient in primary and chronic untreated HIV-infected patients exhibiting high levels of immune activation. CD39 expression on Tregs increases during HIV infection and is associated with disease progression. Indeed, CD39 activity leads to elevated adenosine levels which activates the A2aR pathway involved in T-cell anergy and unresponsiveness. On the other hand, CD39+ Tregs might decrease viral replication in activated CD4 T cells through a cAMP-dependent mechanism.
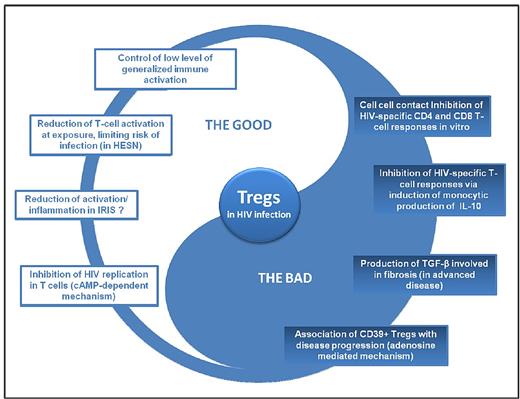
Dual role of Tregs in HIV infection. HESN indicates HIV-exposed seronegative individuals.
Dual role of Tregs in HIV infection. HESN indicates HIV-exposed seronegative individuals.
Although Tregs are able to suppress HIV-specific cellular immune responses in vitro, there is no clear evidence that these responses are suppressed in vivo, at least in viremic patients who exhibit high CD8 T-cell responses. Regarding the control of immune activation, Tregs seem to be somewhat efficient in controlling residual immune activation in patients with ART-mediated viral suppression. However, in viremic patients, even if Treg cells maintain a suppressive function, the inadequate increase in the proportion of Tregs and/or the decrease in the absolute number of Tregs result in a failure to control high immune activation.
Residual immune activation in ART-treated patients with long-term viral suppression, together with lifestyle factors and drug toxicity, may be associated with the development of complications including cardiovascular disease and cancer related to premature ageing.101 Expansion of Tregs may be beneficial in this context. However, expansion of peripheral Tregs following IL-2 treatment did not result in a clinical benefit.58 Further research should aim at better characterizing (1) Treg subsets according to the expression of new markers such as HELIOS and CD147, and (2) other regulatory cell populations by means of their respective ability to suppress immune activation versus to suppress protective T-cell responses. The identification of a particular cell subset able to decrease activation without suppressing pathogen-specific immune responses would be of great interest for immune-based therapy in chronic infectious diseases.
Acknowledgments
The authors thank G. Pancino and D. Scott-Algara (Institut Pasteur) for critical reading of the manuscript. They also thank Jose Pablo Llongueras (John Hopkins University) for carefully reviewing the manuscript.
This work was supported by the Agence Nationale de Recherches sur le SIDA et les Hépatites Virales (ANRS), Sidaction, and the Assistance Publique–Hôpitaux de Paris. M.F.C. is the recipient of a fellowship from the ANRS.
Authorship
Contribution: L.W and M.F.C wrote the manuscript and designed the figures.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Laurence Weiss, MD, PhD, Institut Pasteur, Regulation des infections retrovirales, 20 rue Leblanc, Paris, France 75015; e-mail: laurence.weiss@egp.aphp.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal