Key Points
Neutrophils differentiate into neutrophil–dendritic cell hybrids upon recruitment to sites of inflammation or infection.
Hybrids play dual protective roles against bacterial infection by rapidly clearing bacteria and presenting bacterial antigens to T cells.
Abstract
Although unusual neutrophils expressing major histocompatibility complex class II (MHC II) and costimulatory molecules have been detected at inflammatory sites in mice and humans, their identity, origin, and function remain unclear. We have demonstrated that, when cultured with granulocyte macrophage-colony-stimulating factor, neutrophils can give rise to a unique hybrid population exhibiting dual phenotypic and functionality of neutrophils and dendritic cells (DCs). Here we report that hybrid cells expressing surface markers of neutrophils (Ly6G, L-selectin, CXC chemokines receptor 2, and 7/4) and DCs (CD11c, MHC II, CD80, and CD86) become detectable in the peritoneal cavity, skin, lung, and lymph nodes under inflammatory conditions. Importantly, 20% to 30% of the adoptively transferred neutrophils acquired CD11c and MHC II expression when recovered from inflammatory lesions, demonstrating neutrophil → hybrid conversion in living animals. Using Escherichiacoli strains expressing green fluorescent protein and ovalbumin, we further show hybrids play dual protective roles by rapidly clearing bacteria and presenting bacterial antigens to CD4 T cells. These results indicate that some of the neutrophils recruited to inflammatory lesions can differentiate into neutrophil-DC hybrids, thus challenging the classic view of neutrophils as terminally differentiated leukocytes destined to die or to participate primarily in host innate immunity.
Introduction
Neutrophils and monocytes represent 2 circulating leukocyte populations that play crucial roles in the clearance of microbial pathogens and tissue remodeling. Neutrophils and monocytes not only share a clonogenic common myeloid progenitor1 but also play overlapping roles. They are both rapidly recruited to inflammatory sites, although neutrophils are the first to arrive. They both serve as professional phagocytes, although formation of the neutrophil extracellular traps (NETs) has been observed only with neutrophils. In 1994, monocytes were reported to differentiate into dendritic cells (DCs) when cultured with granulocyte macrophage–colony-stimulating factor (GM-CSF) and interleukin (IL) 4,2 but the in vivo relevance of this phenomenon has long remained unclear. Using DC-SIGN (CD209) and macrophage mannose receptor (CD206) as markers of monocyte-derived DCs (Mo-DCs), Cheong et al3 recently demonstrated that intravenous administration of lipopolysaccharide (LPS) or Escherichia coli induces rapid and robust accumulation of Mo-DCs in skin-draining lymph nodes (LNs) and that selective depletion of monocytes abrogates the emergence of Mo-DCs. Thus, Mo-DCs develop from a large reservoir of peripheral blood monocytes in response to microbial infection.
In the accompanying manuscript,4 we report that when cultured in the presence of GM-CSF, some of the immature and mature neutrophils purified from mouse bone marrow (BM) differentiate into a unique hybrid population, termed neutrophil-DC hybrid, displaying the dual phenotypic and functional properties of neutrophils and DCs. To recapitulate the essence, we have found neutrophil-DC hybrids to (1) express selected markers of both neutrophils (Ly6G, 7/4, L-selectin [CD62L], and CXC chemokine receptor 2 [CXCR2]) and DCs (CD11c, major histocompatibility complex class II [MHC II], CD80, and CD86); (2) exhibit key functionality of antigen-presenting cells (APCs) to release IL-12 and other cytokines upon stimulation with Toll-like receptor agonists and to present various forms of ovalbumin (OVA) antigen to CD4 T cells; and (3) preserve the intrinsic functionality of professional phagocytes to incorporate particulate and soluble materials from the extracellular space, to extrude NETs, and to kill internalized bacteria rapidly in a cathelicidin-dependent manner.4 Our in vitro findings corroborate the previous reports showing that human neutrophils acquire APC markers (eg, MHC II, CD1, and costimulatory molecules) and APC-like activities when cultured in the presence of selected growth factors, such as GM-CSF, interferon-γ, tumor necrosis factor α, and IL-4.5-10 Thus, large numbers of circulating neutrophils may serve as a second reservoir for generating APCs at sites of infection or inflammation.
Although Ly6C is expressed by both neutrophils and inflammatory monocytes, Ly6G is expressed exclusively by neutrophils.11 To the best of our knowledge, none of the currently recognized DC subsets has been reported to express Ly6G. Therefore, neutrophil-DC hybrids can be defined as CD11chigh/MHC II+ DCs expressing the neutrophil marker Ly6G. Two recent reports demonstrated complete lack of Ly6G expression by CD11chigh DC populations in various tissues in mice.12,13 Because neutrophil recruitment and local production of GM-CSF both take place at sites of inflammation,14,15 we hypothesized that significant numbers of neutrophil-DC hybrids might emerge under inflammatory conditions. The present study was, therefore, conducted to test this hypothesis by using standard mouse models of inflammation. Here we report that neutrophil-DC hybrids become readily detectable in various organs (eg, peritoneal cavity, lung, skin, and lymph nodes) under inflammatory conditions, and that they play dual protective roles against microbial infection by rapidly clearing bacteria and presenting bacterial antigens to T cells.
Materials and methods
Mice
Animals were purchased from Jackson Laboratories, except KC-Tie2 transgenic (TG) mice16 and I-Aβ-EGFP knock-in mice.17 All experiments were performed in accordance with the National Institutes of Health guidelines after approval by the Institutional Animal Care and Use Committee of the University of Toledo.
Cell purification
Gr-1high/CD48− band cells were purified from BM cells with purity of >99.5%. Methods for purification of band cells and other populations are described in the supplemental data (“Methods”; see the Blood Web site) with a list of monoclonal antibodies (mAbs) employed in this study.
Experimental inflammation models
We examined the emergence of neutrophil-DC hybrids in the following inflammation models: acute peritonitis induced by intraperitoneal injection of 3% thioglycollate or live E. coli, chronic skin inflammation in KC-Tie2 TG mice,16 acute lung inflammation induced by intratracheal administration of German cockroach (GC) frass extracts,18,19 and acute bacterial lymphadenitis induced by subcutaneous injection of E. coli. Single cell suspensions were prepared as described previously.18-20 Methods for these inflammation models are described in detail in the supplemental data (“Methods”).
In vivo analysis of neutrophil differentiation
Gr-1high/CD48− band cells purified from C57BL/6 mice (CD45.2) were intravenously injected (3 × 106 cells per animal) into B6 SJL mice (CD45.1) that had been treated with thioglycollate or E. coli 6 hours earlier. The peritoneal exudate cells (PECs) harvested at various time points were examined for surface phenotype and morphology within the CD45.2+/CD45.1− populations. In vivo differentiation was also examined by transferring Gr-1high/CD48− band cells purified from CD11c promoter-driven diphtheria toxin receptor (DTR)–EGFP TG mice21 and from I-Aβ-EGFP knock-in mice into thioglycollate-pretreated B6 SJL mice; the PECs were examined for green fluorescent protein (GFP) fluorescence signals within the CD45.2+/CD45.1− populations.
Bacterial uptake assay
C57BL/6 mice that had received thioglycollate treatment 2 days earlier were challenged with intraperitoneal injection of GFP E. coli (107 colony-forming units [CFUs] per animal), and the PECs isolated 1 hour later were analyzed under fluorescent microscopy or by FACSCalibur (BD Biosciences, San Jose, CA).
Antigen presentation assays
C57BL/6 mice that had received thioglycollate treatment 2 days earlier were challenged with intraperitoneal injection of live TOP10.OVA E. coli or wild-type (WT) E. coli (106 CFUs per animal). One hour later, neutrophil-DC hybrids and Ly6G− traditional DCs were purified from the PECs to test their in vitro abilities to activate OVA-specific CD4 or CD8 T cells from OT-II or OT-I TG mice, respectively. To test in vivo APC function, neutrophil-DC hybrids and traditional DCs were fluorescence-activated cell sorter (FACS) purified from GM-CSF–supplemented BM culture. The samples were pulsed for 60 minutes with OVA-coated latex beads or beads coated with bovine serum albumin (BSA) and then intraperitoneally injected (2 × 104 cells per animal) into B6 SJL mice that had received intravenous injection of carboxyfluorescein diacetate succinimidyl ester (CFSE)–labeled OT-II CD4 T cells (4 × 106 cells per animal) 24 hours earlier. The recipient mice were analyzed 3 days later for the numbers of CFSElo T cells in the CD45.2+/T-cell receptor (TCR) Vα2+ gated populations in pancreatic LNs.
Assessment of physiological roles for neutrophil-DC hybrids
CD11c promoter-driven DTR-EGFP TG mice received 2 intravenous injections of diphtheria toxin (DT) (4 μg/kg) or phosphate-buffered saline (PBS) alone on days 0 and 2. After intraperitoneal injection of thioglycollate (day 1), the mice were challenged with intraperitoneal inoculation of E. coli (2 × 107 CFUs per animal) on day 3. The PECs harvested 30 minutes later were examined for the number of live E. coli. In some of the DT-treated mice, E. coli were intraperitoneally injected together with neutrophil-DC hybrids or traditional DCs (2.5 × 105 cells per animal) purified from the PECs from the second panel of thioglycollate-treated C57BL/6 mice. Alternatively, B6 SJL mice received intravenous injection of anti–Gr-1 mAb (RB6-8C5; BD Biosciences, San Jose, CA) or isotype-matched control IgG (300 μg per animal) on day 0. After intravenous transfer (4 × 106 cells per animal) of CFSE-labeled OT-II CD4 T cells on day 0, the mice received intraperitoneal inoculation (2 × 107 CFUs per animal) of pnir15.OVA E. coli22 on day 1 and were examined for the numbers of CFSElo T cells in the CD45.2+/TCR Vα2+ gated populations in pancreatic LNs on day 3. Some of the mice treated with anti–Gr-1 mAb received intraperitoneal injection of Ly6G+/Ly6C+ neutrophil-DC hybrids or Ly6G−/Ly6C+ traditional DCs (2 × 105 cells per animal) purified from the PECs from the second panel of thioglycollate-treated C57BL/6 mice.
Statistical analyses
All data were processed by GraphPad Prism 5.0 program (GraphPad Software) together with SigmaPlot 10 (Systat Software). Quantitative data are presented as the means ± standard deviation (SD). Statistical significance was assessed based on the unpaired Student t test with 2-tailed distributions or 1-way analysis of variance with the Newman-Keuls multiple comparison test. P values < .05 were considered to be significant.
Results
Identification of neutrophil-DC hybrids in experimentally induced peritonitis lesions
Various lymphoid and nonlymphoid tissues freshly harvested from C57BL/6 mice were examined for the presence of neutrophil-DC hybrids as defined by dual expression of DC markers (CD11c and MHC II) and neutrophil marker Ly6G. Only few, if any, Ly6G+/CD11chigh/MHC II+ cells were found under the steady state, corroborating the recent reports showing the lack of Ly6G expression by CD11chigh DC populations in peripheral blood, spleen, lung, and liver.12,13 We interpreted this negative result to imply that generation of neutrophil-DC hybrids might require inflammatory signals leading to neutrophil recruitment and GM-CSF production.14,15 We tested this hypothesis using a standard peritonitis model induced by intraperitoneal injection of thioglycollate.23 Both of the previously discussed key factors for hybrid cell generation were, indeed, present in this model: thioglycollate treatment induced marked infiltration of Ly6G+/CD11c− neutrophils (Figure 1A), and GM-CSF became detectable, albeit in relatively small amounts (59 ± 30 pg/mL, n = 3), in the peritoneal cavity 2 hours after the treatment. Importantly, Ly6G+/CD11chigh hybrids were also found in thioglycollate-induced peritonitis lesions (Figure 1A). To study time kinetics, we harvested PEC samples at different time points and counted the numbers of Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11chigh/MHC II+ hybrids, and Ly6G−/CD11chigh/MHC II+ traditional DCs. Neutrophil numbers increased rapidly and transiently after thioglycollate treatment with a sharp peak on day 1. On the other hand, the numbers of neutrophil-DC hybrids increased more slowly with a peak on day 2 (Figure 1B). Traditional DCs also emerged slowly with a peak on days 2 to 4. It should be stated that neutrophil-DC hybrids represented a relatively minor population, accounting for <1% of total cell numbers recovered from the inflamed peritoneal cavity. Nevertheless, these observations demonstrated in vivo emergence of neutrophil-DC hybrids under inflammatory conditions.
Identification of neutrophil-DC hybrids in thioglycollate-induced peritonitis lesions. (A-B) After intraperitoneal injection of thioglycollate into C57BL/6 mice, PECs harvested on day 2 (A) or at the indicated time points (B) were analyzed for the expression of Ly6G, CD11c, and MHC II. Data shown are Ly6G and CD11c expression profiles (A) and the numbers of total cells in the PECs, Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11chigh/MHC II+ neutrophil-DC hybrids, and Ly6G−/CD11chigh/MHC II+ traditional DCs (B). (C) Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11chigh/MHC II+ neutrophil-DC hybrids, and Ly6G−/CD11chigh/MHC II+ traditional DCs purified from the PECs (day 2) were examined for morphology after HEMA-3 staining (bars represent 20 μm). (D) PECs harvested 2 days after thioglycollate treatment were examined for the expression profiles for the indicated leukocyte markers within the Ly6G+/CD11c− neutrophil population, the Ly6G+/CD11chigh hybrid population, and Ly6G−/CD11chigh traditional DC population. Data are representative of at least 5 independent experiments.
Identification of neutrophil-DC hybrids in thioglycollate-induced peritonitis lesions. (A-B) After intraperitoneal injection of thioglycollate into C57BL/6 mice, PECs harvested on day 2 (A) or at the indicated time points (B) were analyzed for the expression of Ly6G, CD11c, and MHC II. Data shown are Ly6G and CD11c expression profiles (A) and the numbers of total cells in the PECs, Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11chigh/MHC II+ neutrophil-DC hybrids, and Ly6G−/CD11chigh/MHC II+ traditional DCs (B). (C) Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11chigh/MHC II+ neutrophil-DC hybrids, and Ly6G−/CD11chigh/MHC II+ traditional DCs purified from the PECs (day 2) were examined for morphology after HEMA-3 staining (bars represent 20 μm). (D) PECs harvested 2 days after thioglycollate treatment were examined for the expression profiles for the indicated leukocyte markers within the Ly6G+/CD11c− neutrophil population, the Ly6G+/CD11chigh hybrid population, and Ly6G−/CD11chigh traditional DC population. Data are representative of at least 5 independent experiments.
Ly6G+/CD11chigh/MHC II+ hybrids and Ly6G−/CD11chigh/MHC II+ traditional DCs purified from the inflamed peritoneal cavity both showed typical DC-like morphology characterized by oval-shaped nuclei and dendritic processes (Figure 1C). By contrast, the Ly6G+/CD11c−/MHC II− neutrophil fraction exhibited a typical appearance of polymorphonuclear neutrophils (PMNs) characterized by small cell size and segmented nuclei. When PEC samples were incubated for 2 hours on tissue culture plates, most of the hybrids (93.4 ± 0.5%, n = 3) were recovered as nonadherent cells. An overwhelming majority of adherent cells isolated by a standard method for macrophages24 exhibited numerous cytoplasmic vacuoles (supplemental Figure 1A). Neutrophil-DC hybrids recovered from inflammatory sites, defined by the phenotype of Ly6G+/CD11chigh, uniformly expressed all tested neutrophil markers: CD62L, CXCR2, 7/4, and Ly6C (Figure 1D). Although CD62L and 7/4 were detectable on some of the Ly6G− traditional DCs, CXCR2 was undetectable in this population. The hybrids also differed from neutrophils by the expression of DC markers CD11c, MHC II, CD80, and CD86. By contrast, macrophages isolated from the PEC samples uniformly displayed CD11b and F4/80 but expressed none of the tested neutrophil markers (supplemental Figure 1B). Thus, neutrophil-DC hybrids emerging in thioglycollate-induced peritonitis lesions differ morphologically and phenotypically from neutrophils, traditional DCs, and macrophages recovered from the same lesions.
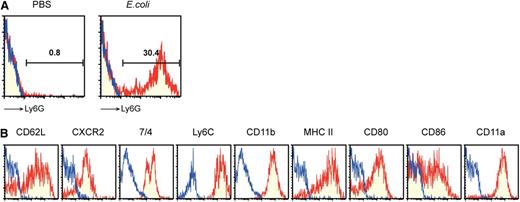
We next sought to determine whether neutrophil-DC hybrids also become detectable after intraperitoneal injection of viable E. coli. Ly6G expression was detected in significant fractions (∼30%) of the CD11chigh/MHC II+ DC fractions recovered from the peritonitis lesions (Figure 2A). Moreover, these hybrid cells isolated from the bacterial infection sites, defined by the phenotype of Ly6G+/CD11chigh, expressed neutrophil markers (CD62L, CXCR2, 7/4, and Ly6C), as well as DC markers (MHC II, CD80, and CD86) (Figure 2B). Importantly, the hybrid population we identified in thioglycollate-induced and E. coli–induced peritonitis lesions was virtually indistinguishable from the in vitro generated counterpart in terms of morphology and surface phenotype.4
Bacterial infection triggers the emergence of neutrophil-DC hybrids. (A) PECs harvested from C57BL/6 mice 24 hours after intraperitoneal injection of PBS alone or E. coli were examined for surface expression of Ly6G, CD11c, and MHC II. Data shown are staining profiles with anti-Ly6G mAb (red) or control IgG (blue) within the CD11chigh/MHC II+ gated populations. (B) The same PEC samples harvested after E. coli injection were examined for expression of the indicated leukocyte markers within the Ly6G+/CD11chigh gated population. Data are representative of 5 independent experiments.
Bacterial infection triggers the emergence of neutrophil-DC hybrids. (A) PECs harvested from C57BL/6 mice 24 hours after intraperitoneal injection of PBS alone or E. coli were examined for surface expression of Ly6G, CD11c, and MHC II. Data shown are staining profiles with anti-Ly6G mAb (red) or control IgG (blue) within the CD11chigh/MHC II+ gated populations. (B) The same PEC samples harvested after E. coli injection were examined for expression of the indicated leukocyte markers within the Ly6G+/CD11chigh gated population. Data are representative of 5 independent experiments.
Neutrophil-DC hybrids emerge in other organs under inflammatory conditions
We examined the development of neutrophil-DC hybrids in 2 additional inflammatory disease models. Chronic skin inflammation occurs spontaneously in KC-Tie2 TG mice, in which keratinocytes overexpress the angiopoietin receptor Tie2.16 Ear skin samples with scaly erythematous changes harvested from 10- to 14-week-old KC-Tie2 TG mice were examined for Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11chigh/MHC II+ hybrids, and Ly6G−/CD11chigh/MHC II+ traditional DCs. The inflammatory skin lesions contained substantial numbers of neutrophil-DC hybrids (corresponding to ∼25% of traditional DCs), in addition to large numbers of neutrophils (Figure 3A). By contrast, almost no hybrids were present in control ear skin samples harvested from age-matched WT mice. Rapid and profound recruitment of neutrophils occurs in lung after intratracheal administration of GC frass extracts as reported previously.18,19 Significant, albeit modest, numbers of hybrids (corresponding to ∼2% of traditional DCs) were found in those acute inflammatory lesions (Figure 3B). By contrast, control lung samples harvested after PBS inhalation contained almost no hybrid cells.
Emergence of neutrophil-DC hybrids in various organs under inflammatory conditions. Single cell suspensions were prepared from ear skin samples harvested from KC-Tie2 TG mice or age-matched WT mice (3 mice per panel) (A), from lung samples harvested from BALB/c mice (3 mice per panel) 48 hours after intratracheal instillation of GC frass or PBS alone (B), or from skin-draining LN samples harvested from C57BL/6 mice (5 mice per panel) 24 hours after subcutaneous inoculation of live E. coli or PBS alone (C). Data shown are the numbers (means ± SD) of Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11chigh/MHC II+ neutrophil-DC hybrids, and Ly6G−/CD11chigh/MHC II+ traditional DCs. *P < .05; **P < .01 between the indicated samples. (D) The same LN samples harvested after E. coli injection were examined for expression of the indicated leukocyte markers within the Ly6G+/CD11chigh gated population. Data are representative of 5 independent experiments.
Emergence of neutrophil-DC hybrids in various organs under inflammatory conditions. Single cell suspensions were prepared from ear skin samples harvested from KC-Tie2 TG mice or age-matched WT mice (3 mice per panel) (A), from lung samples harvested from BALB/c mice (3 mice per panel) 48 hours after intratracheal instillation of GC frass or PBS alone (B), or from skin-draining LN samples harvested from C57BL/6 mice (5 mice per panel) 24 hours after subcutaneous inoculation of live E. coli or PBS alone (C). Data shown are the numbers (means ± SD) of Ly6G+/CD11c−/MHC II− neutrophils, Ly6G+/CD11chigh/MHC II+ neutrophil-DC hybrids, and Ly6G−/CD11chigh/MHC II+ traditional DCs. *P < .05; **P < .01 between the indicated samples. (D) The same LN samples harvested after E. coli injection were examined for expression of the indicated leukocyte markers within the Ly6G+/CD11chigh gated population. Data are representative of 5 independent experiments.
After subcutaneous inoculation of live E. coli, Ly6G+/CD11chigh/MHC II+ neutrophil-DC hybrids became readily detectable in skin-draining LNs in the numbers equivalent to 3% to 5% of neutrophil numbers (or 5% to 7% of traditional DC numbers) (Figure 3C). Once again, hybrid cells isolated from the inflamed LNs, defined by the phenotype of Ly6G+/CD11chigh, expressed neutrophil markers (CD62L, CXCR2, 7/4, and Ly6C), as well as DC markers (MHC II, CD80, and CD86) (Figure 3D). In summary, we have observed rapid and substantial emergence of neutrophil-DC hybrids in various disease models, including thioglycollate-induced peritonitis, bacterial peritonitis, chronic skin inflammation, allergen-induced acute lung inflammation, and bacterial lymphadenitis.
Origin of in vivo emerging neutrophil-DC hybrids
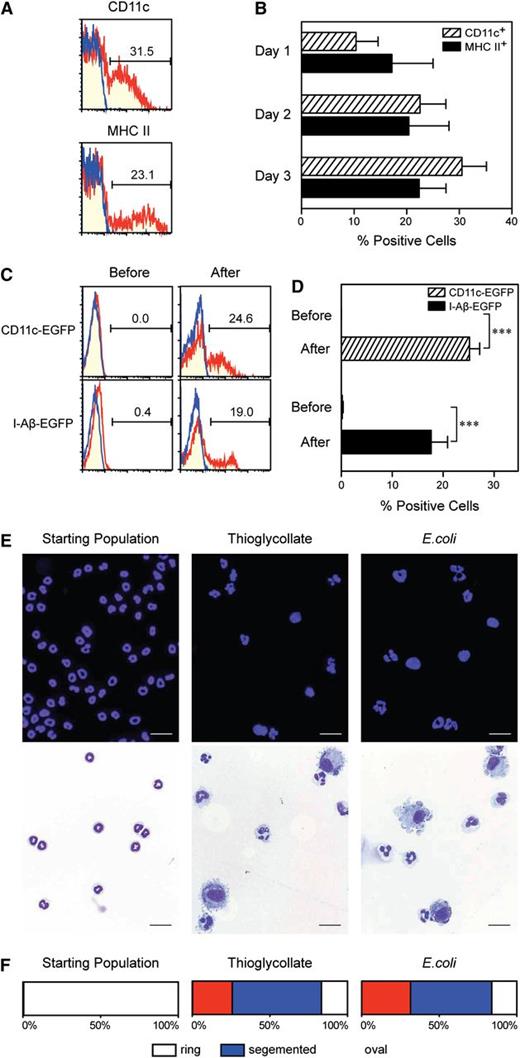
With regard to the origin, the following lines of evidence support our central hypothesis that neutrophil-DC hybrid cells are generated from neutrophils. First, after coculturing Gr-1high/CD48− band cells (from CD45.2 mice) with BM feeder cells (from CD45.1 mice) in the presence of GM-CSF, we observed time-dependent development of neutrophil-DC hybrids within the CD45.2+/CD45.1− population. Second, those experiments were carried out with the starting band cell preparations showing >99.5% purity in postsort analyses as well as remarkable homogeneity in terms of surface phenotype and morphology. Third, in addition to the previously discussed Gr-1high/CD48− band cell population, Gr-1high/CD11blow immature neutrophil and Gr-1high/CD11bhigh mature neutrophil populations both acquired DC-like markers in the previously discussed cocultures, while maintaining Ly6G expression. Finally, CD45.2+ monocytes differentiated into Mo-DCs, but not into neutrophil-DC hybrids.4 To determine the origin of in vivo emerging hybrids, we carried out adoptive transfer experiments. Briefly, Gr-1high/CD48− band cells freshly purified from CD45.2 donor mice were intravenously injected into CD45.1 recipient mice that had received thioglycollate treatment. Surface expression of CD11c and MHC II was observed in some of the CD45.2+/CD45.1− cells recovered 3 days later from the inflamed peritoneal cavity (Figure 4A). Acquisition of CD11c and MHC II expression was already detectable 1 day after transfer, and 20% to 30% of the transferred cells showed surface expression of these DC markers on days 2 to 3 (Figure 4B).
Band cell origin of in vivo emerging neutrophil-DC hybrids. (A-B) Gr-1high/CD48− band cells purified from C57BL/6 (CD45.2) mice were intravenously injected into B6 SJL (CD45.1) recipient mice that had received thioglycollate treatment 6 hours earlier. The PECs harvested on day 3 (A) or at the indicated time points (B) were examined for CD11c and MHC II expression profiles and the percentages (means ± SD, n = 3) of CD11c+ cells and MHC II+ cells within the CD45.2+/CD45.1− gated population. (C-D) Gr-1high/CD48− band cells purified from CD11c promoter-driven DTR-EGFP TG mice (C, red in upper panels), I-Aβ-EGFP knock-in mice (C, red in lower panels), or WT mice (C, blue in all panels) were examined for GFP fluorescence signals before and 3 days after adoptive transfer into B6 SJL recipient mice as in (A). Data shown are GFP expression profiles and the percentages (means ± SD, n = 3) of GFP+ cells within the CD45.2+/CD45.1− gated population. (E-F) Gr-1high/CD48− band cells purified from C57BL/6 (CD45.2) mice were adoptively transferred to B6 SJL recipients that had received intraperitoneal injection of thioglycollate (middle panels) or E. coli 6 hours earlier (right panels). The starting band cell preparations (left panels) and the CD45.2+/CD45.1− fractions FACS purified from the peritonitis lesions were examined for morphology. (E) Data shown are representative images (bars indicate 20 μm). (F) The percent of cells showing the indicated nuclear shapes were determined by analyzing >500 cells per sample under the microscopy. All data are representative of at least 2 independent 2experiments.
Band cell origin of in vivo emerging neutrophil-DC hybrids. (A-B) Gr-1high/CD48− band cells purified from C57BL/6 (CD45.2) mice were intravenously injected into B6 SJL (CD45.1) recipient mice that had received thioglycollate treatment 6 hours earlier. The PECs harvested on day 3 (A) or at the indicated time points (B) were examined for CD11c and MHC II expression profiles and the percentages (means ± SD, n = 3) of CD11c+ cells and MHC II+ cells within the CD45.2+/CD45.1− gated population. (C-D) Gr-1high/CD48− band cells purified from CD11c promoter-driven DTR-EGFP TG mice (C, red in upper panels), I-Aβ-EGFP knock-in mice (C, red in lower panels), or WT mice (C, blue in all panels) were examined for GFP fluorescence signals before and 3 days after adoptive transfer into B6 SJL recipient mice as in (A). Data shown are GFP expression profiles and the percentages (means ± SD, n = 3) of GFP+ cells within the CD45.2+/CD45.1− gated population. (E-F) Gr-1high/CD48− band cells purified from C57BL/6 (CD45.2) mice were adoptively transferred to B6 SJL recipients that had received intraperitoneal injection of thioglycollate (middle panels) or E. coli 6 hours earlier (right panels). The starting band cell preparations (left panels) and the CD45.2+/CD45.1− fractions FACS purified from the peritonitis lesions were examined for morphology. (E) Data shown are representative images (bars indicate 20 μm). (F) The percent of cells showing the indicated nuclear shapes were determined by analyzing >500 cells per sample under the microscopy. All data are representative of at least 2 independent 2experiments.
Considering the possibility that neutrophils might have simply acquired CD11c and MHC II proteins from neighboring APCs under the inflammatory conditions, we repeated the previous experiments by using 2 reporter mouse strains, that is, the CD11c promoter-driven DTR-EGFP TG mice (in which EGFP fluorescence signals are expressed by all CD11c+ populations)21 and the I-Aβ-EGFP knock-in mice (in which EGFP is expressed by all MHC II+ populations).17 Gr-1high/CD48− band cells freshly purified from these reporter mouse strains (of CD45.2 background) showed no EGFP signals (Figure 4C, left panels). We then transferred them into WT CD45.1 recipients that had received thioglycollate treatment. GFP signals became readily detectable in the CD45.2+/CD45.1− populations when recovered from the inflamed peritoneal cavity (Figure 4C, red lines in right panels). By contrast, band cells purified from WT mice failed to acquire GFP signals (Figure 4C, blue lines in right panels). Appropriately 20% of the adoptively transferred cells from either of the 2 reporter strains exhibited GFP signals when recovered from inflamed peritoneal cavities (Figure 4D). Taken together, our data demonstrate that some neutrophils can indeed differentiate into neutrophil-DC hybrids after extravasation at inflammatory sites.
To determine the fate of the remaining cells that failed to differentiate into hybrids, we FACS purified the CD45.2+/CD45.1− fraction from the thioglycollate-induced peritonitis lesions for morphological analyses. In marked contrast to the starting population showing typical band cell morphology (Figure 4E, left panels), this fraction recovered from inflammatory sites contained a population showing DC-like morphology characterized by oval-shaped nuclei and dendritic processes and a second population resembling band cells or PMNs (Figure 4E, middle panels). By analyzing >500 cells per sample for nuclear shape, we estimated that ∼25% of the adoptively transferred band cells had acquired oval-shaped nuclei in thioglycollate-induced peritonitis lesions, whereas the remaining cells showed either ring-shaped or segmented nuclei (Figure 4F, middle panel). We repeated the same experiments by inducing peritonitis by intraperitoneal inoculation of E. coli. Again, the CD45.2+/CD45.1− fraction purified from the PECs contained a population displaying DC-like morphology and a second population with the typical appearance of either band cell or PMNs (Figure 4E, right panels). We estimated that ∼30% of the transferred cells had acquired oval-shaped nuclei in bacterial peritonitis lesions. Again, the remaining 70% showed either ring-shaped nuclei or segmented nuclei (Figure 4F, right panel). Thus, upon recruitment into inflammatory sites, band cells appear to undergo either trans-differentiation into neutrophil-DC hybrids or maturation into PMNs.
Neutrophil-DC hybrids participate in rapid bacterial clearance at infection sites
Neutrophil-DC hybrids purified from GM-CSF–supplemented BM cultures exhibit dual functionality to serve as professional phagocytes and APCs. For example, they show potent endocytotic potentials, cathelicidin-dependent bacterial killing, and NET formation. At the same time, they exhibited striking abilities to release IL-12 and other cytokines upon stimulation with Toll-like receptor agonists and to present OVA antigens in all tested forms to CD4 T cells.4 Thus, it was of particular interest to determine whether neutrophil-DC hybrids emerging in vivo at inflammatory sites retain this dual functionality. To test the endocytotic capacity of hybrid cells, we inoculated E. coli expressing GFP into the thioglycollate-induced peritonitis lesions. Strikingly, Ly6G+/CD11chigh hybrids in the PECs harvested 1 hour later had incorporated large numbers of GFP+ bacteria as measured by fluorescence signals (Figure 5A) and as visualized under fluorescence microscopy (Figure 5B). By contrast, Ly6G−/CD11chigh traditional DCs showed only modest bacterial uptake. Unexpectedly, rather limited GFP signals were detected in Ly6G+/CD11c− neutrophils; it is conceivable that GFP-expressing bacteria were digested in neutrophils rapidly and completely, thereby losing their fluorescence signals. In this E. coli infection model, intraperitoneally inoculated bacteria were efficiently cleared in a time-dependent manner, with ∼60% clearance achieved at 30 minutes (Figure 5C, open circles). To assess potential contributions of neutrophil-DC hybrids to this rapid bacterial clearance, we employed the CD11c promoter-driven DTR-EGFP TG mice, in which all CD11c-positive populations (including both the hybrids and traditional DCs) can be eliminated by systemic injection of DT.21 DT-induced ablation of CD11c+ cells in the TG mice resulted in significantly impaired clearance of E. coli (Figure 5D, top 2 bars). Importantly, the same DT treatment had no effect in WT mice (Figure 5C, closed circles). These observations implied that in addition to conventional CD11c− phagocytes, CD11c+ populations contribute to bacterial clearance. We next reinstated Ly6G+/CD11chigh/MHC II+ hybrids or Ly6G−/CD11chigh/MHC II+ traditional DCs back individually to the DT-treated mice by transferring the corresponding populations purified from the thioglycollate-induced peritonitis lesions in WT mice. Bacterial clearance was significantly restored by neutrophil-DC hybrids, as well as by traditional DCs, albeit to a lesser extent (Figure 5D, bottom 2 bars). We have interpreted these results to suggest that neutrophil-DC hybrids participate in rapid bacterial clearance in living animals.
Neutrophil-DC hybrids function as professional phagocytes in bacterial peritonitis lesions. (A-B) At 48 hours after thioglycollate treatment, C57BL/6 mice received intraperitoneal inoculation of E. coli expressing GFP. (A) One hour later, PECs were examined for GFP fluorescence signals (means ± SD, n = 3) for Ly6G+/CD11c− neutrophils, Ly6G+/CD11chigh neutrophil-DC hybrids, and Ly6G−/CD11chigh traditional DCs. (B) Fluorescence (left), phase contract (middle), and merged images (right) are shown for the FACS-purified Ly6G+/CD11chigh hybrid population (bar indicates 20 μm). (C-D) C57BL/6 mice (C) or CD11c promoter-driven DTR-EGFP TG mice (D) received systemic injections of DT or PBS alone on days 0 and 2 and thioglycollate treatment on day 1. The mice were challenged with intraperitoneal inoculation of E. coli on day 3 and then examined for the numbers (means ± SD from 3 mice per panel) of live bacteria recovered from the peritoneal cavities at the indicated time points (C) or after 30 minutes (D). (D) Ly6G+/CD11chigh/MHC II+ neutrophil-DC hybrids or Ly6G−/CD11chigh/MHC II+ DCs purified from the PECs from the second panel of thioglycollate-treated C57BL/6 mice were intraperitoneally injected together with E. coli to test their ability to restore bacterial clearance in the DC-depleted mice. *P < .05; **P < .01 between the indicated samples. Data are representative of at least 2 independent experiments.
Neutrophil-DC hybrids function as professional phagocytes in bacterial peritonitis lesions. (A-B) At 48 hours after thioglycollate treatment, C57BL/6 mice received intraperitoneal inoculation of E. coli expressing GFP. (A) One hour later, PECs were examined for GFP fluorescence signals (means ± SD, n = 3) for Ly6G+/CD11c− neutrophils, Ly6G+/CD11chigh neutrophil-DC hybrids, and Ly6G−/CD11chigh traditional DCs. (B) Fluorescence (left), phase contract (middle), and merged images (right) are shown for the FACS-purified Ly6G+/CD11chigh hybrid population (bar indicates 20 μm). (C-D) C57BL/6 mice (C) or CD11c promoter-driven DTR-EGFP TG mice (D) received systemic injections of DT or PBS alone on days 0 and 2 and thioglycollate treatment on day 1. The mice were challenged with intraperitoneal inoculation of E. coli on day 3 and then examined for the numbers (means ± SD from 3 mice per panel) of live bacteria recovered from the peritoneal cavities at the indicated time points (C) or after 30 minutes (D). (D) Ly6G+/CD11chigh/MHC II+ neutrophil-DC hybrids or Ly6G−/CD11chigh/MHC II+ DCs purified from the PECs from the second panel of thioglycollate-treated C57BL/6 mice were intraperitoneally injected together with E. coli to test their ability to restore bacterial clearance in the DC-depleted mice. *P < .05; **P < .01 between the indicated samples. Data are representative of at least 2 independent experiments.
Neutrophil-DC hybrids also function as APCs at bacterial infection sites
To determine whether neutrophil-DC hybrids that have captured bacteria at the infection sites can process the bacterial antigens for presentation, we inoculated live E. coli expressing OVA into the thioglycollate-induced peritonitis lesions. Ly6G+/CD11chigh hybrids purified from the peritoneal cavity 1 hour later induced robust proliferation of OVA-specific OT-II CD4 T cells, compared with the second hybrid population purified in parallel from control mice infected with WT E. coli (Figure 6A, left panels). Neutrophil-DC hybrids were significantly more efficient than Ly6G−/CD11chigh traditional DCs purified from the same peritoneal cavity, perhaps reflecting the difference in bacterial uptake. The same hybrid population purified from OVA–E. coli infection sites induced only modest proliferation of OVA-specific OT-I CD8 T cells (Figure 6A, right panels), suggesting their limited ability to cross-present exogenous antigen.
Neutrophil-DC hybrids also function as APCs in living animals. (A) At 48 hours after thioglycollate treatment, C57BL/6 mice received intraperitoneal inoculation of OVA E. coli. One hour later, Ly6G+/CD11chigh/MHC II+ neutrophil-DC hybrids and Ly6G−/CD11chigh/MHC II+ traditional DCs were purified from the PECs and then cocultured with OT-II CD4 T cells (left panels) or OT-I CD8 T cells (right panels). The 2 populations purified from a second panel of mice receiving intraperitoneal inoculation of WT E. coli served as controls. Data shown are the levels (means ± SD from triplicate cultures) of 3H-thymidine uptake. (B) Ly6G+/CD11chigh/MHC II+ hybrids purified from GM-CSF–supplemented BM culture (day 6) were labeled with CFSE and then subcutaneously injected into C57BL/6 mice. Skin-draining LN samples harvested 48 hours later were examined for the presence of CFSE+ cells. Cryostat sections were examined under fluorescence microscopy after counterstaining with APC-conjugated anti-CD3 mAb (bar indicates 50 μm). (C-E) Ly6G+/CD11chigh/MHC II+ hybrids and Ly6G−/CD11chigh/MHC II+ traditional DCs purified from GM-CSF–supplemented BM culture from C57BL/6 (CD45.2) mice were pulsed for 30 minutes with OVA-coated latex beads (C) or BSA-coated beads (D). These APC preparations were then intraperitoneally injected into B6 SJL (CD45.1) mice that had received intravenous injection of CFSE-labeled OT-II CD4 T cells (CD45.2) 24 hours earlier. The recipient mice were analyzed 3 days later for the expansion of CD45.2+/TCR Vα2+ T cells in pancreatic LNs. Data shown are actual CFSE profiles (C-D) and the numbers (means ± SD from 3 mice per panel) of CFSElo T cells (E). (F-G) B6 SJL mice received systemic injection of anti–Gr-1 mAb or isotype-matched control IgG and intravenous injection of CFSE-labeled OT-II CD4 T cells both on day 0. (F) These mice (6 mice per panel) received intraperitoneal inoculation of OVA E. coli on day 1 and were examined for the expansion of CD45.2+/TCR Vα2+ T cells in pancreatic LNs. (G) Ly6G+/Ly6C+/CD11chigh neutrophil-DC hybrids or Ly6G−/Ly6C+/CD11chigh traditional DCs purified from the PECs from the second panel of thioglycollate-treated B6 SJL mice were intraperitoneally injected together with OVA E. coli to test their ability to restore the APC function in the previously discussed mice treated with anti–Gr-1 mAb. *P < .05; **P < .01 between the indicated samples. Data are representative of at least 2 independent experiments.
Neutrophil-DC hybrids also function as APCs in living animals. (A) At 48 hours after thioglycollate treatment, C57BL/6 mice received intraperitoneal inoculation of OVA E. coli. One hour later, Ly6G+/CD11chigh/MHC II+ neutrophil-DC hybrids and Ly6G−/CD11chigh/MHC II+ traditional DCs were purified from the PECs and then cocultured with OT-II CD4 T cells (left panels) or OT-I CD8 T cells (right panels). The 2 populations purified from a second panel of mice receiving intraperitoneal inoculation of WT E. coli served as controls. Data shown are the levels (means ± SD from triplicate cultures) of 3H-thymidine uptake. (B) Ly6G+/CD11chigh/MHC II+ hybrids purified from GM-CSF–supplemented BM culture (day 6) were labeled with CFSE and then subcutaneously injected into C57BL/6 mice. Skin-draining LN samples harvested 48 hours later were examined for the presence of CFSE+ cells. Cryostat sections were examined under fluorescence microscopy after counterstaining with APC-conjugated anti-CD3 mAb (bar indicates 50 μm). (C-E) Ly6G+/CD11chigh/MHC II+ hybrids and Ly6G−/CD11chigh/MHC II+ traditional DCs purified from GM-CSF–supplemented BM culture from C57BL/6 (CD45.2) mice were pulsed for 30 minutes with OVA-coated latex beads (C) or BSA-coated beads (D). These APC preparations were then intraperitoneally injected into B6 SJL (CD45.1) mice that had received intravenous injection of CFSE-labeled OT-II CD4 T cells (CD45.2) 24 hours earlier. The recipient mice were analyzed 3 days later for the expansion of CD45.2+/TCR Vα2+ T cells in pancreatic LNs. Data shown are actual CFSE profiles (C-D) and the numbers (means ± SD from 3 mice per panel) of CFSElo T cells (E). (F-G) B6 SJL mice received systemic injection of anti–Gr-1 mAb or isotype-matched control IgG and intravenous injection of CFSE-labeled OT-II CD4 T cells both on day 0. (F) These mice (6 mice per panel) received intraperitoneal inoculation of OVA E. coli on day 1 and were examined for the expansion of CD45.2+/TCR Vα2+ T cells in pancreatic LNs. (G) Ly6G+/Ly6C+/CD11chigh neutrophil-DC hybrids or Ly6G−/Ly6C+/CD11chigh traditional DCs purified from the PECs from the second panel of thioglycollate-treated B6 SJL mice were intraperitoneally injected together with OVA E. coli to test their ability to restore the APC function in the previously discussed mice treated with anti–Gr-1 mAb. *P < .05; **P < .01 between the indicated samples. Data are representative of at least 2 independent experiments.
The next question concerned whether neutrophil-DC hybrids can home to draining LNs, where antigen presentation actually takes place. To test this, Ly6G+/CD11chigh/MHC II+ hybrids purified from GM-CSF–supplemented BM culture were labeled with CFSE and then subcutaneously injected into WT recipients. Relatively small, but significant, fractions (0.20 ± 0.14%, 4 mice per panel) of the injected hybrid cells were recovered from the draining LNs, and they were found primarily in the T-cell areas (Figure 6B). Ly6G− traditional DCs purified from our BM culture also showed LN-directed homing with a similar frequency (0.19 ± 0.15%, n = 4), which was in complete agreement with the reported homing efficiency for CD11c+ DCs.25
To examine in vivo APC function, Ly6G+/CD11chigh/MHC II+ hybrids purified from GM-CSF–supplemented BM culture were pulsed with latex particles coated with OVA protein and then introduced into the thioglycollate-induced peritonitis lesions in the CD45.1 recipient mice. The immunological outcome was then assessed by measuring the proliferation of CFSE-labeled OT-II CD4 T cells (CD45.2 background) that had been administered into the recipients. OVA-loaded hybrids induced significant expansion of OT-II T cells as measured by counting the number of CFSElo dividing T cells in the CD45.2+/TCR Vα2+ population harvested from pancreatic LNs (Figure 6C). The control hybrid population loaded with latex particles coated with BSA failed to induce T-cell expansion (Figure 6D). Ly6G− traditional DCs were less efficient in this assay (Figure 6E), perhaps reflecting their limited ability to incorporate latex beads.4 These observations validate the ability of in vitro generated hybrid cells to present a model antigen to CD4 T cells in living animals.
To assess the relative level of functional contribution of neutrophil-DC hybrids to the induction of CD4 T-cell responses to bacterial antigens, we depleted Ly6G+ hybrids (as well as other leukocyte populations expressing Ly6G and/or Ly6C) from CD45.1 mice by systemic injection of anti–Gr-1 mAb that recognizes both Ly6G and Ly6C. After receiving CFSE-labeled OT-II CD4 T cells, the CD45.1 mice were challenged with intraperitoneal inoculation of OVA-expressing E. coli. The mice treated with anti–Gr-1 mAb showed significantly diminished expansion of OVA-specific T cells compared with a control group treated with PBS alone, as well as a second control group treated with isotype-matched control IgG (Figure 6F). Importantly, the magnitude of T-cell expansion was significantly restored by transferring Ly6G+/Ly6C+ neutrophil-DC hybrids purified from the thioglycollate-induced peritonitis lesions in nontreated mice back to the mice treated with anti–Gr-1 (Figure 6G). The Ly6G−/Ly6C+ DC population failed to show a significant activity in this assay, perhaps reflecting the poor ability to ingest live bacteria. In summary, our results imply that neutrophil-DC hybrids play dual protective roles against bacterial infection, by participating in rapid bacterial clearance and bacterial antigen presentation.
Discussion
In the present study, we demonstrate that some neutrophils that extravasate at sites of inflammation or infection differentiate into a unique hybrid population that expresses surface markers of both neutrophils (Ly6G, 7/4, CXCR2, and CD62L) and DCs (CD11c, MHC II, and costimulatory molecules). Moreover, neutrophil-DC hybrids exhibit dual functionality in living animals by clearing bacteria rapidly and by presenting antigens to CD4 T cells. Our in vivo data duplicate our in vitro observations that highly purified neutrophils can give rise to neutrophil-DC hybrids when cultured in the presence of GM-CSF and that those in vitro generated hybrid cells can function as professional phagocytes and APCs.4 Taken together, our findings now demonstrate that full maturation into PMNs represents one, but not the only, differentiation option for neutrophils, thus challenging the classic view of neutrophils as terminally differentiated leukocytes destined to die or to participate primarily in host innate immunity.
Our findings may not be totally unexpected considering the recent notion that neutrophils may function as APCs.14,26,27 For example, human neutrophils have been shown to acquire the abilities to present superantigen to CD4 T cells and to activate allogeneic T cells when cultured in the presence of selected cytokines.5,6 A potent APC capacity to present foreign protein antigen to CD4 and CD8 T cells has been observed for Ly6G+ neutrophil fractions purified from thioglycollate-induced peritonitis lesions in mice.28,29 It is conceivable that those Ly6G+ fractions contain substantial numbers of neutrophil-DC hybrids expressing Ly6G. In addition to activation of T cells, neutrophils have been reported to promote immunoglobulin class switching and antibody production by B cells,30 IgG-mediated systemic anaphylaxis,31 and development and function of natural killer cells.32 These observations, together with our findings with neutrophil-DC hybrids, imply that neutrophils can make a wide variety of contributions to the host immunity.
Cheong et al3 have recently demonstrated in vivo differentiation of circulating monocytes into CD209+/CD206+ Mo-DCs. We show that intraperitoneal inoculation of E. coli induces the emergence of neutrophil-DC hybrids in the inflamed peritoneal cavity, whereas they demonstrate that intravenous injection of E. coli triggers the recruitment of Mo-DCs to the T-cell area of skin-draining LNs. Neutrophil-DC hybrids differ from Mo-DCs in surface phenotypes; only the former express Ly6G, Ly6C, and CD205,4 whereas CD209 and CD14 are detectable only in the latter.3 Functionally, Mo-DCs purified from the LNs after intravenous injection of OVA-producing E. coli induced marked proliferation of OVA-reactive CD8 T cells,3 whereas neutrophil-DC hybrids purified from the peritoneal cavity after intraperitoneal injection of OVA–E. coli activated OVA-reactive CD4 T cells, but not CD8 T cells. Thus, it is tempting to speculate that bacterial infection triggers simultaneous differentiation of 2 professional phagocyte populations (ie, neutrophils and monocytes) into 2 distinct APC populations equipped with unique functional properties (ie, hybrids capable of entrapping, capturing, and killing bacteria and Mo-DCs capable of cross-presenting bacterial antigens).
In the accompanying manuscript,4 we report that in vitro differentiation of neutrophils into hybrids is promoted by GM-CSF, but not by other growth factors, including Flt3L, macrophage CSF, and granulocyte CSF. In this study, we detected GM-CSF in the peritoneal cavity after thioglycollate injection, corroborating the reports in a casein-induced peritonitis model.33,34 Unlike Flt3L serving as a key growth factor for steady-state DC development from nonmonocytic precursors in BM,35-37 GM-CSF is generally believed to promote differentiation of monocytes into Mo-DCs.2,38 More recently, macrophage-CSF receptor, but not the GM-CSF receptor, has been shown to be required for the differentiation of inflammatory monocytes into Mo-DCs in inflammatory sites.39 On the other hand, GM-CSF receptor and/or GM-CSF are also indispensable for development of CD103+/CD11b+ intestinal DCs40,41 or CD103+/CD207+ dermal DCs,42 respectively. Studies are in progress in our laboratory to determine growth factor requirements for in vivo differentiation of neutrophils into hybrids in a systematic manner.
It is important to state the limitations of this study. First, not all the band cells differentiated into neutrophil-DC hybrids at inflammatory sites. More specifically, only 20% to 30% of the adoptively transferred band cells exhibited surface expression of CD11c and MHC II when recovered from the inflamed peritoneal cavity. We have confirmed this by injecting the band cell preparations purified from CD11c promoter-driven DTR-EGFP TG mice and from I-Aβ-EGFP knock-in mice; again, only 20% to 30% of the band cells acquired GFP signals. The remaining cells that failed to differentiate into hybrid cells mostly matured into PMNs. Thus, it will be important to define mechanisms governing neutrophil→hybrid conversion under inflammatory conditions. Second, our cell depletion/reconstitution experiments were not designed to determine relative contributions of neutrophil-DC hybrids vs traditional DCs; although thioglycollate-induced peritonitis lesions contained ∼10-fold more traditional DCs than the hybrids, the 2 APC populations were reinstated back with the same cell numbers per animal. Obviously, a better experimental system for selective depletion of neutrophil-DC hybrids is required to define their physiological roles in a more definitive manner. Finally, although we have characterized the phenotype and function of neutrophil-DC hybrids emerging at inflammatory sites, their gene expression profiles remain to be determined. A recent, large-scale gene expression profiling study has characterized signature genes expressed by 26 distinct DC populations isolated from various organs in mice.43 Thus, it will be interesting to determine whether neutrophil-DC hybrids resemble 1 or more of those DC populations based on signature gene expression profiles.
Neutrophil-DC hybrids were found, albeit in varying numbers, in all tested disease models, that is, thioglycollate-induced peritonitis, bacterial peritonitis, chronic skin inflammation in KC-Tie2 TG mice, cockroach allergen–induced lung inflammation, and bacterial lymphadenitis. These models were selected arbitrarily based on a single criterion (ie, abundant neutrophil infiltration). Thus, we predict that emergence of neutrophil-DC hybrids is a relatively common feature observed in a broader spectrum of inflammatory conditions and diseases. In fact, unusual neutrophils expressing APC markers have also been found in inflammatory lesions. For example, neutrophils isolated from the synovial fluid of patients with rheumatoid arthritis have been shown to express MHC II and CD83.44,45 MHC II+ neutrophils have also been detected in peripheral blood samples from autoimmune vasculitis patients.46,47 Furthermore, MHC II+/CD86+ neutrophils have been identified experimentally induced colitis lesions in mice.48 Although our data suggest dual protective roles for neutrophil-DC hybrids against bacterial infection, their potential contribution(s) to the pathophysiology of inflammatory diseases remain totally unknown. In the K/B x N mouse model of rheumatoid arthritis, anti-Ly6G mAb administration (which depletes both neutrophils and hybrids) not only completely prevented the disease onset but also reversed disease progression.49 Thus, it is tempting to speculate that neutrophil-DC hybrids may contribute to the pathophysiology of autoimmune inflammatory diseases. We believe that the present study provides both conceptual and technical frameworks for studying the immunobiology of neutrophil-DC hybrids in various disease models and even in human patients. Such studies may ultimately lead to the development of novel therapeutic approaches that are specifically designed to control neutrophil→hybrid conversion.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Mr Sean Linkes and Dr Kristen Williams for technical assistance. The authors also thank Dr Casey T. Weaver and Dr Marianne Boes for providing the pnir15.OVA E. coli strain and I-Aβ-EGFP knock-in mice, respectively.
This work was supported by grants from the National Institutes of Health (R01 AR053355-S, RC1ES018026, R01 AR053355, R01 AI1055885, and R01 AR043777) and the Bill and Melinda Gates Foundation.
National Institutes of Health
Authorship
Contribution: S.G. and H.M. performed most experiments, analyzed the data, and drafted the manuscript; T.O., Y.Y., and R.L. made significant contributions to animal experiments; K.P., R.M.B., N.L.W., and T.M. provided key materials; and A.T. designed the study, interpreted the data, and finalized the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Akira Takashima, Department of Medical Microbiology and Immunology, University of Toledo College of Medicine, 3000 Arlington Ave, Toledo, OH 43614-5806; e-mail: akira.takashima@utoledo.edu.
References
Author notes
S.G. and H.M. contributed equally to this study.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal