Key Points
B-cell subpopulation as biomarker for NIH-defined BOS.
Abstract
Bronchiolitis obliterans syndrome (BOS), pathognomonic for chronic graft-versus-host disease (cGVHD) of the lung, is a progressive and often fatal complication after allogeneic hematopoietic cell transplantation (HCT). Biomarkers for the prediction and diagnosis of BOS are urgently needed to improve patients’ prognosis. We prospectively evaluated B-cell subpopulations and B-cell activating factor (BAFF) in 136 patients (46 BOS, 41 no cGVHD, 49 cutaneous cGVHD) to define novel biomarkers for early diagnosis of National Institutes of Health-defined BOS diagnosed a median of 11 mo after HCT. Patients with newly diagnosed BOS had significantly higher percentages of CD19+CD21low B cells (25.5 versus 6.6%, P < .0001), BAFF (7.3 versus 3.5 ng/mL, P = .02), and BAFF/CD19+ ratio (0.18 versus 0.02 ng/103 CD19+ B cells, P = .007) compared with patients without cGVHD. The area under the receiver operating curve for CD19+CD21low B cells was 0.97 (95% confidence interval, 0.94-0.99) and a cutoff point >9% was optimal for diagnosing BOS in patients with first drop of pulmonary function tests with a sensitivity of 96% and a negative predictive value of 94%. Thus, elevated levels of CD19+CD21low B cells are a potential novel biomarker for HCT patients at risk for developing BOS at an early stage and could allow improvement of patient outcome.
Introduction
Chronic graft-versus-host disease (cGVHD) is a frequent and serious complication of allogeneic hematopoietic cell transplantation (HCT) associated with high morbidity and nonrelapse mortality (NRM), functional impairment, prolonged duration of immunosuppressive medication, and poor quality of life.1-4 Affected patients present unique diagnostic clinical features as defined by the National Institutes of Health (NIH)5 and most frequently the skin, oral mucosa, eyes, and liver are involved.3,6 Bronchiolitis obliterans syndrome (BOS) is the only single pathopneumonic manifestation of cGVHD according to the NIH consensus.5 BOS is characterized by the new development of airflow obstruction with a prevalence ranging from 2% to 26% depending on the definition used.7-10 Lung biopsy specimens demonstrate bronchiolitis involving the small airways and fibrinous obliteration of the lumen of respiratory bronchioles and inflammatory cell infiltrates and variable degrees of intralumental or peribronchiolar fibrosis.7 Currently, the cause of BOS is still unknown and risk factors associated with BOS include the use of peripheral blood (PB) stem cells, use of busulfan or methotrexate (MTX) during transplant, intensity of the conditioning regimen, impaired pretransplant lung function, low serum immunoglobulin (Ig) levels, and respiratory tract infections during the first 100 d after HCT.7,9,11-13 The clinical factor most commonly associated with the development of BOS is the presence of cGVHD in another organ.7,8,10,14 The NIH cGVHD consensus project developed a definition of BOS in an effort to standardize definitions for comparisons of data among research centers.5 A subsequent modification of these criteria required absence of infection, another manifestation of cGVHD, and forced expiratory volume in 1 s (FEV1, <75% predicted or decline >10%) and either signs of obstruction as seen in FEV1/slow vital capacity <0.7 or obstruction in the pulmonary function test (PFT) defined as residual volume or residual volume/total lung capacity >120% and signs of air trapping on expiratory high-resolution computed tomography (HR-CT).5,7,10,15 The pathologic finding of obliterative bronchiolitis (BO) in lung biopsy is considered to be a diagnostic feature of pulmonary cGVHD.5,7,10,15
The prognosis of BOS is poor, with an overall survival (OS) of 44% at 2 y and 13% at 5 y.7,8,10,15 Despite advances in immunosuppressive treatment and supportive care, the dismal prognosis of BOS has remained unchanged. Better identification of patients at risk for BOS and those who have developed very early disease at a stage when the allo-injury may be more amenable to immunosuppressive therapies could improve patients’ survival and avoid irreversible organ damage. The early diagnosis of BOS remains challenging. Because patients are asymptomatic in the early stages, diagnosis is often delayed until significant airflow obstruction has occurred, leading to dyspnea on exertion and poor exercise tolerance.5,7,15 No biomarkers for BOS have yet been validated in large prospective cohorts.
B cells are increasingly assigned an important role in the pathogenesis of cGVHD.16-19 Previously, we demonstrated abnormalities of B-cell function in patients with active cGVHD seen as a distortion of B-cell homeostasis with an elevation of CD19+CD21low B cells and CD19+CD21int-highCD38high IgMhigh transitional B cells as well as an excess of B-cell activating factor (BAFF).16,17 Furthermore, assessment of B-cell subpopulations and BAFF/B-cell ratios in cGVHD patients allowed a distinction of different impairments of humoral immunity seen as either immunodeficiency or autoimmunity.17 BAFF is a cytokine of the tumor necrosis family that plays an important role in B-cell homeostasis20 and elevated BAFF levels correlated with the occurrence of cGVHD and therapeutic response.18,21,22 Furthermore, genetic variation in recipient BAFF reportedly modulates the phenotype of GVHD.23 Recently, Allen and colleagues24 observed a heightened metabolic state and resistance to apoptosis in B cells as well as ongoing signaling through the AKT and extracellular signal-regulated kinase pathway, suggesting a mechanistic link between increased BAFF levels, aberrant survival of B cells, and disease pathogenesis in patients with cGVHD.
Here, we investigated B-cell subpopulations and plasma BAFF levels as potential noninvasive biomarkers for diagnosis of BOS and patients’ prognosis by analyzing cGVHD patients with NIH-defined BOS in comparison with allogeneic HCT recipients without cGVHD and patients with cGVHD of the skin without lung involvement. Findings in newly diagnosed BOS patients were confirmed in patients with refractory BOS.
Patients and methods
Patients and trial conduct
Since June 2005, all consecutive patients given an allogeneic HCT at our institution and alive by d 100 were asked to participate in a prospective study to investigate the incidence and severity of cGVHD according to NIH-defined criteria and patient outcome.6 Patients gave written informed consent in accordance with the Declaration of Helsinki. The study had the approval of the Ethics Committee of the Medical University of Vienna. No medical interventions were included in the study and transplant clinicians in charge of the respective patients had no access to laboratory results. During routine follow-up visits in the outpatient clinic, patients were assessed for the presence of cGVHD, including physical exams and laboratory tests. All patients had PFTs with arterial blood gas analysis prior to the start of conditioning therapy and starting on d 100 after HCT every 3 mo for 3 y; in the case of a decrease of FEV1 >10% compared with the value prior to HCT, HR-CT of the lungs and bronchoalveolar lavage (BAL) with transbronchial biopsy were performed.
So far, a total of 188 patients have been enrolled in this study. Here, we compared the subset of 46 patients developing BOS (supplemental Table 1) to 41 patients without evidence of cGVHD and 49 with cutaneous cGVHD without lung involvement enrolled during the same study period. The patient and transplant characteristics are shown in Table 1. Five patients (nos. 16, 20, 23, 25, and 31 in supplemental Table 1) had impairment of PFTs prior to HCT (FEV1 < 75%). Twenty-one patients with BOS were previously reported.17
Patient and transplant characteristics
| . | All n (%) . | BOS n (%) . | cGVHD skin n (%) . | No cGVHD n (%) . | P . |
|---|---|---|---|---|---|
| Number of patients | 136 | 46 | 49 | 41 | |
| Median age in years (range) | 41 (19-72) | 37 (19-63) | 43 (21-72) | 46 (20-65) | |
| Gender | |||||
| Male | 62 (46) | 24 (52) | 20 (41) | 18 (44) | |
| Female | 74 (54) | 22 (48) | 29 (59) | 23 (56) | |
| Diagnosis | |||||
| Acute leukemia | 99 (71) | 34 (74) | 36 (75) | 29 (70.7) | |
| Chronic myeloid leukemia | 6 (4) | 3 (6.5) | 3 (6) | 0 | |
| Malignant lymphoma | 16 (12) | 7 (15.2) | 4 (8) | 5 (12.2) | |
| Other† | 15 (11) | 2 (4.3) | 6 (12) | 7 (17.1) | |
| Disease status at HCT | |||||
| Standard risk‡ | 61 (45) | 25 (54) | 15 (31)** | 21 (51)** | *.054 |
| High risk | 75 (55) | 21 (46) | 34 (69) | 20 (49) | |
| Conditioning regimens | |||||
| Myeloablative | 88 (65) | 32 (70)* | 38 (78) | 18 (44)* | *.018 |
| Reduced intensity | 48 (35) | 14 (30) | 11 (25)** | 23 (56)** | **.002 |
| Stem cell donors | |||||
| Related | 34 (25) | 11 (24) | 13 (27) | 10 (24) | |
| Unrelated | 102 (75) | 35 (76) | 36 (73) | 31 (76) | |
| HLA-identical | 115 (84) | 36 (78)* | 40 (82) | 39 (95)* | *.02 |
| HLA-mismatch | 21 (16) | 10 (22) | 9 (18) | 2 (5) | |
| Stem cell source | |||||
| Bone marrow | 6 (4) | 2 (4) | 3 (6) | 1 (2) | |
| PB | 128 (94) | 43 (93) | 45 (92) | 40 (98) | |
| Umbilical cord | 2 (1) | 1 (2.2) | 1 (2) | 0 | |
| Median number of CD34+ cells ×106/kg transplanted (range) | 6.5 (0.9-9.9) | 6.5 (0.9-9.5) | 6.5 (2.8-9.9) | 6.5 (1.4-9.9) | |
| Prophylaxis of acute GVHD | |||||
| CSA | 3 (2) | 1 (2) | 1 (2) | 1 (2) | |
| CSA + MTX | 94 (69) | 39 (85)* | 37 (76) | 18 (44)* | *.0001 |
| CSA + MMF | 39 (29) | 6 (13) | 11 (22)** | 22 (54)** | **.003 |
| . | All n (%) . | BOS n (%) . | cGVHD skin n (%) . | No cGVHD n (%) . | P . |
|---|---|---|---|---|---|
| Number of patients | 136 | 46 | 49 | 41 | |
| Median age in years (range) | 41 (19-72) | 37 (19-63) | 43 (21-72) | 46 (20-65) | |
| Gender | |||||
| Male | 62 (46) | 24 (52) | 20 (41) | 18 (44) | |
| Female | 74 (54) | 22 (48) | 29 (59) | 23 (56) | |
| Diagnosis | |||||
| Acute leukemia | 99 (71) | 34 (74) | 36 (75) | 29 (70.7) | |
| Chronic myeloid leukemia | 6 (4) | 3 (6.5) | 3 (6) | 0 | |
| Malignant lymphoma | 16 (12) | 7 (15.2) | 4 (8) | 5 (12.2) | |
| Other† | 15 (11) | 2 (4.3) | 6 (12) | 7 (17.1) | |
| Disease status at HCT | |||||
| Standard risk‡ | 61 (45) | 25 (54) | 15 (31)** | 21 (51)** | *.054 |
| High risk | 75 (55) | 21 (46) | 34 (69) | 20 (49) | |
| Conditioning regimens | |||||
| Myeloablative | 88 (65) | 32 (70)* | 38 (78) | 18 (44)* | *.018 |
| Reduced intensity | 48 (35) | 14 (30) | 11 (25)** | 23 (56)** | **.002 |
| Stem cell donors | |||||
| Related | 34 (25) | 11 (24) | 13 (27) | 10 (24) | |
| Unrelated | 102 (75) | 35 (76) | 36 (73) | 31 (76) | |
| HLA-identical | 115 (84) | 36 (78)* | 40 (82) | 39 (95)* | *.02 |
| HLA-mismatch | 21 (16) | 10 (22) | 9 (18) | 2 (5) | |
| Stem cell source | |||||
| Bone marrow | 6 (4) | 2 (4) | 3 (6) | 1 (2) | |
| PB | 128 (94) | 43 (93) | 45 (92) | 40 (98) | |
| Umbilical cord | 2 (1) | 1 (2.2) | 1 (2) | 0 | |
| Median number of CD34+ cells ×106/kg transplanted (range) | 6.5 (0.9-9.9) | 6.5 (0.9-9.5) | 6.5 (2.8-9.9) | 6.5 (1.4-9.9) | |
| Prophylaxis of acute GVHD | |||||
| CSA | 3 (2) | 1 (2) | 1 (2) | 1 (2) | |
| CSA + MTX | 94 (69) | 39 (85)* | 37 (76) | 18 (44)* | *.0001 |
| CSA + MMF | 39 (29) | 6 (13) | 11 (22)** | 22 (54)** | **.003 |
CSA, cyclosporine A; MMF, mycophenolate mofetil.
Indicates significant difference between the BOS cohort and the no-cGVHD cohort.
Indicates significant difference between the cGVHD skin cohort and the no-cGVHD cohort.
Other diagnoses included myelodysplastic syndrome and chronic lymphocytic leukemia.
‡Standard risk was defined as acute leukemia in first or second complete remission or chronic myeloid leukemia in first chronic phase. High risk disease included myelodysplastic syndrome and advanced stage of acute and chronic leukemia.
Besides clinical assessment, PB samples were analyzed by flow cytometry on d 100 after HCT, at onset of cGVHD, at onset of pulmonary involvement, and thereafter for up to 3 y. Serum Ig and BAFF levels were serially analyzed at the same time points and correlated with the severity of cGVHD. In patients without cGVHD, PB samples for analysis of B-cell subpopulations and BAFF levels were obtained from d 100 after HCT every 3 mo for 2 y.
Transplant regimens
All patients underwent HCT according to standard-of-care or institutional review board-approved protocols. Significantly more patients with BOS had received myeloablative conditioning (P = .018) and GVHD prophylaxis with cyclosporine A (CSA) and MTX (P = .0001) compared with patients without cGVHD. Significantly more patients with an HLA-identical stem cell donor were in the no-cGVHD cohort (P = .019). Significantly more patients in the no-cGVHD cohort had a standard risk disease (P = .054), received reduced-intensity conditioning (P = .002) and had GVHD prophylaxis with CSA and mycophenolate mofetil (MMF) (P = .003) compared with cutaneous cGVHD. GVHD prophylaxis consisted of CSA with MTX in all patients given myeloablative conditioning and CSA with or without MMF after reduced-intensity conditioning. All patients received antiinfectious prophylaxes according to institutional guidelines.
Acute GVHD was graded weekly until d 100 after HCT and thereafter every 2 to 3 wk.25 Chronic GVHD was graded according to the NIH consensus criteria5 by assessing patients during routine clinical visits in the outpatient clinic (Table 2).
Characteristics of patients with BOS
| . | At onset of BOS (n = 46) . | At maximum grade of BOS (n = 46) . |
|---|---|---|
| n (%) | ||
| NIH-defined cGVHD lung severity score | ||
| 1 | 25 (54) | 16 (35) |
| 2 | 18 (39) | 21 (45) |
| 3 | 3 (7) | 9 (20) |
| LFS | ||
| 0 (< 3) | 1 (2) | 0 |
| 1 (3-5) | 11 (24) | 9 (20) |
| 2 (6-9) | 31 (67) | 28 (60) |
| 3 (10-12) | 3 (7) | 9 (20) |
| Median LFS, range | 7 (2-10) | 8 (4-12) |
| Lung symptoms scale* | ||
| 0 | 37 (80) | 14 (30) |
| 1 | 9 (20) | 21 (46) |
| 2 | 0 | 8 (17) |
| 3 | 0 | 3 (7) |
| Median FEV1, range | 61 (36-75) | 52 (16-75) |
| Patients with FEV1 decline >10% | 42 (91) | 46 (100) |
| Patients with FEV1 ≤55% | 12 (26) | 23 (50) |
| Median FEV1/SVC ratio, range | 0.69 (0.41-0.94) | 0.62 (0.22-0.88) |
| Patients with FEV1/SVC ratio <0.7 | 25 (54) | 28 (61) |
| Median RV %, range | 136 (71-222) | 145 (63-270) |
| Patients with RV >120% | 32 (70) | 35 (76) |
| Median RV/TLC ratio, range | 137 (96-208) | 136 (85-223) |
| Patients with RV/TLC >120% | 32 (70) | 32 (70) |
| NIH-defined HR-CT changes | ||
| Air trapping | 35 (76)** | 39 (85) |
| Bronchiectasis | 2 (4) | 7 (15) |
| Small airway thickening | 25 (54) | 36 (78) |
| Transbronchial biopsies | ||
| Constrictive bronchiolitis | 23/36 (64) | |
| Alveolitis | 13/36 (36) | |
| Median time from d 0 to BOS, mo, range | 11 (3-30) | |
| Median therapies, range | 2.5 (1-7) | |
| . | At onset of BOS (n = 46) . | At maximum grade of BOS (n = 46) . |
|---|---|---|
| n (%) | ||
| NIH-defined cGVHD lung severity score | ||
| 1 | 25 (54) | 16 (35) |
| 2 | 18 (39) | 21 (45) |
| 3 | 3 (7) | 9 (20) |
| LFS | ||
| 0 (< 3) | 1 (2) | 0 |
| 1 (3-5) | 11 (24) | 9 (20) |
| 2 (6-9) | 31 (67) | 28 (60) |
| 3 (10-12) | 3 (7) | 9 (20) |
| Median LFS, range | 7 (2-10) | 8 (4-12) |
| Lung symptoms scale* | ||
| 0 | 37 (80) | 14 (30) |
| 1 | 9 (20) | 21 (46) |
| 2 | 0 | 8 (17) |
| 3 | 0 | 3 (7) |
| Median FEV1, range | 61 (36-75) | 52 (16-75) |
| Patients with FEV1 decline >10% | 42 (91) | 46 (100) |
| Patients with FEV1 ≤55% | 12 (26) | 23 (50) |
| Median FEV1/SVC ratio, range | 0.69 (0.41-0.94) | 0.62 (0.22-0.88) |
| Patients with FEV1/SVC ratio <0.7 | 25 (54) | 28 (61) |
| Median RV %, range | 136 (71-222) | 145 (63-270) |
| Patients with RV >120% | 32 (70) | 35 (76) |
| Median RV/TLC ratio, range | 137 (96-208) | 136 (85-223) |
| Patients with RV/TLC >120% | 32 (70) | 32 (70) |
| NIH-defined HR-CT changes | ||
| Air trapping | 35 (76)** | 39 (85) |
| Bronchiectasis | 2 (4) | 7 (15) |
| Small airway thickening | 25 (54) | 36 (78) |
| Transbronchial biopsies | ||
| Constrictive bronchiolitis | 23/36 (64) | |
| Alveolitis | 13/36 (36) | |
| Median time from d 0 to BOS, mo, range | 11 (3-30) | |
| Median therapies, range | 2.5 (1-7) | |
LFS, lung function score; RV, residual volume; SVC, slow vital capacity; TLC, total lung capacity.
Symptoms reported by the patients as published by Filipovich and colleagues.5 This score distinguished between no symptoms, mild symptoms (shortness of breath after climbing one flight of steps), moderate symptoms (shortness of breath after walking on flat ground), and severe symptoms (shortness of breath at rest, requiring O2).
In 3 patients HR-CT scan of the lungs was not valuable due to poor patient compliance.
Transbronchial biopsies were performed in 41 patients; however, valid diagnosis of biopsy specimens was possible in only 36 patients.
PFT
All PFTs were performed at the Department of Pulmonology in accordance with the guidelines of the American Thoracic Society and the European Respiratory Society task force on standardization of lung function testing,26-28 using the Autobox DL 6200 (Sensor Medics, Vienna, Austria). All diffusion capacity of the lung for carbon monoxide (DLCO) measurements were corrected for hemoglobin values obtained at the time of determination of the diffusion capacity.28 All PFT values, except the FEV1/vital capacity ratio, were expressed as a percentage of predicted values and categorically assessed.
In accordance with NIH recommendations, the lung function score (LFS) was calculated according to FEV1 and DLCO, each of which was categorized as follows: 1 = ≥80%, 2 = 70% to 79%, 3 = 60% to 69%, 4 = 50% to 59%, 5 = 40% to 49%, and 6 = <40%.10,15 The scores for FEV1 and DLCO were then summed and categorized as 0-3 according to NIH recommendations as follows: LFS 2 = category 0 (normal), LFS 3-5 = category 1 (mildly abnormal), LFS 6-9 = category 2 (moderately abnormal), and LFS 10-12 = category 3 (severely abnormal). BOS was defined according to modified NIH criteria.5,7,10,15 In the case of missing histological proof of BO, at least one other distinctive manifestation of cGVHD in a separate organ system was required. In cases of decreased PFT, all patients had a routine work-up to exclude respiratory tract infections, including CT scans, microbiologic and fungal cultures from BAL, and viral screen.
Treatment of BOS
As soon as a diagnosis of BOS was established, all patients received immunosuppressive therapy with methylprednisolone at 0.5 to 1 mg/kg. Additional treatment consisted of calcineurin inhibitors in 28 patients and/or extracorporeal photopheresis in 10 patients. All patients were evaluated in 2- to 4-wk intervals during treatment of BOS. If no improvement after 3 mo or progressive disease after 1 mo was observed, the systemic immunosuppressive therapy was changed. Patients received a median of 2.5 (range, 1-7) treatment regimens for BOS. Salvage immunosuppressive therapy of steroid-refractory BOS patients included extracorporeal photopheresis (n = 17), sirolimus (n = 10), MMF (n = 6), tacrolimus (n = 10), rituximab (n = 5), and imatinib (n = 6). The majority of patients received topical treatment consisting of an inhalative steroid and salmeterol as a long-acting bronchodilator. All patients were given additional immunomodulatory treatment with a macrolide in the course of disease.29
Isolation of blood cells, immunophenotyping, and flow cytometry
Immunophenotyping of whole blood cells was performed as reported.16 We used optimal concentrations of the directly conjugated monoclonal antibodies CD3-APC (S4.1), CD4-FITC (VIT4), CD8-PE (VIT 8), CD10-APC (H110a), CD14-PE (MEM-18), CD16-PE (3G8), CD19-FITC (SJ25-C1), CD21-PE (BU32), CD38-FITC (HIT2), CD56-PE (MEM-188), CD45-FITC (VIT200), CD27-FITC (VIT14), and appropriate isotype controls for characterization of B, T, and NK cell subpopulations. Three-color data acquisition was performed on a fluorescence-activated cell sorter Calibur flow cytometer (Becton Dickinson, San Jose, CA). The flow cytometric gating algorithm on circulating CD19+ B cells is shown in Figure 1. The subset of CD19+CD21low B cells contains both CD21negative as well as CD19+CD21low B cells as defined.30
Flow cytometric gating algorithm on PB B-cell subpopulations in patients without cGVHD (no cGVHD) and BOS. Whole blood was analyzed by multicolor flow cytometry. B cells as defined by CD19 positivity and side scatter characteristics (SSC) were further stained in various combinations for IgD, CD21, CD27, CD38, and CD10. Numbers indicate percent of cells within respective gates.
Flow cytometric gating algorithm on PB B-cell subpopulations in patients without cGVHD (no cGVHD) and BOS. Whole blood was analyzed by multicolor flow cytometry. B cells as defined by CD19 positivity and side scatter characteristics (SSC) were further stained in various combinations for IgD, CD21, CD27, CD38, and CD10. Numbers indicate percent of cells within respective gates.
Assessment of BAFF by enzyme-linked immunosorbent assay
Patients’ plasma samples were examined for soluble BAFF by enzyme-linked immunosorbent assay (R&D Systems, Minneapolis, MN) according to the manufacturer’s instructions.
Assessment of PB Ig levels
Serum levels of IgG, IgM, and IgA were quantified by nephelometry (BNH, Dade Behring, Stratham, NH). The normal values were: IgG, 700 to 1600 mg/dL; IgA, 70 to 400 mg/dL; and IgM, 40 to 230 mg/dL. In all patients with elevation of Ig levels, standard serum protein electrophoresis was performed to exclude oligoclonal B-cell reconstitution.
Statistical analysis
The study was designed as a prospective cohort study including 136 patients that were followed longitudinally. NRM was defined as any death in the absence of the underlying malignancy. Cumulative incidence of NRM was estimated by using the Kaplan-Meier method with adjustment for relapse as a competing risk event.31 Relapse was defined as recurrence of malignancy after achievement of complete remission or any progression of malignant disease. OS was calculated from d 0 of HCT to the day of death from any cause or last follow-up. Patients were censored at the date of last contact. OS was modeled using Cox regression methods.
Statistical pairwise comparisons of B-cell subpopulations and BAFF levels with 2 patient cohorts were made using the Student t test. Differences in diagnostic parameters between BOS patients and those without cGVHD or cutaneous cGVHD without lung involvement were assessed with Fisher’s exact test for continuous values and Student t tests of association for categorical values. Of note, PFT values and other covariates for BOS were considered as categorical variables in the analyses. Areas under the curve (AUCs) and corresponding 95% confidence intervals (CIs) were computed nonparametrically.
Differences were considered statistically significant at a P value < .05. The data were calculated using SPSS Version 17.0 (IBM, New York, NY).
Results
We prospectively compared 46 patients with BOS to 41 without cGVHD and 49 with cutaneous cGVHD without lung involvement (Table 1) and analyzed clinical, cellular, and plasma variables as potential biomarkers for the occurrence and severity of BOS. Onset of cGVHD was observed a median of 143 d (range, 74-732 d) after allogeneic HCT consisting of mild disease in 9, moderate disease in 34, and severe disease in 3 patients (supplemental Table 2). The most frequently affected organs at onset of cGVHD were skin (89%), eyes (87%), oral mucosa (76%), and liver (80%). Seventy percent of patients had NIH-defined classic cGVHD and 33% had the progressive onset type of cGVHD.
Clinical characterization of BOS
During the prospective and serial assessments of all patients, BOS was diagnosed in 46 at a median of 11 mo (range, 3-30 mo) after HCT (Table 2). The overall cumulative incidence of BOS at 3 and 5 y was 27% (95% CI, 20% to 34%) and 30% (95% CI, 22% to 38%), respectively. At the time of diagnosis of BOS, all patients had other manifestations of cGVHD, including skin (n = 28), eyes (n = 30), mouth (n = 11), and liver (n = 28) (supplemental Table 2). In 15 patients (33%), lung involvement was already present at the onset of cGVHD. At onset, 54% of patients had NIH-defined mild lung involvement and the mean of total lung capacity and DLCO were 96% (range, 77% to 119%) and 59% (range, 34% to 89%), respectively. Only 9 patients (20%) had respiratory symptoms at onset [lung symptom scale (LSS) 1] compared with 70% at maximum grade of BOS. At onset, distinctive signs of BOS in HR-CT were present in 35 patients (76%).5 Among 36 patients with transbronchial biopsy suitable for assessment, 23 (64%) had histological confirmation of BO (supplemental Tables 2 and 3).32
During a median follow-up of 35 mo (range, 11.6-66 mo) after HCT, the maximum severity of pulmonary cGVHD according to NIH lung scores was mild, moderate, and severe in 35%, 45%, and 20% of patients, respectively. The median LFS at onset and maximum of pulmonary cGVHD were 7 (range, 2-10) and 8 (range, 4-12), respectively. The median FEV1 values at onset and maximum of BOS were 61% (range, 36% to 75%) and 52% (range, 16% to 75%), respectively.
Relative numbers of CD19+CD21low B cells as potential diagnostic biomarker of new onset BOS
Early diagnosis of BOS is clinically challenging, because afflicted patients can be clinically asymptomatic for prolonged periods of time and in symptomatic patients, differential diagnoses such as infections and toxicities have to be kept in mind. Thus, an objective biomarker for diagnosis of BOS could allow timely clinical intervention and may lead to improved patient outcome.
Serum IgG levels were significantly lower in newly diagnosed BOS patients (533 vs 1172 mg/dL, P < .0001) compared with ones without cGVHD. Comparison of different T-cell subpopulations revealed significant differences solely in CD4+ T cells (23.6% vs 17.5%, P = .004) in univariate analyses.
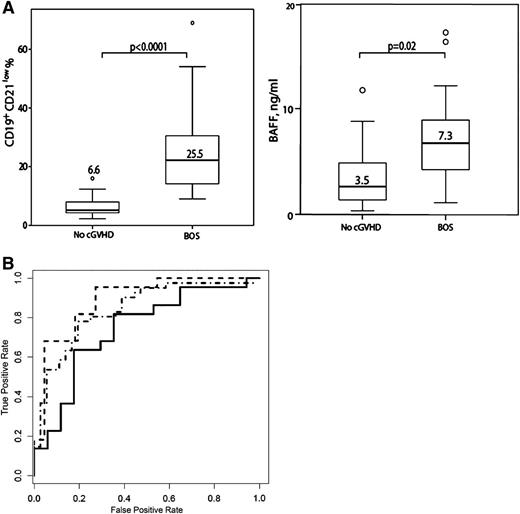
Analyses of BAFF levels and B-cell subpopulations were performed in 46 patients at first decline of PFTs in combination with distinct changes in HR-CT of the lungs, and the results were compared with those obtained in 41 patients without cGVHD analyzed at the same time, a median of 11 mo after HCT (Table 3). Patients with newly diagnosed BOS had significantly higher BAFF levels compared with patients without cGVHD (7.3 vs 3.5 ng/mL, P = .02). Absolute numbers (146 vs 317 × 106/L, P < .0001) as well as percentages (10.2% vs 19.7%, P < .0001) of CD19+ B cells were significantly lower in cGVHD patients at onset of BOS compared with those without cGVHD. The BAFF/CD19+B-cell ratio was significantly higher in the newly diagnosed BOS cohort (0.18 vs 0.02 ng BAFF/CD19+ × 103 B cells, P = .007) compared with patients without cGVHD. Patients with newly diagnosed BOS demonstrated significantly higher percentages of CD19+CD21low B cells (25.5% vs 6.6%, P < .0001) compared with the cohort without cGVHD (Figure 2A). CD19+CD21low B cells were comprised of cells with a CD38high and CD38low phenotype. CD19+CD21lowCD38high B cells consisted mostly of transitional B cells with high IgM expression levels, CD10 positivity, and CD27 negativity, whereas the fraction of CD19+CD21low CD38highIgMlow plasmablasts was small. In some BOS patients, a CD19+CD21lowCD38low B-cell population was significantly expanded. Only one-quarter (22.1 ± 9.8%) of these cells coexpressed CD27 but all were CD10negative. Patients with BOS had significantly higher percentages of CD38high (7.1% vs 3.8%, P = .003) and CD38low (11.6% vs 4.5%, P = .0004) CD19+CD21low B cells compared with patients without cGVHD (Figure 3).
Significant differences in cellular and plasma biomarkers between newly diagnosed BOS and no-cGVHD patients in univariate analysis
| . | All BOS . | NIH-BOS . | Other BOS . | No cGVHD . | cGVHD skin . | P NIH-BOS vs other BOS . | P BOS vs no cGVHD . | P BOS vs skin GVHD . |
|---|---|---|---|---|---|---|---|---|
| Number of patients | 46 | 40 | 6 | 41 | 49 | |||
| BAFF, ng/mL | 7.3 | 7.5 | 7.0 | 3.5 | 4.5 | .63 | .02 | .03 |
| CD19+ B cells × 106/L | 146 | 141 | 178 | 317 | 231 | .5 | <.0001 | .05 |
| CD19+ B cells, % of lymphocytes | 10.2 | 10.1 | 16.3 | 19.7 | 11.2 | .14 | <.0001 | .67 |
| Ratio ng BAFF/CD19+ × 103 B cells | 0.18 | 0.19 | 0.15 | 0.02 | 0.05 | .8 | .007 | .003 |
| CD19+CD21low B cells, % of CD19+ B cells | 25.5 | 26.4 | 16.5 | 6.6 | 7.2 | .18 | <.0001 | <.0001 |
| IgG, mg/dL | 533 | 522 | 605 | 1172 | 754 | .5 | <.0001 | .03 |
| CD4+ T-cells, % of lymphocytes | 23.6 | 23.2 | 26.7 | 17.5 | 24.4 | .41 | .004 | .7 |
| . | All BOS . | NIH-BOS . | Other BOS . | No cGVHD . | cGVHD skin . | P NIH-BOS vs other BOS . | P BOS vs no cGVHD . | P BOS vs skin GVHD . |
|---|---|---|---|---|---|---|---|---|
| Number of patients | 46 | 40 | 6 | 41 | 49 | |||
| BAFF, ng/mL | 7.3 | 7.5 | 7.0 | 3.5 | 4.5 | .63 | .02 | .03 |
| CD19+ B cells × 106/L | 146 | 141 | 178 | 317 | 231 | .5 | <.0001 | .05 |
| CD19+ B cells, % of lymphocytes | 10.2 | 10.1 | 16.3 | 19.7 | 11.2 | .14 | <.0001 | .67 |
| Ratio ng BAFF/CD19+ × 103 B cells | 0.18 | 0.19 | 0.15 | 0.02 | 0.05 | .8 | .007 | .003 |
| CD19+CD21low B cells, % of CD19+ B cells | 25.5 | 26.4 | 16.5 | 6.6 | 7.2 | .18 | <.0001 | <.0001 |
| IgG, mg/dL | 533 | 522 | 605 | 1172 | 754 | .5 | <.0001 | .03 |
| CD4+ T-cells, % of lymphocytes | 23.6 | 23.2 | 26.7 | 17.5 | 24.4 | .41 | .004 | .7 |
Patients with cGVHD had erythematous lesions alone (n = 37), erythematous lesions and moveable sclerosis (n = 4), moveable sclerosis alone (n = 2), or superficial and deep sclerotic features (n = 6). NIH-BOS, BOS defined according to the modified NIH criteria7,15 ; other BOS, patients not fulfilling all criteria of the NIH definition but having BOS according to pulmonary specialist assessment.
(A) BAFF and CD19+CD21low B cells at onset of BOS compared with patients without cGVHD (no cGVHD). Patients with newly diagnosed BOS have significantly higher BAFF levels and percentages of CD19+CD21low B cells compared with the no cGVHD cohort. Data are shown as box plots with medians and ranges, and P values are indicated. (B) Receiver operating characteristic curves for percentages of CD19+CD21low B cells (dotted line), the ratio of BAFF/CD19+ B cells (dotted and dashed line), and BAFF (solid line). Individual receiver operating characteristic curves are shown.
(A) BAFF and CD19+CD21low B cells at onset of BOS compared with patients without cGVHD (no cGVHD). Patients with newly diagnosed BOS have significantly higher BAFF levels and percentages of CD19+CD21low B cells compared with the no cGVHD cohort. Data are shown as box plots with medians and ranges, and P values are indicated. (B) Receiver operating characteristic curves for percentages of CD19+CD21low B cells (dotted line), the ratio of BAFF/CD19+ B cells (dotted and dashed line), and BAFF (solid line). Individual receiver operating characteristic curves are shown.
CD38 expression of CD19+CD21low B cells. BOS patients have significantly higher percentages of CD38high and CD38low B cells compared with patients without cGVHD. PB mononuclear cells were stained with monoclonal antibodies against CD19, CD21, and CD38. Data are shown as box plots with medians and ranges, and P values are indicated.
CD38 expression of CD19+CD21low B cells. BOS patients have significantly higher percentages of CD38high and CD38low B cells compared with patients without cGVHD. PB mononuclear cells were stained with monoclonal antibodies against CD19, CD21, and CD38. Data are shown as box plots with medians and ranges, and P values are indicated.
Because 6 patients (nos. 1, 7, 12, 16, 22, and 30 in supplemental Table 1) did not fulfill all the criteria of the modified NIH definition7,15 but had BOS according to a pulmonary specialist’s assessment and thus were included in our prospective study, we compared these to 40 patients having NIH-defined BOS. No significant differences in potential cellular and plasma biomarkers were observed between NIH-defined BOS and other BOS patients (Table 3). Therefore, all following analyses were performed with the whole cohort of 46 BOS patients.
Receiver operating characteristic curves of these biomarkers distinguished BOS from non-GVHD. The AUC for relative numbers of CD19+CD21low B cells was 0.97 (95% CI, 0.94-0.99) and a cutoff point >9% was found optimal for diagnosing BOS in patients with first decline of PFTs corresponding to a sensitivity of 96% and a negative predictive value (NPV) of 94% (Figure 2B). The AUC for the ratio of BAFF/CD19+ B cells was 0.89 (95% CI, 0.79-0.98) and a cutoff point <0.05 had a sensitivity of 68% and a NPV of 70%. The AUC for BAFF was 0.74 (95% CI, 0.58-0.90) and a cutoff point >3 ng/mL had a sensitivity of 82% and a NPV of 73%, as shown in Figure 2B. The AUC for relative numbers of CD4+ T cells was 0.68 (95% CI, 0.56-0.80).
BAFF and B-cell subpopulations in BOS and cutaneous cGVHD
We prospectively compared 46 patients with newly diagnosed BOS to 49 patients with cutaneous manifestations of cGVHD without lung involvement who were analyzed a median of 11.6 mo (range, 3-21 mo) after HCT (Table 3). BAFF levels (7.3 vs 4.5 ng/mL, P = .03), CD19+CD21low B cells (25.5% vs 7.2%, P < .0001), ratios of BAFF/CD19+ B cells (0.18 vs 0.05 ng BAFF/CD19+ × 103 B cells, P = .003) as well as serum IgG levels (533 vs 754 mg/dL, P = .03) were significantly different between the 2 cohorts. Thus, relative numbers of CD19+CD21low B cells can serve as potential diagnostic biomarkers for new onset BOS.
Correlation of cellular biomarker with severity of BOS
No correlation between CD19+CD21low B cells, BAFF, CD19+ B cells, and ratios of BAFF and B-cell subpopulations and NIH-defined severity scores of pulmonary cGVHD were observed. However, asymptomatic patients with a drop in PFTs and NIH-defined changes in HR-CT (LSS 0) could be distinguished from patients without cGVHD due to significantly higher CD19+CD21low B cells (25.4% vs 6.6%, P < .0001) and ratio of BAFF/CD19+ B cells (0.15 vs 0.02, P = .01). Furthermore, these patients had significantly lower serum IgG levels (535 vs 1172 mg/dL, P = .0001). Thus, clinically asymptomatic patients with elevated numbers of CD19+CD21low B cells and a distortion of B-cell subpopulations already have newly diagnosed BOS and can by biomarkers be distinguished from patients without cGVHD.
Distinction of BOS from respiratory tract infections
We prospectively compared 49 patients with BOS to 46 patients with lung infection occurring a median of 12.6 mo (range, 3-31 mo) after HCT (Table 4). In patients without cGVHD, no significant differences in BAFF and B-cell subpopulations were observed when comparing patients with or without respiratory tract infections. Patients with cGVHD without lung involvement and respiratory tract infection had significantly fewer CD19+CD21low B cells (12.35% vs 25.5%, P = .001), more CD19+ B cells (220 vs 146 × 106/L, P = .04), and higher serum IgG levels (895 vs 533 mg/dL, P = .0007) when compared with newly diagnosed BOS patients. Thus, relative numbers of CD19+CD21low B cells can distinguish BOS from respiratory tract infections both in patients without cGVHD as well as in extrapulmonary cGVHD.
Significant differences in cellular and plasma biomarkers between newly diagnosed BOS and patients with respiratory tract infections in univariate analysis
| . | Newly diagnosed BOS . | Infection and cGVHD . | Infection and no cGVHD . | P BOS vs infection and cGVHD . | P BOS vs infection and no cGVHD . |
|---|---|---|---|---|---|
| Number of patients | 46 | 29 | 17 | ||
| BAFF, ng/mL | 7.3 | 4.6 | 3.7 | .08 | .03 |
| CD19+ B cells × 106/L | 146 | 220 | 330 | .04 | .0001 |
| CD19+ B cells, % of lymphocytes | 10.2 | 12 | 19.2 | .5 | .0021 |
| Ratio ng BAFF/CD19+ × 103 B cells | 0.18 | 0.08 | 0.01 | .08 | .02 |
| CD19+CD21low B cells, % of CD19+ B cells | 25.5 | 12.35 | 7.8 | .001 | .0002 |
| IgG, mg/dL | 533 | 895 | 961 | .0007 | .0004 |
| CD4+ T cells, % of lymphocytes | 23.6 | 21.6 | 23.4 | .4 | .9 |
| . | Newly diagnosed BOS . | Infection and cGVHD . | Infection and no cGVHD . | P BOS vs infection and cGVHD . | P BOS vs infection and no cGVHD . |
|---|---|---|---|---|---|
| Number of patients | 46 | 29 | 17 | ||
| BAFF, ng/mL | 7.3 | 4.6 | 3.7 | .08 | .03 |
| CD19+ B cells × 106/L | 146 | 220 | 330 | .04 | .0001 |
| CD19+ B cells, % of lymphocytes | 10.2 | 12 | 19.2 | .5 | .0021 |
| Ratio ng BAFF/CD19+ × 103 B cells | 0.18 | 0.08 | 0.01 | .08 | .02 |
| CD19+CD21low B cells, % of CD19+ B cells | 25.5 | 12.35 | 7.8 | .001 | .0002 |
| IgG, mg/dL | 533 | 895 | 961 | .0007 | .0004 |
| CD4+ T cells, % of lymphocytes | 23.6 | 21.6 | 23.4 | .4 | .9 |
Cellular biomarkers for prediction of BOS and during the course of BOS
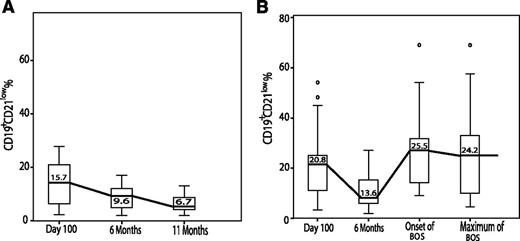
Analyses of B-cell subpopulations and BAFF levels on d 100 after HCT revealed no significant differences between patients developing BOS at later time points and patients not experiencing cGVHD. On d 100, CD19+CD21low B cells (Figure 4) were elevated in both patient cohorts (20.8% in BOS compared with 15.7% in the no cGVHD cohort, P = .18), confirming that the CD21low transitional B cells are the first to leave the BM and subsequently give rise to more mature B-cell subpopulations during improved B-cell reconstitution after HCT.33 At 6 mo after HCT, relative numbers of CD19+CD21low B cells decreased to 13.6% and 9.6% (P = .16). Whereas in patients without cGVHD, CD19+CD21low B cells further decreased to 6.7%, patients with newly diagnosed BOS showed a renewed increase of CD19+CD21low B cells to 25.5% and these remained elevated throughout the course of BOS.
Serial analyses of CD19+CD21low B cells from d 100 after allogeneic HCT until onset of and during the course of BOS (B) compared with patients without cGVHD (no cGVHD) (A). In both patient cohorts, CD19+CD21low B cells decreased 6 mo after HCT, indicating B-cell reconstitution, because CD21low B cells reportedly are the first B-cell population to be exported from the bone marrow into the periphery and decrease during B-cell maturation.33 Whereas in patients without cGVHD (A) percentages of CD19+CD21low B cells further decreased until 11 mo after HCT, in patients with newly diagnosed BOS (B) CD19+CD21low B cells increased again and remained elevated throughout the course of BOS. Data are shown as box plots with medians and ranges. ° indicates outliers.
Serial analyses of CD19+CD21low B cells from d 100 after allogeneic HCT until onset of and during the course of BOS (B) compared with patients without cGVHD (no cGVHD) (A). In both patient cohorts, CD19+CD21low B cells decreased 6 mo after HCT, indicating B-cell reconstitution, because CD21low B cells reportedly are the first B-cell population to be exported from the bone marrow into the periphery and decrease during B-cell maturation.33 Whereas in patients without cGVHD (A) percentages of CD19+CD21low B cells further decreased until 11 mo after HCT, in patients with newly diagnosed BOS (B) CD19+CD21low B cells increased again and remained elevated throughout the course of BOS. Data are shown as box plots with medians and ranges. ° indicates outliers.
Confirmation of potential cellular and plasma biomarkers in patients with long-lasting BOS
Significant differences in B-cell subpopulations and BAFF levels observed between patients with newly diagnosed BOS and those never experiencing cGVHD were confirmed by serial follow-up analyses of BOS patients not responding to systemic immunosuppressive treatment and thus experiencing more severe and long-lasting disease. Significantly elevated BAFF levels, BAFF/CD19+ B-cell ratios, and percentages of CD19+ and CD19+CD21low B cells as well as CD4+ T cells were observed in all patient cohorts with long-lasting BOS compared with patients without cGVHD (Table 5). Of note, relative numbers of CD19+CD21low B cells were 25.5%, 27.8%, 26.5%, 22%, and 25.6% in patients with newly diagnosed BOS, FEV1 ≤ 55%, histologically confirmed BO, LFS 3, and LSS 2 and 3, respectively, and thus significantly elevated both at onset of BOS as well as in patients with long-lasting BOS.
Confirmation of cellular and plasma biomarkers in patients with long-lasting BOS
| . | Onset of BOS . | FEV1 ≤ 55% . | Histological BO . | LFS 3 . | LSS 2+3 . | No cGVHD . |
|---|---|---|---|---|---|---|
| Number of patients | 46 | 23 | 23 | 9 | 11 | 41 |
| BAFF, ng/mL | 7.3* | 8.0* | 9.1* | 8.0* | 7.7* | 3.5 |
| CD19+ B cells × 106/L | 146* | 120* | 117* | 90* | 129 | 317 |
| CD19+ B cells, % | 10.2* | 8.5* | 8.7* | 8.9* | 8.5* | 19.7 |
| Ratio BAFF/CD19+ | 0.18* | 0.19* | 0.18* | 0.47* | 0.21* | 0.02 |
| CD19+CD21low, % | 25.5* | 27.8* | 26.5* | 22* | 25.6* | 6.6 |
| CD4+ T cells, % | 23.62* | 21.2* | 21.8* | 24.3* | 23.2* | 17.5 |
| IgG, mg/dL | 533* | 580* | 512* | 584 | 449* | 1172 |
| . | Onset of BOS . | FEV1 ≤ 55% . | Histological BO . | LFS 3 . | LSS 2+3 . | No cGVHD . |
|---|---|---|---|---|---|---|
| Number of patients | 46 | 23 | 23 | 9 | 11 | 41 |
| BAFF, ng/mL | 7.3* | 8.0* | 9.1* | 8.0* | 7.7* | 3.5 |
| CD19+ B cells × 106/L | 146* | 120* | 117* | 90* | 129 | 317 |
| CD19+ B cells, % | 10.2* | 8.5* | 8.7* | 8.9* | 8.5* | 19.7 |
| Ratio BAFF/CD19+ | 0.18* | 0.19* | 0.18* | 0.47* | 0.21* | 0.02 |
| CD19+CD21low, % | 25.5* | 27.8* | 26.5* | 22* | 25.6* | 6.6 |
| CD4+ T cells, % | 23.62* | 21.2* | 21.8* | 24.3* | 23.2* | 17.5 |
| IgG, mg/dL | 533* | 580* | 512* | 584 | 449* | 1172 |
Indicates significant difference compared with patients without cGVHD.
No prognostic impact of potential cellular and plasma biomarkers on survival and NRM of patients with BOS
In our prospective study, the 5-y probability of OS of cGVHD patients with BOS was 77% compared with 69% in patients without cGVHD (Figure 5A). In univariate analysis, only patients with LSS 1 at onset (44.4% vs 89.2%, P = .001) and LSS 3 at maximum (33% vs 93%, P = .027) had significantly worse OS. Furthermore, patients with an NIH-defined severe lung score at maximum had significantly worse 5-y OS (56% vs 87%, P = .021) compared with moderate and mild disease. No PFT values at BOS peak had a significant impact on OS.
(A) Probability of OS of patients with BOS (dotted line) compared with ones without cGVHD (solid line); (B) Cumulative incidence of NRM of patients with BOS (dotted line) compared with ones without cGVHD (solid line).
(A) Probability of OS of patients with BOS (dotted line) compared with ones without cGVHD (solid line); (B) Cumulative incidence of NRM of patients with BOS (dotted line) compared with ones without cGVHD (solid line).
Nine patients died, including 3 (33.3%) of relapse, 4 (44.5%) of BOS, and 2 (22.2%) of cardiovascular failure. The 5-y cumulative NRM with relapse as a competing risk for all patients was 11% (95% CI, 4% to 19%). It was 15% (95% CI, 4% to 27%) in patients with BOS compared with 7% (95% CI, 2% to 18%) in patients without cGVHD (Figure 5B). Besides a higher LSS at onset of BOS (HR 5.5; 95% CI, 1.2-24; P = .025), no other variable including cellular and plasma biomarkers had a significant influence on NRM in univariate analysis.
The 3- and 5-y cumulative relapse rates with NRM as competing risk for all patients were 14% (95% CI, 6% to 22%) and 30% (95% CI, 11% to 49%). It was 7% (95% CI, 0% to 16%) and 23% (95% CI, 0% to 54%) in patients with BOS compared with 21% (95% CI, 7% to 36%) and 41% (95% CI, 18% to 64%) in patients without cGVHD, respectively.
Discussion
BOS is a serious complication of allogeneic HCT with a dismal prognosis; the OS rate is 13% at 5 y.7-9,11,12,15 Unfortunately, the survival and treatment of patients with BOS have not improved over the last 20 y.12,34,35 Challenges to progress in the medical management of BOS include little knowledge about pathogenesis to direct effective therapies and delay in diagnosis, because patients with lung involvement by cGVHD can remain asymptomatic for prolonged periods of time. We serially performed PFTs starting on d 100 after HCT and in cases of BOS diagnosis, corticosteroid therapy was administered. The main emphasis of this prospective study was on the early diagnosis of BOS, assuming that detection of BOS at an early stage when the immunologic injury of pulmonary tissues may be more amenable to treatment may change the course of the disease’s natural history. This could lead to improved patient outcome. Previous publications expressed this hope; however, no prospective interventional studies have been reported to confirm this assumption. 7,15 A recent survey revealed that screening asymptomatic patients for early detection of pulmonary cGVHD had only been performed by ∼50% of HCT centers.36
We identified, to our knowledge, the first potential diagnostic biomarker for BOS after HCT, namely CD19+CD21low B cells, which were significantly higher in patients with newly diagnosed BOS compared with patients without cGVHD. A cutoff point >9% corresponded to a sensitivity of 96% and a NPV of 94%. Thus, analysis of CD19+CD21low B cells in cGVHD is able to identify almost all patients with incipient lung involvement when 80% of them are still clinically asymptomatic. Patients with respiratory tract infections had significantly fewer CD19+CD21low B cells compared with BOS, which is of major clinical importance, because clinical and radiologic symptoms of pulmonary infection can mimic early BOS.
Significantly higher CD19+CD21low B cells, BAFF, and BAFF/CD19+ B-cell ratios were observed in all cohorts with long-lasting BOS compared with patients without cGVHD, confirming the validity of our potential B-cell biomarkers for early diagnosis of BOS. Patients with cutaneous cGVHD had significantly lower CD19+CD21low B cells, BAFF, and BAFF/CD19+ B-cell ratio compared with BOS patients. Thus, a high percentage of CD19+CD21low B cells can be regarded as a potential predictive marker for the recent development of BOS in patients suffering from cGVHD. Here, the cumulative incidence of BOS was 30%, whereas Arai and colleagues3 recently reported a lower incidence of BOS but 50% of lung involvement by cGVHD in 298 patients with cGVHD prospectively assessed at study enrollment. Because the Chronic GVHD Consortium of the U.S. did not provide any details on the definitions used and had a substantially shorter follow-up time, it must remain unanswered whether longer follow-up would translate into a higher rate of BOS as seen in our study. Historically, BOS was characterized as irreversible, progressive, treatment-refractory obstruction. In our study, the 5-y OS for BOS was 77% and NRM was 15%. Historically, survival of BOS patients was poor7-10,13-15 ; however, previous publications included patients with severe, long-lasting BOS diagnosed at later time points using various diagnostic criteria that may not be comparable with the NIH definition and thus hinder a valid comparison. Arai and colleagues3 reported a 2-y OS of 86% and 62% for patients with moderate and severe NIH-defined cGVHD. Almost 60% of our study population had severe cGVHD and thus, our survival rates compare favorably with published results. Significantly, survival of BOS patients diagnosed early reached a level similar to that of patients without cGVHD. This is most likely due to the fact that NRM was not significantly increased in the BOS cohort, whereas risk for relapse was significantly increased in patients without cGVHD as previously reported.6,37-39
In patients with BOS, CD19+CD21lowCD38high immature transitional B cells defined as previously reported16,17,40 were significantly higher compared with patients without cGVHD. We also observed in some patients with BOS a CD19+CD21lowCD38lowCD10neg B-cell population lacking CD27 coexpression which, so far, has only been identified in HIV patients with high plasma viremia41 and patients with common variable immunodeficiency (CVID).42 In CVID, these CD21low B cells are believed to belong to a polyclonal population of innate-like B cells of the non-memory pool that are enriched in PB, synovial fluid of patients with rheumatoid arthritis, and BAL fluid in the lungs of patients with CVID.42 CD21 represents an important receptor for one of the potent immunoregulatory fragments of complement component 3, ie, C-terminal C3d. CD21/complement receptor 2 has several important functional roles within the immune system.43 In mice, uncoupling of CD21 from the CD19/CD81 complex led to enhanced apoptosis of germinal center B cells, diminished secondary antibody titers and thus overall impairment of humoral immunity.44 Therefore, CD21 not only delivers an important signal for fine-tuning the threshold of B-cell activation but also mediates an important survival signal for B cells within germinal centers. Low to negative expression of CD21 on PB B cells indicates that these cells are not in the position to exert such functions. This represents the rationale for why we regard CD21neg/low B cells as the group of B cells with altered co-receptor capabilities and thus have subsumed the respective populations as one entity.
Little is known regarding the pathogenesis of BOS after HCT. Allorecognition of lung antigens is suspected, because new airflow obstruction generally develops during or after recent withdrawal of immunosuppression. Severe neutrophilic inflammation was observed in the early phase of BOS, whereas prolonged immune activation is known to induce a fibrotic process resulting in airway obliteration.45 During airway wall destruction, bronchus-associated lymphoid tissue is generated and B cells generate autoimmune antibodies as reported against collagen V.46 Srinivasan et al.47 observed CD4+ T-cell and donor-derived B220+ B-cell infiltrations with alloantibody deposition in murine lungs with BO after HCT. In our BOS cohort, BAFF levels and the BAFF/B-cell ratio were significantly higher compared with patients without cGVHD. BAFF promotes B-cell survival and proliferation and is known to be produced by a variety of cells recruited during inflammation, including activated airway epithelial cells leading to local accumulation and activation of B cells.19,48
Although our results are promising, there are limitations to be mentioned. This prospective cohort included only 46 patients with BOS that were serially analyzed at defined time points after HCT and the results were compared with those obtained in patients with cutaneous cGVHD and patients without cGVHD matched for time after HCT. A greater number of patients screened by PFTs, radiology, and biomarker studies is thus warranted to confirm our findings. Per design, this study was not an interventional one; however, our findings suggest that a decrease in PFTs and elevation of CD19+CD21low B cells confirming the early diagnosis of BOS identified patients with cGVHD who responded to early immunosuppressive treatment with corticosteroids. Additional prospective studies will have to be performed to address the significance of clinically asymptomatic lung disease such as NIH-defined mild pulmonary involvement by cGVHD in terms of patient outcome and long-term prognosis.
In conclusion, CD19+CD21low B cells may serve as a potential diagnostic biomarker for early onset of BOS, enable the distinction of BOS from respiratory tract infection and other organ manifestations of cGVHD, and could allow improvement of patient outcome.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Harald Fuehrer from the University of Vienna for his assistance in statistical analyses.
This work was supported by European Commission grant 037703 STEMDIAGNOSTICS and a grant by Austrotransplant.
Authorship
Contribution: H.T.G. and W.F.P. designed the research study, analyzed and interpreted the data, and coauthored the manuscript; Z.K. and R.W. performed the clinical research and collected and analyzed data; P.K. and M.M. performed clinical assessments of cGVHD according to the NIH criteria; K.K. and V.P. performed PFTs and clinical assessments; U.K. and A.R. performed flow cytometric analyses; and L.P. evaluated HR-CT scans and G.D. assessed transbronchial biopsies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hildegard T. Greinix, Medizinische Universitaet Wien, Klinik fuer Innere Medizin I, Knochenmarktransplantation, Waehringer Guertel 18-20, A-1090 Vienna, Austria; e-mail: hildegard.greinix@meduniwien.ac.at; and Winfried F. Pickl, Medizinische Universitaet Wien, Institut fuer Immunologie, Borschkegasse 8a, A-1090 Vienna, Austria; e-mail: winfried.pickl@meduniwien.ac.at.
References
Author notes
H.T.G. and W.F.P. contributed equally to this study.
Presented at the Annual Meeting of the European Group for Blood and Marrow Transplantation, Paris, France, April 2011 and at the Annual Meeting of the American Society of Hematology, Orlando, FL, December 2010.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal