Key Points
Both VEGFA and VEGFB and their receptors, Kdr and Flt1, are involved in retinal neovascularization.
Abstract
To understand the mechanisms of Src-PLD1-PKCγ-cPLA2 activation by vascular endothelial growth factor A (VEGFA), we studied the role of Kdr and Flt1. VEGFA, while having no effect on Flt1 phosphorylation, induced Kdr phosphorylation in human retinal microvascular endothelial cells (HRMVECs). Depletion of Kdr attenuated VEGFA-induced Src-PLD1-PKCγ-cPLA2 activation. Regardless of its phosphorylation state, downregulation of Flt1 also inhibited VEGFA-induced Src-PLD1-PKCγ-cPLA2 activation, but only modestly. In line with these findings, depletion of either Kdr or Flt1 suppressed VEGFA-induced DNA synthesis, migration, and tube formation, albeit more robustly with Kdr downregulation. Hypoxia induced tyrosine phosphorylation of Kdr and Flt1 in mouse retina, and depletion of Kdr or Flt1 blocked hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation and retinal neovascularization. VEGFB induced Flt1 tyrosine phosphorylation and Src-PLD1-PKCγ-cPLA2 activation in HRMVECs. Hypoxia induced VEGFA and VEGFB expression in retina, and inhibition of their expression blocked hypoxia-induced Kdr and Flt1 activation, respectively. Furthermore, depletion of VEGFA or VEGFB attenuated hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation and retinal neovascularization. These findings suggest that although VEGFA, through Kdr and Flt1, appears to be the major modulator of Src-PLD1-PKCγ-cPLA2 signaling in HRMVECs, facilitating their angiogenic events in vitro, both VEGFA and VEGFB mediate hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation and retinal neovascularization via activation of Kdr and Flt1, respectively.
Introduction
Ischemic retinal diseases such as diabetic retinopathy, retinal vein occlusion, and retinopathy of prematurity may precipitate retinal neovascularization.1,2 Pathological retinal angiogenesis, in turn, may cause vitreous hemorrhage, retinal detachment, and/or neovascular glaucoma affecting vision.3 Among the many molecules produced by the hypoxic retina, vascular endothelial growth factor A (VEGFA) is a potent angiogenic and vascular permeability factor.4,5 VEGFA induces angiogenesis via its ability to stimulate growth, migration, and capillary-like structure formation of endothelial cells (ECs) and enhance their permeability.6-9 Among the 5 VEGF molecules identified, VEGFA and VEGFD are reported to be the most potent angiogenic factors.10 Similarly, among the 3 VEGF receptors characterized thus far, namely Flt1, Kdr, and Flt4 (also known as VEGFR1, VEGFR2, and VEGFR3, respectively), VEGFA binds to both Flt1 and Kdr.11-13 On the other hand, VEGFB and VEGFD bind only to Flt1, and VEGFC and VEGFE bind to Kdr as well as Flt4.14-18 Although VEGFA binds to both Flt1 and Kdr, its cellular effects appear to be predominantly mediated by Kdr in ECs.7-9 It was further suggested that even though VEGFA binds to Flt1 with high affinity, activation might not occur because the receptor shows only weak kinase activity.13 Despite its weak kinase activity in response to VEGFA, Flt1 has been shown to suppress tip-cell formation19 and its deletion led to embryonic lethality from excessive vessel overgrowth,20 suggesting that this receptor negatively affects angiogenesis, particularly developmental angiogenesis, although some investigations dispute this assertion.21,22 Recent studies, however, have demonstrated that Flt1 still could mediate some cellular effects of VEGFA such as EC migration.23,24 In addition, the development of antiangiogenic therapies targeting Flt1 for the treatment of tumors and ischemic diseases suggests that Flt1 does play a role in angiogenesis, at least in pathological angiogenesis.25-27 However, due to functional redundancies among these various VEGF molecules and the complexities in their receptor selectivity, antiangiogenic therapies targeting VEGFA or its receptors, Kdr or Flt1, though effective significantly,28-31 appear to be inadequate, because resistance to these therapies develops in both tumors and ischemic diseases.25,32,33 Thus, these inadequacies in current antiangiogenic therapies necessitate further studies to identify the comprehensive mechanisms by which these VEGF molecules and other factors modulate pathological angiogenesis.
We have previously demonstrated that VEGFA activates Src-PLD1-PKCγ-cPLA2 signaling in human retinal microvascular endothelial cells (HRMVECs), facilitating their growth, migration, and tube formation and, in the mouse retina, mediating hypoxia-induced neovascularization.34,35 Because our previous findings showed that activation of Src-PLD1-PKCγ-cPLA2 signaling is required for VEGFA-induced angiogenic effects both in vitro and in vivo, we questioned which VEGF receptor(s) mediates this signaling axis in ECs and retina. In this communication, we report that although VEGFA stimulates tyrosine phosphorylation of Kdr but not Flt1 in HRMVECs, both of these receptors mediate its effects on Src-PLD1-PKCγ-cPLA2 activation, facilitating HRMVEC growth, migration, and tube formation. On the other hand, hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation and retinal neovascularization appear to depend on both VEGFA and VEGFB production as well as Kdr and Flt1 stimulation. Based on these observations, Src-PLD1-PKCγ-cPLA2 signaling could be considered as a drugable pathway for the treatment of ischemic diseases such as retinal neovascularization.
Materials and methods
Reagents
Growth-factor–reduced Matrigel (354230) was obtained from BD Biosciences (Bedford, MA). Recombinant human VEGF165 (293-VE) and recombinant human VEGF-B167 (751-VE) were bought from R&D Systems (Minneapolis, MN). Anti–β-tubulin (SC-9104), anti-cPLA2 (SC-454), anti-PKCγ (SC-211), anti-VEGFB (SC-80442), and anti-VEGFA (SC-152) antibodies were purchased from Santa Cruz Biotechnology (Santa Cruz, CA). Anti-phospho-cPLA2 (2831), anti–phospho-PKCγ (9379), anti–phospho-PLD1 (3831), anti–phospho-Src (2101), anti-KDR (VEGFR2) (2479), and anti-PLD1 (3832) antibodies were obtained from Cell Signaling Technology (Beverly, MA). Anti-Src (05-184) and anti-phosphotyrosine antibodies (05-777) were bought from Millipore (Temecula, CA). Anti-CD31 (550274) antibody was purchased from BD Pharmingen (Palo Alto, CA). Anti–phospho-cPLA2 (ab53105), anti–Ki-67 (ab15580), and anti-FLT1 (VEGFR1) (ab32152) antibodies were obtained from Abcam (Cambridge, MA). Cytosolic PLA2 assay kit (765021) was bought from Cayman Chemicals (Ann Arbor, MI). Control nontargeting small interfering RNA (siRNA) (D-001810-10), human FLT1 siRNA (ON-TARGET plus SMARTpool L-003136-00-0010), human KDR siRNA (ON-TARGET plus SMARTpool L-003148-00-0010), mouse FLT1 siRNA (ON-TARGET plus SMARTpool L-040636-00-0010), mouse KDR siRNA (ON-TARGET plus SMARTpool L-040634-00-0010), mouse VEGFA siRNA (ON-TARGET plus SMARTpool L-040812-00-0010), and mouse VEGFB siRNA (ON-TARGET plus SMARTpool L-047407-01-0010) were obtained from Thermo Scientific (Chicago, IL). Alexa Fluor 488 goat anti-rat immunoglobulin G, Alexa Fluor 568 goat anti-rabbit immunoglobulin G, Hoechst 33342, isolectin B4-594, and Prolong Gold antifade reagent were bought from Molecular Probes (Eugene, OR). [3H]-Thymidine (S. A. 20 Ci/mM) was obtained from Perkin Elmer (Boston, MA).
Cell culture
HRMVECs (catalog number ACBRI 181) were purchased from Applied Cell Biology Research Institute (Kirkland, WA) and grown in medium 131 containing microvascular growth supplements (MVGS), 10 μg/mL gentamycin, and 0.25 μg/mL amphotericin B. Cultures were maintained at 37°C in a humidified 95% air and 5% CO2 atmosphere. Growth arrest was induced in HRMVECs with passage numbers between 5 and 10 by incubating in medium 131 without MVGS for 24 hours and used to perform the experiments unless otherwise indicated.
Cell migration
Cell migration was measured using modified Boyden chamber method as described previously,36 and the values are expressed as number of migrated cells per field.
DNA synthesis
DNA synthesis was measured by [3H]-thymidine incorporation as described previously37 and expressed as counts/min/dish.
Tube formation
Tube formation was measured as described previously.34 The tube-like structures were observed under a phase-contrast light microscope (Nikon Eclipse TS100; type, ADL; original magnification ×10/NA 0.25) and the images were captured by a charged-couple device camera (KP-D20AU, Hitachi) using Apple iMovie 7.1.4 software. The tube length was calculated using NIH ImageJ version 1.43 and expressed in micrometers.
Western blotting
Cell or tissue extracts containing an equal amount of protein were resolved by electrophoresis on 0.1% (weight/volume [w/v]) sodium dodecyl sulfate and 10% (w/v) polyacrylamide gels. The proteins were transferred electrophoretically to a nitrocellulose membrane. After blocking in either 5% (w/v) nonfat dry milk or 5% (w/v) bovine serum albumin, the membrane was probed with the appropriate primary antibodies followed by incubation with horseradish-peroxidase-conjugated secondary antibodies. The antigen-antibody complexes were detected using an enhanced chemiluminescence detection reagent kit (Amersham Biosciences).
Transfections
HRMVECs were transfected with scrambled or target gene siRNA molecules at a final concentration of 100 nM using Lipofectamine 2000 transfection reagent according to the manufacturer’s instructions. After transfections, cells were growth arrested in MVGS-free medium 131 for 24 hours and then used as required. In the case of transfections in vivo, siRNA molecules at 1 µg/0.5 µL/eye were injected intravitreally using a 33G needled syringe.
Retinal angiogenesis
All experiments involving the use of animals were approved by the Animal Care and Use Committee of the University of Tennessee Health Science Center, Memphis, TN. Oxygen-induced retinopathy (OIR) was performed as described by Smith et al38 and quantified according to the method of Connor et al.39 C57BL/6 mice pups (P7) with dams were exposed to 75% oxygen for 5 days and then returned to room air at P12. Mice pups of the same age kept at room air were used as controls. After exposure to hyperoxia, mice pups were administered scrambled siRNA, FLT1 siRNA, KDR siRNA, VEGFA siRNA, or VEGFB siRNA (1 μg/0.5 μL/eye) at P12, P13, and P15 by intravitreal injections using a 33G needle. The mice pups were sacrificed at P17 and eyes were enucleated and fixed in 4% (v/v) paraformaldehyde for 1 hour at room temperature. Retinas were isolated, stained with isolectin B4, flat mounted, placed under a coverslip, and examined by a Zeiss inverted fluorescence microscope (AxioVision AX10). Retinal vasculature was quantified by calculating the ratio of fluorescence intensity to total retinal area. Retinal neovascularization was quantified by first setting a scale with a tolerance point of 50 based on the fluorescence intensity in the screenshot using Nikon NIS-Elements software version AR 3.1. Neovascularity was highlighted in red and then quantified by dividing the fluorescence intensity in the highlighted area by the total fluorescence intensity in the screenshot (n = 6 eyes).
Immunofluorescence staining
After hyperoxia, mouse pups were returned to room air for 3 days, after which time they were sacrificed, eyes enucleated and fixed in optimal cutting temperature compound, and cryosections were prepared. To identify proliferating ECs, after blocking in normal goat serum, the cryosections were probed with rabbit anti-mouse Ki-67 antibodies (1:100) and rat anti-mouse CD31 antibodies (1:100) followed by incubation with Alexa Fluor 568-conjugated goat anti-rabbit and Alexa Fluor 488–conjugated goat anti-rat secondary antibodies. The sections were observed under Zeiss inverted microscope (Zeiss AxioVision AX10; type, LD plan-Neofluar; original magnification ×40/NA 0.6 or type, plan-Apochromat; original magnification ×10/NA 0.45) and the fluorescence images were captured by Zeiss AxioCam MRm camera using the microscope operating and image analysis software AxioVision 4.7.2 (Carl Zeiss Imaging Solutions GmbH). The retinal EC proliferation was quantified by counting Ki67- and CD31-positive cells that extended anterior to the inner limiting membrane per section (n = 6 eyes, 3 sections/eye).
Statistics
All experiments were repeated 3 times and data are presented as mean ± standard deviation (SD). The treatment effects were analyzed by 2-tailed Student t test, and P values < .05 were considered statistically significant. In the case of western blot analysis, immunofluorescence, staining, and retinal angiogenesis, 1 data set is presented.
Results
Kdr and Flt1 mediate VEGFA-induced HRMVEC DNA synthesis, migration, and tube formation
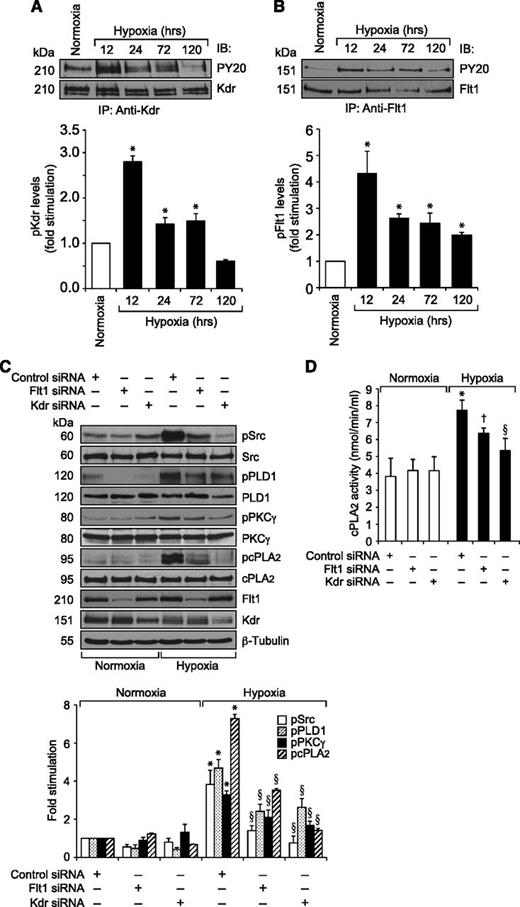
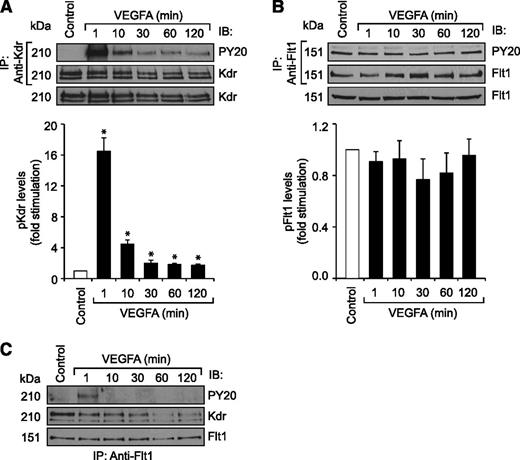
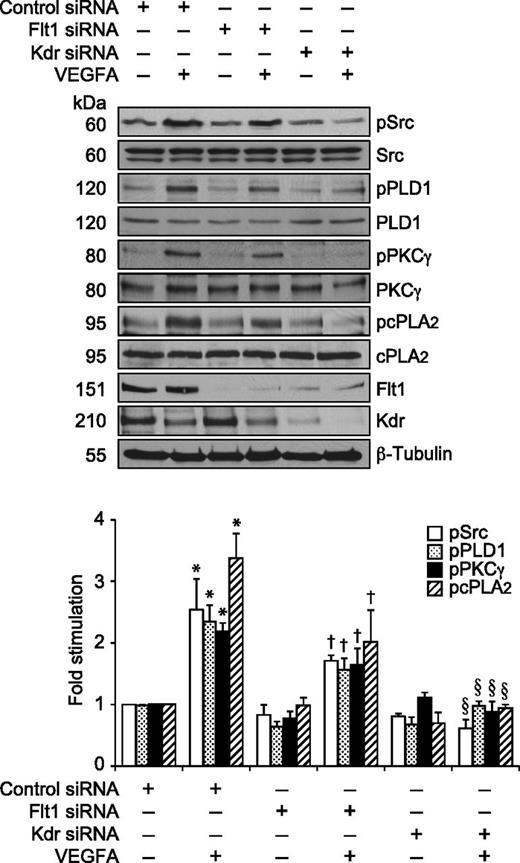
Previously, we have reported that VEGFA activates Src-PLD1-PKCγ-cPLA2 signaling in mediating pathological retinal angiogenesis.34,35 To understand the upstream mechanisms of Src-PLD1-PKCγ-cPLA2 activation by VEGFA in HRMVECs, its effects on Kdr and Flt1 tyrosine phosphorylation were measured. VEGFA induced tyrosine phosphorylation of Kdr in a time-dependent manner with a maximum 15-fold increase at 1 minute and declining thereafter, reaching almost basal level at 2 hours (Figure 1A). On the other hand, VEGFA had no effect on Flt1 tyrosine phosphorylation (Figure 1B). To understand any interactions between Kdr and Flt1, we examined their capacity to form macromolecular complexes. Coimmunoprecipitation experiments revealed that Kdr and Flt1 exist as a heterodimer in quiescent cells, and, upon stimulation with VEGFA, Kdr is tyrosine phosphorylated and dissociates from the complex (Figure 1C). To investigate the role of these receptors in VEGFA-induced Src-PLD1-PKCγ-cPLA2 activation, their steady-state levels were downregulated using their respective siRNA, and the effect of their depletion on Src-PLD1-PKCγ-cPLA2 activation was tested in response to VEGFA. Downregulation of Kdr levels blocked VEGFA-induced activation of Src-PLD1-PKCγ-cPLA2 signaling substantially (Figure 2). In contrast to the lack of effect of VEGFA on Flt1 tyrosine phosphorylation, downregulation of Flt1 levels also inhibited VEGFA-induced Src, PLD1, PKCγ, and cPLA2 phosphorylation, although only modestly (Figure 2). As expected, upon treatment with VEGFA, Kdr levels were depleted, perhaps due to receptor internalization and degradation. Kdr downregulation also led to attenuation of Flt1 levels. In line with the effect of Kdr depletion on Src-PLD1-PKCγ-cPLA2 activation, siRNA-mediated reduction in its steady-state levels suppressed VEGFA-induced HRMVEC DNA synthesis, migration, and tube formation more robustly (Figure 3A-C). Regarding the role of Flt1 in VEGFA-induced angiogenic events, downregulation of Flt1 levels also inhibited VEGFA-induced HRMVEC DNA synthesis, migration, and tube formation modestly (Figure 3A-C), which is consistent with its mild effects on Src, PLD1, PKCγ, and cPLA2 phosphorylation.
VEGFA stimulates Kdr but not Flt1 tyrosine phosphorylation in HRMVECs. (A,B) Quiescent HRMVECs were treated with and without VEGFA (40 ng/mL) for the indicated time periods and cell extracts were prepared. An equal amount of protein from the control and each treatment group was immunoprecipitated with anti-Kdr or anti-Flt1 antibodies and the immunocomplexes were analyzed by western blotting using anti-PY20, anti-Kdr, or anti-Flt1 antibodies. An equal amount of protein from control and each treatment was also analyzed by western blotting for total Kdr and Flt1 levels using their specific antibodies. (C) All the conditions were the same as in panel A except that an equal amount of protein from the control and each treatment group was immunoprecipitated with anti-Flt1 antibodies. The immunocomplexes were analyzed by western blotting using anti-PY20 antibodies, and the blot was reprobed sequentially with anti-Kdr and anti-Flt1 antibodies. The bar graphs represent the quantitative analysis of 3 independent experiments. The values are presented as mean ± SD. *P < .01 vs control. Anti-PY20 antibodies, phosphotyrosine antibodies.
VEGFA stimulates Kdr but not Flt1 tyrosine phosphorylation in HRMVECs. (A,B) Quiescent HRMVECs were treated with and without VEGFA (40 ng/mL) for the indicated time periods and cell extracts were prepared. An equal amount of protein from the control and each treatment group was immunoprecipitated with anti-Kdr or anti-Flt1 antibodies and the immunocomplexes were analyzed by western blotting using anti-PY20, anti-Kdr, or anti-Flt1 antibodies. An equal amount of protein from control and each treatment was also analyzed by western blotting for total Kdr and Flt1 levels using their specific antibodies. (C) All the conditions were the same as in panel A except that an equal amount of protein from the control and each treatment group was immunoprecipitated with anti-Flt1 antibodies. The immunocomplexes were analyzed by western blotting using anti-PY20 antibodies, and the blot was reprobed sequentially with anti-Kdr and anti-Flt1 antibodies. The bar graphs represent the quantitative analysis of 3 independent experiments. The values are presented as mean ± SD. *P < .01 vs control. Anti-PY20 antibodies, phosphotyrosine antibodies.
Kdr but not Flt1 mediates VEGFA-induced Src-PLD1-PKCγ-cPLA2 activation in HRMVECs. HRMVECs transfected with control, Kdr, or Flt1 siRNA (100 nM) and quiesced were treated with and without VEGFA (40 ng/mL) for 30 minutes and cell extracts were prepared. An equal amount of protein from the control and each treatment group was analyzed for pSrc, pPLD1, pPKCγ, and pcPLA2 levels using their phosphospecific antibodies. The blots were reprobed with anti-Src, anti-PLD1, anti-PKCγ, anti-cPLA2, anti-Flt1, anti-Kdr, and anti-β-tubulin antibodies for normalization. The bar graph represents the quantitative analysis of 3 independent experiments. The values are presented as mean ± SD. *P < .01 vs control siRNA; †P < .05 vs control siRNA + VEGFA; §P < .01 vs control siRNA + VEGFA.
Kdr but not Flt1 mediates VEGFA-induced Src-PLD1-PKCγ-cPLA2 activation in HRMVECs. HRMVECs transfected with control, Kdr, or Flt1 siRNA (100 nM) and quiesced were treated with and without VEGFA (40 ng/mL) for 30 minutes and cell extracts were prepared. An equal amount of protein from the control and each treatment group was analyzed for pSrc, pPLD1, pPKCγ, and pcPLA2 levels using their phosphospecific antibodies. The blots were reprobed with anti-Src, anti-PLD1, anti-PKCγ, anti-cPLA2, anti-Flt1, anti-Kdr, and anti-β-tubulin antibodies for normalization. The bar graph represents the quantitative analysis of 3 independent experiments. The values are presented as mean ± SD. *P < .01 vs control siRNA; †P < .05 vs control siRNA + VEGFA; §P < .01 vs control siRNA + VEGFA.
Both Kdr and Flt1 mediate VEGFA-induced HRMVEC DNA synthesis, migration, and tube formation. (A-C) HRMVECs that were transfected with control, Kdr, or Flt1 siRNA (100 nM) and quiesced were treated with and without VEGFA (40 ng/mL) and either DNA synthesis (A), migration (B), or tube formation (C) was measured. DNA synthesis was measured by [3H]-thymidine incorporation. Cell migration was measured by modified Boyden chamber method. The representative HRMVEC migration and tube formation images are shown in panels B and C, respectively. The bar graphs represent the quantitative analysis of 3 independent experiments. The values are presented as mean ± SD. *P < .01 vs control siRNA; †P < .05 vs control siRNA + VEGFA; §P < .01 vs control siRNA + VEGFA.
Both Kdr and Flt1 mediate VEGFA-induced HRMVEC DNA synthesis, migration, and tube formation. (A-C) HRMVECs that were transfected with control, Kdr, or Flt1 siRNA (100 nM) and quiesced were treated with and without VEGFA (40 ng/mL) and either DNA synthesis (A), migration (B), or tube formation (C) was measured. DNA synthesis was measured by [3H]-thymidine incorporation. Cell migration was measured by modified Boyden chamber method. The representative HRMVEC migration and tube formation images are shown in panels B and C, respectively. The bar graphs represent the quantitative analysis of 3 independent experiments. The values are presented as mean ± SD. *P < .01 vs control siRNA; †P < .05 vs control siRNA + VEGFA; §P < .01 vs control siRNA + VEGFA.
Kdr and Flt1 mediate hypoxia-induced retinal neovascularization
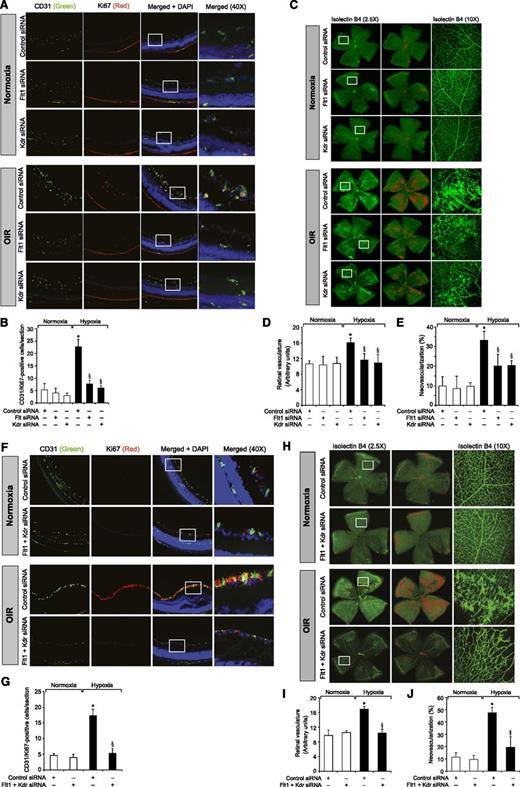
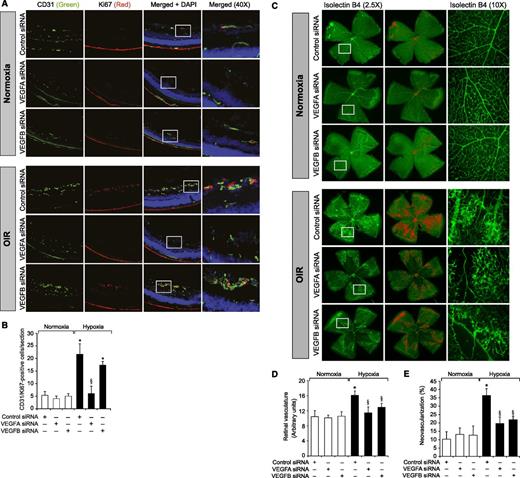
To understand the role of Kdr and Flt1 receptors in both hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation and retinal neovascularization, we first studied the effect of hypoxia on their phosphorylation. Hypoxia induced tyrosine phosphorylation of both Kdr and Flt1 in a time-dependent manner with maximum 3-fold increase at 12 hours and declining thereafter, reaching almost basal levels by 5 days (Figure 4A-B). To test the role of Kdr and Flt1 in hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation, we used an siRNA approach. siRNA-mediated downregulation of either Kdr or Flt1 levels negated the effect of hypoxia on Src-PLD1-PKCγ-cPLA2 phosphorylation almost to the same level (Figure 4C). In line with their involvement in Src-PLD1-PKCγ-cPLA2 activation, siRNA-mediated depletion of either receptor level reduced hypoxia-induced cPLA2 activity (Figure 4D). Because activation of Src-PLD1-PKCγ-cPLA2 signaling is essential for hypoxia-induced retinal neovascularization,34,35 we next tested the role of these receptors in hypoxia-induced retinal angiogenesis. Downregulation of either Kdr or Flt1 levels inhibited hypoxia-induced retinal EC proliferation (Figure 5A,B). In addition, depletion of either receptor level also inhibited hypoxia-induced retinal neovascularization as observed by decreased tortuosity, tufts, and dilation (Figure 5C-E). Based on the above findings, depletion of both Kdr and Flt1 simultaneously substantially inhibited hypoxia-induced retinal EC proliferation and neovascularization (Figure 5F-J).
Both Kdr and Flt1 mediate hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation in the mouse retina. (A,B) C57BL/6 mice pups were exposed to 75% oxygen from P7 to P12, at which time they were returned to normoxia to develop relative hypoxia. At the indicated time periods of hypoxia, their eyes were enucleated and retinas isolated, and tissue extracts were prepared. An equal amount of protein from normoxic and various periods of hypoxic retinal extracts was immunoprecipitated with anti-Kdr or anti-Flt1 antibodies, and the immunocomplexes were analyzed by western blotting using anti-PY20 antibodies. The blots were reprobed with anti-Kdr or anti-Flt1 antibodies for normalization. (C) After exposure to hyperoxia, mice pups were returned to normoxia and administered 1 μg/0.5 μL control, Kdr, or Flt1 siRNA at P12 and P13 by intravitreal injections. At P15, retinas were isolated and extracts prepared and analyzed by western blotting for pSrc, pPLD1, pPKCγ, and pcPLA2 levels using their phosphospecific antibodies. The blots were reprobed with anti-Src, anti-PLD1, anti-PKCγ, anti-cPLA2, anti-Flt1, anti-Kdr, or anti-β-tubulin antibodies for normalization or to show the effect of siRNAs on their target molecules. (D) All the conditions were the same as in panel C except that the retinal extracts were analyzed for cPLA2 activity. The bar graphs represent the quantitative analysis of 3 independent experiments. The values are presented as mean ± SD. *P < .01 vs normoxia or control siRNA; †P < .05 vs control siRNA + hypoxia; §P < .01 vs control siRNA + hypoxia.
Both Kdr and Flt1 mediate hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation in the mouse retina. (A,B) C57BL/6 mice pups were exposed to 75% oxygen from P7 to P12, at which time they were returned to normoxia to develop relative hypoxia. At the indicated time periods of hypoxia, their eyes were enucleated and retinas isolated, and tissue extracts were prepared. An equal amount of protein from normoxic and various periods of hypoxic retinal extracts was immunoprecipitated with anti-Kdr or anti-Flt1 antibodies, and the immunocomplexes were analyzed by western blotting using anti-PY20 antibodies. The blots were reprobed with anti-Kdr or anti-Flt1 antibodies for normalization. (C) After exposure to hyperoxia, mice pups were returned to normoxia and administered 1 μg/0.5 μL control, Kdr, or Flt1 siRNA at P12 and P13 by intravitreal injections. At P15, retinas were isolated and extracts prepared and analyzed by western blotting for pSrc, pPLD1, pPKCγ, and pcPLA2 levels using their phosphospecific antibodies. The blots were reprobed with anti-Src, anti-PLD1, anti-PKCγ, anti-cPLA2, anti-Flt1, anti-Kdr, or anti-β-tubulin antibodies for normalization or to show the effect of siRNAs on their target molecules. (D) All the conditions were the same as in panel C except that the retinal extracts were analyzed for cPLA2 activity. The bar graphs represent the quantitative analysis of 3 independent experiments. The values are presented as mean ± SD. *P < .01 vs normoxia or control siRNA; †P < .05 vs control siRNA + hypoxia; §P < .01 vs control siRNA + hypoxia.
Downregulation of Kdr or Flt1 levels blocks hypoxia-induced retinal EC proliferation and neovascularization. (A) After exposure to 75% oxygen, the mice pups were returned to normoxia, administered 1 μg/0.5 μL control, Kdr, or Flt1 siRNA at P12 and P13 by intravitreal injections, and the retinas were isolated at P15 and fixed; cross-sections were made and analyzed by immunofluorescence staining for CD31 and Ki67. The right column shows the higher magnification (×40) of the selected areas (rectangular boxes) of the images shown in the left column. (B) Retinal EC proliferation was measured by counting CD31- and Ki67-positive cells that extended anterior to the inner limiting membrane per section (n = 6 eyes, 3 sections/eye). (C) All the conditions were the same as in panel A except that intravitreal injections of control, Kdr, or Flt1 siRNAs were given at P12, P13, and P15, and at P17 the mice pups were anesthetized, sacrificed, and enucleated, and retinas were isolated and stained with isolectin B4; flat mounts were made and examined for retinal neovascularization. Retinal vascularization is shown in the first row. Neovascularization is highlighted with red in the second row. The third row shows selected rectangular areas of the images shown in the first row under ×10 original magnification. (D-E) Retinal vascularization (D) and the neovascularization (E) were determined as described in “Materials and methods.” (F-J) All the conditions were the same as in panels A-E, respectively, except that mice pups were administered with Kdr and Flt1 siRNA simultaneously at 1 μg each in a total volume of 0.5 μL/eye. The bar graphs represent the quantitative analysis of 6 retinas. The values are presented as mean ± SD. *P < .01 vs normoxia + control siRNA; §P < .01 vs hypoxia + control siRNA.
Downregulation of Kdr or Flt1 levels blocks hypoxia-induced retinal EC proliferation and neovascularization. (A) After exposure to 75% oxygen, the mice pups were returned to normoxia, administered 1 μg/0.5 μL control, Kdr, or Flt1 siRNA at P12 and P13 by intravitreal injections, and the retinas were isolated at P15 and fixed; cross-sections were made and analyzed by immunofluorescence staining for CD31 and Ki67. The right column shows the higher magnification (×40) of the selected areas (rectangular boxes) of the images shown in the left column. (B) Retinal EC proliferation was measured by counting CD31- and Ki67-positive cells that extended anterior to the inner limiting membrane per section (n = 6 eyes, 3 sections/eye). (C) All the conditions were the same as in panel A except that intravitreal injections of control, Kdr, or Flt1 siRNAs were given at P12, P13, and P15, and at P17 the mice pups were anesthetized, sacrificed, and enucleated, and retinas were isolated and stained with isolectin B4; flat mounts were made and examined for retinal neovascularization. Retinal vascularization is shown in the first row. Neovascularization is highlighted with red in the second row. The third row shows selected rectangular areas of the images shown in the first row under ×10 original magnification. (D-E) Retinal vascularization (D) and the neovascularization (E) were determined as described in “Materials and methods.” (F-J) All the conditions were the same as in panels A-E, respectively, except that mice pups were administered with Kdr and Flt1 siRNA simultaneously at 1 μg each in a total volume of 0.5 μL/eye. The bar graphs represent the quantitative analysis of 6 retinas. The values are presented as mean ± SD. *P < .01 vs normoxia + control siRNA; §P < .01 vs hypoxia + control siRNA.
VEGFA and VEGFB mediate hypoxia-induced retinal angiogenesis
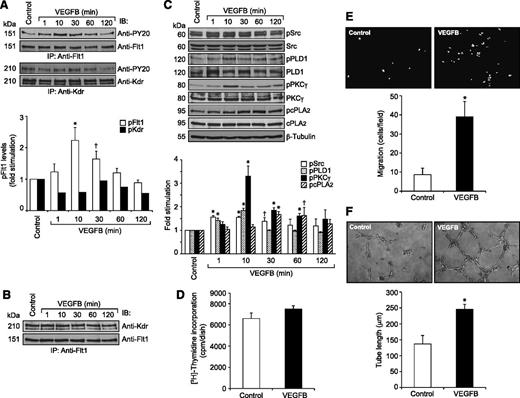
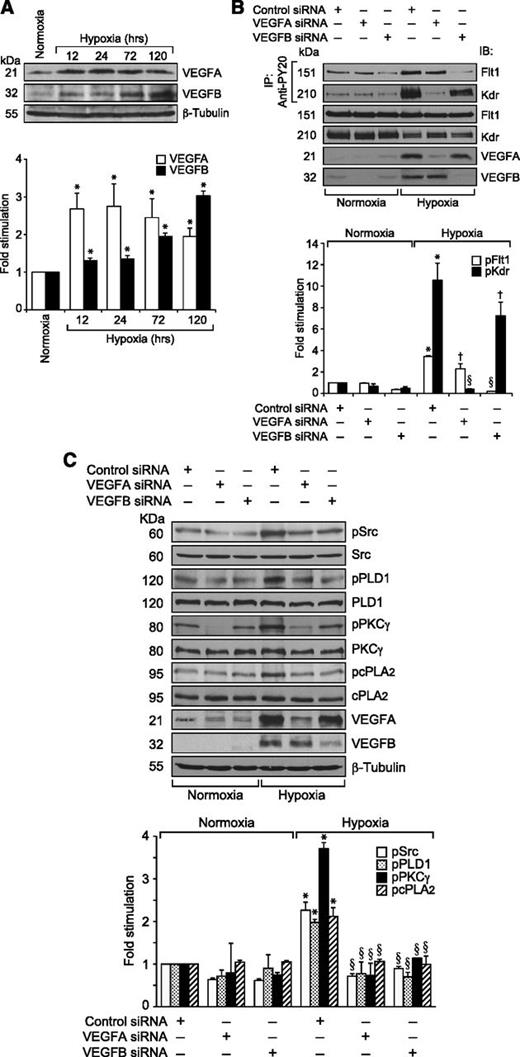
Because VEGFA did not stimulate the tyrosine phosphorylation of Flt1 in HRMVECs but hypoxia induced its tyrosine phosphorylation in the mouse retina, we wanted to identify the potential ligands for its activation in hypoxic retina. Because many reports show that VEGFB binds to Flt1 preferentially,15,16 we tested the effect of VEGFB on Flt1 tyrosine phosphorylation in HRMVECs. VEGFB, while having no apparent effect on Kdr tyrosine phosphorylation, did induce tyrosine phosphorylation of Flt1 in a time-dependent manner with maximum 2-fold increase at 10 minutes and thereafter declining to its basal level by 1 hour (Figure 6A). However, in contrast to VEGFA, VEGFB did not cause any dissociation of Kdr and Flt1 heterodimers (Figure 6B). To determine whether VEGFB mimics VEGFA in the stimulation of intracellular signaling events, we tested its effects on Src, PLD1, PKCγ, and cPLA2 activation. VEGFB exhibited small but consistent effects on phosphorylation of Src, PLD1, PKCγ, and cPLA2 in HRMVECs (Figure 6C). Interestingly, VEGFB, while having no apparent effect on DNA synthesis, did induce migration and tube formation of HRMVECs (Figure 6D-F). Next, we tested the effect of hypoxia on the expression of VEGFA and VEGFB in the retina. Hypoxia induced the expression of both VEGFA and VEGFB in the retina in a time-dependent manner (Figure 7A). Having observed that hypoxia induces the expression of both VEGFA and VEGFB, we wanted to determine which of these VEGF molecules accounts for hypoxia-induced Kdr and Flt1 tyrosine phosphorylation. Downregulation of VEGFA, while attenuating Flt1 tyrosine phosphorylation weakly, completely blocked hypoxia-induced Kdr tyrosine phosphorylation (Figure 7B). Similarly, depletion of VEGFB, while inhibiting Kdr tyrosine phosphorylation modestly, completely suppressed hypoxia-induced Flt1 tyrosine phosphorylation (Figure 7B). These findings reveal that while VEGFA mediates hypoxia-induced Kdr tyrosine phosphorylation, VEGFB causes Flt1 tyrosine phosphorylation to a major level. Based on these observations, we have now tested the role of VEGFA and VEGFB in hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation. Downregulation of either VEGFA or VEGFB levels attenuated hypoxia-induced Src-PLD1-PKCγ-cPLA2 phosphorylation, though more robustly with VEGFA-depletion (Figure 7C). On the other hand, depletion of only VEGFA but not VEGFB levels substantially attenuated hypoxia-induced retinal EC proliferation (Figure 8A-B). Nonetheless, downregulation of either VEGFA or VEGFB levels inhibited hypoxia-induced retinal neovascularization as measured by decreased tortuosity, tufts, and dilation (Figure 8C-E).
VEGFB stimulates Flt1 tyrosine phosphorylation and Src-PLD1-PKCγ-cPLA2 activation in HRMVECs as well as the migration and tube formation of these cells. (A) Quiescent HRMVECs were treated with and without VEGFB (40 ng/mL) for the indicated time periods and cell extracts were prepared. An equal amount of protein from control and each treatment group was immunoprecipitated with anti-Flt1 or anti-Kdr antibodies and the immunocomplexes were analyzed by western blotting using anti-PY20 antibodies. The blots were reprobed with anti-Flt1 or anti-Kdr antibodies for normalization. (B) All the conditions were the same as in panel A except that the cell extracts consisting of an equal amount of protein from control and each treatment were immunoprecipitated with anti-Flt1 antibodies and the immunocomplexes were immunoblotted with anti-Kdr antibodies. (C) All conditions were the same as in panel A except that an equal amount of protein from control and each treatment group was analyzed for pSrc, pPLD1, pPKCγ, and pcPLA2 levels using their phosphospecific antibodies. The blots were reprobed with anti-Src, anti-PLD1, anti-PKCγ, anti-cPLA2, or anti-β-tubulin antibodies for normalization. (D-F) Quiescent HRMVECs were treated with and without VEGFB (40 ng/mL) and subjected to DNA synthesis (D), migration (E), or tube formation (F) as described in the Figure 3 legend. The representative HRMVEC migration and tube formation images are shown in panels E and F, respectively. The bar graphs in panels A and C-F represent the quantitative analysis of 3 independent experiments. The values are presented as mean ± SD. *P < .01 vs control; †P < 0.05 vs control.
VEGFB stimulates Flt1 tyrosine phosphorylation and Src-PLD1-PKCγ-cPLA2 activation in HRMVECs as well as the migration and tube formation of these cells. (A) Quiescent HRMVECs were treated with and without VEGFB (40 ng/mL) for the indicated time periods and cell extracts were prepared. An equal amount of protein from control and each treatment group was immunoprecipitated with anti-Flt1 or anti-Kdr antibodies and the immunocomplexes were analyzed by western blotting using anti-PY20 antibodies. The blots were reprobed with anti-Flt1 or anti-Kdr antibodies for normalization. (B) All the conditions were the same as in panel A except that the cell extracts consisting of an equal amount of protein from control and each treatment were immunoprecipitated with anti-Flt1 antibodies and the immunocomplexes were immunoblotted with anti-Kdr antibodies. (C) All conditions were the same as in panel A except that an equal amount of protein from control and each treatment group was analyzed for pSrc, pPLD1, pPKCγ, and pcPLA2 levels using their phosphospecific antibodies. The blots were reprobed with anti-Src, anti-PLD1, anti-PKCγ, anti-cPLA2, or anti-β-tubulin antibodies for normalization. (D-F) Quiescent HRMVECs were treated with and without VEGFB (40 ng/mL) and subjected to DNA synthesis (D), migration (E), or tube formation (F) as described in the Figure 3 legend. The representative HRMVEC migration and tube formation images are shown in panels E and F, respectively. The bar graphs in panels A and C-F represent the quantitative analysis of 3 independent experiments. The values are presented as mean ± SD. *P < .01 vs control; †P < 0.05 vs control.
Hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation requires the production of both VEGFA and VEGFB. (A) An equal amount of protein from normoxic or various time periods of hypoxic retinal extracts was analyzed by western blotting for VEGFA and VEGFB levels using their respective antibodies. The blot was reprobed with anti-β-tubulin antibodies for normalization. (B) All the conditions were the same as in Figure 4C except that the mice pups were administered with control, VEGFA, or VEGFB siRNA at P10 and P11 by intravitreal injections, and at P12 the pups were returned to normoxia. After 12 hours the retinas were isolated, extracts prepared, and an equal amount of protein from normoxic and hypoxic retinas was immunoprecipitated with anti-PY20 antibodies; the immunocomplexes were analyzed by western blotting for Flt1 and Kdr using their specific antibodies. An equal amount of protein from normoxic and hypoxic retinas was also analyzed by western blotting for Flt1, Kdr, VEGFA, and VEGFB levels using their specific antibodies. (C) All the conditions were the same as in Figure 4C except that VEGFA or VEGFB siRNAs were administered at P12 and P13 and retinal extracts were analyzed for phosphorylation of Src, PLD1, PKCγ, and cPLA2 using their phosphospecific antibodies. The blots were reprobed with anti-Src, anti-PLD1, anti-PKCγ, anti-cPLA2, anti-VEGFA, anti-VEGFB, or anti-β-tubulin antibodies either for normalization or to show the downregulation of VEGFA and VEGFB by their respective siRNAs. The bar graphs in panels A-C represent the quantitative analysis of 3 independent experiments. The values are presented as mean ± SD. *P < .01 vs normoxia + control siRNA; †P < .05 vs hypoxia + control siRNA; §P < .01 vs hypoxia + control siRNA.
Hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation requires the production of both VEGFA and VEGFB. (A) An equal amount of protein from normoxic or various time periods of hypoxic retinal extracts was analyzed by western blotting for VEGFA and VEGFB levels using their respective antibodies. The blot was reprobed with anti-β-tubulin antibodies for normalization. (B) All the conditions were the same as in Figure 4C except that the mice pups were administered with control, VEGFA, or VEGFB siRNA at P10 and P11 by intravitreal injections, and at P12 the pups were returned to normoxia. After 12 hours the retinas were isolated, extracts prepared, and an equal amount of protein from normoxic and hypoxic retinas was immunoprecipitated with anti-PY20 antibodies; the immunocomplexes were analyzed by western blotting for Flt1 and Kdr using their specific antibodies. An equal amount of protein from normoxic and hypoxic retinas was also analyzed by western blotting for Flt1, Kdr, VEGFA, and VEGFB levels using their specific antibodies. (C) All the conditions were the same as in Figure 4C except that VEGFA or VEGFB siRNAs were administered at P12 and P13 and retinal extracts were analyzed for phosphorylation of Src, PLD1, PKCγ, and cPLA2 using their phosphospecific antibodies. The blots were reprobed with anti-Src, anti-PLD1, anti-PKCγ, anti-cPLA2, anti-VEGFA, anti-VEGFB, or anti-β-tubulin antibodies either for normalization or to show the downregulation of VEGFA and VEGFB by their respective siRNAs. The bar graphs in panels A-C represent the quantitative analysis of 3 independent experiments. The values are presented as mean ± SD. *P < .01 vs normoxia + control siRNA; †P < .05 vs hypoxia + control siRNA; §P < .01 vs hypoxia + control siRNA.
Downregulation of VEGFA and VEGFB blocks hypoxia-induced retinal EC proliferation and neovascularization. (A-E) All the conditions were the same as in Figure 5 except that the mice pups were administered with control, VEGFA, or VEGFB siRNA. Retinal EC proliferation and neovascularization were measured as described in “Materials and methods.” (A) Retinal EC proliferation was detected by coimmunofluorescence staining of the retinal sections for CD31 and Ki67. The right column shows the higher magnification (×40) of the selected areas (rectangular boxes) from the images shown in the left column. (B) Retinal EC proliferation was measured by counting CD31- and Ki67-positive cells that extended anterior to the inner limiting membrane per section (n = 6 eyes, 3 sections/eye). (C) Retinal vascularization is shown in the first row. Neovascularization is highlighted with red color in the second row. The third row shows selected rectangular areas of the images shown in the first row under ×10 original magnification. (D) The bar graph shows quantification of retinal vascularization. (E) The bar graph shows quantification of retinal neovascularization. The values are presented as mean ± SD (n = 6 retinas). *P < .01 vs normoxia + control siRNA; §P < .01 vs hypoxia + control siRNA.
Downregulation of VEGFA and VEGFB blocks hypoxia-induced retinal EC proliferation and neovascularization. (A-E) All the conditions were the same as in Figure 5 except that the mice pups were administered with control, VEGFA, or VEGFB siRNA. Retinal EC proliferation and neovascularization were measured as described in “Materials and methods.” (A) Retinal EC proliferation was detected by coimmunofluorescence staining of the retinal sections for CD31 and Ki67. The right column shows the higher magnification (×40) of the selected areas (rectangular boxes) from the images shown in the left column. (B) Retinal EC proliferation was measured by counting CD31- and Ki67-positive cells that extended anterior to the inner limiting membrane per section (n = 6 eyes, 3 sections/eye). (C) Retinal vascularization is shown in the first row. Neovascularization is highlighted with red color in the second row. The third row shows selected rectangular areas of the images shown in the first row under ×10 original magnification. (D) The bar graph shows quantification of retinal vascularization. (E) The bar graph shows quantification of retinal neovascularization. The values are presented as mean ± SD (n = 6 retinas). *P < .01 vs normoxia + control siRNA; §P < .01 vs hypoxia + control siRNA.
Discussion
Previously, we have shown that VEGFA stimulates Src-PLD1-PKCγ-cPLA2 signaling in the release of arachidonic acid in HRMVECs. In addition, Src-PLD1-PKCγ-cPLA2-mediated arachidonic acid release is needed for VEGFA-induced HRMVEC growth, migration, and tube formation.34,35 In accordance with these observations, hypoxia also induced Src-PLD1-PKCγ-cPLA2 activation in this order, and blockade of this signaling abrogated hypoxia-induced retinal neovascularization.34,35 To understand the far-upstream signaling mechanisms of Src-PLD1-PKCγ-cPLA2 activation by hypoxia, in the present study we have examined the role of Kdr and Flt1. Although VEGFA binds to Flt1 with high affinity,12,13,40 it only induced tyrosine phosphorylation of Kdr in HRMVECs. In addition, downregulation of Kdr levels significantly suppressed VEGFA-induced Src-PLD1-PKCγ-cPLA2 activation. In accordance with these observations, inhibition of Kdr levels suppressed VEGFA-induced HRMVEC growth, migration, and tube formation. Together, these observations reinforce a major role for Kdr in VEGFA-induced angiogenic events. However, although VEGFA does not stimulate the tyrosine phosphorylation of Flt1, downregulation of Flt1 levels inhibited VEGFA-induced Src-PLD1-PKCγ-cPLA2 activation in HRMVECs. Similarly, depletion of Flt1 levels modestly inhibited HRMVEC DNA synthesis, migration, and tube formation. These findings suggest that, in addition to Kdr, Flt1 also plays a role in VEGFA-induced Src-PLD1-PKCγ-cPLA2 activation in HRMVECs, mediating their growth, migration, and tube formation. It should be noted that many studies have reported a role for Flt1 in VEGFA-induced angiogenesis. For instance, it was shown that Flt1 plays a role in the assembly of vascular endothelium as well as pathological angiogenesis.20,27 It was also reported that Flt1 that lacks tyrosine kinase activity is sufficient in the modulation of normal development and angiogenesis.21 However, despite its role in VEGFA-induced developmental and pathological angiogenesis, the mechanisms by which Flt1 mediates these effects were not clear. Toward this end, the present findings reveal that although VEGFA does not stimulate the tyrosine phosphorylation of Flt1, the receptor still plays a role in VEGFA-induced angiogenic events such as EC growth, migration, and tube formation. The other notable observation of the present study is that both Kdr and Flt1 exist as a complex in quiescent HRMVECs, and Kdr is tyrosine phosphorylated and released from the complex in response to VEGFA but not VEGFB. Based on these observations, one may argue that the uncoupled Flt1 may be involved in mediating some of the VEGFA-induced intracellular signaling events, such as activation of Src-PLD1-PKCγ-cPLA2 signaling axis, perhaps in a spatiotemporal manner, facilitating EC migration, proliferation, tube formation, and/or survival, at least modestly. A spatiotemporal involvement for Flt1 in Kdr-mediated VEGFA-induced angiogenesis has also been suggested by other investigations.22 Additional support for the role of Flt1 in VEGFA-induced angiogenesis could be drawn from a report showing that Flt1 but not Kdr forms a complex with protein tyrosine kinase 7 and enhances VEGFA-induced angiogenesis.23 Similarly, it was reported that Flt1 modulates VEGFA-induced EC migration via RACK1-dependent activation of PI3K-Akt.24 In view of these findings, it may be speculated that the role of Flt1 in VEGFA-induced angiogenesis may be secondary to Kdr activation, at least in the absence of other VEGF molecules. Furthermore, a computational study predicted that Kdr and Flt1 form heterodimers in response to VEGF.41 In this regard, although our findings provide evidence for the heterodimerization of Kdr and Flt1, they appear to be quite opposite of those predicted by this computational model. Specifically, our results reveal that both Kdr and Flt1 exist as a heterodimer in quiescent condition and, upon challenging with their agonist, VEGFA, they dissociate from each other, suggesting that the heterodimers are antiangiogenic. On the other hand, the computational model states that Kdr and Flt1 form a heterodimer in response to their agonist. Many studies have demonstrated that soluble Flt1 (sFlt1), the alternative splice variant of Flt1 gene, could act as a decoy receptor for VEGFA and may thereby limit Kdr signaling, affecting developmental and pathological angiogenesis.42,43 Because sFlt1 not only blocks VEGFA signaling but may also sequester the binding of VEGFB and PIGF to Flt1, it can be argued that, in addition to inhibiting VEGFA-mediated angiogenesis, it may also be suppressing both VEGFB and PIGF-induced formation of new blood vessels. Based on these possible interpretations of the effects of sFlt1, Flt1 may be viewed as a positive modulator of angiogenesis. Therefore, some of the beneficial effects associated with the use of sFlt1 therapy may be due to inhibition of its own signaling events.
To validate the in vitro observations in vivo, we used an OIR model. Hypoxia induced the tyrosine phosphorylation of both Kdr and Flt1 with a similar time course. Interestingly, downregulation of either receptor decreased hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation, although the decrease was more robust in the Kdr-depleted retina. Concurring with these observations, downregulation of either receptor also led to a decrease in hypoxia-induced retinal neovascularization. This finding indicates that both receptors are activated by hypoxia and are involved in hypoxia-induced retinal neovascularization. Indeed, a more robust inhibition of hypoxia-induced EC retinal proliferation and neovascularization by simultaneous depletion of Kdr and Flt1 levels further supports a role for both the receptors in OIR. However, in vitro in HRMVECs, VEGFA does not cause tyrosine phosphorylation of Flt1, suggesting that hypoxia-induced tyrosine phosphorylation of Flt1 may be mediated by factors other than VEGFA in the retina. Convincing evidence suggests that VEGFB preferentially activates Flt1.14,15 Furthermore, VEGFB has been shown to stimulate EC proliferation in vitro and angiogenesis in vivo.44,45 Mice lacking VEGFB have smaller hearts and dysfunctional coronary vasculature and exhibited impaired recovery following myocardial ischemia.46 In this study, we show that VEGFB stimulates Flt1 tyrosine phosphorylation as well as Src-PLD1-PKCγ-cPLA2 activation in HRMVECs, facilitating the migration and tube formation of these cells. In addition, hypoxia induced the expression of both VEGFA and VEGFB in the mouse retina in a time-dependent manner. Because VEGFA and VEGFB stimulate the tyrosine phosphorylation of Kdr and Flt1, respectively, in HRMVECs, it is likely that these VEGF molecules also mediate the hypoxia-induced tyrosine phosphorylation of Kdr and Flt1, respectively. Indeed, downregulation of VEGFA levels, while modestly effecting Flt1 tyrosine phosphorylation, completely blocked hypoxia-induced Kdr tyrosine phosphorylation. Similarly, depletion of VEGFB levels, while weakly effecting Kdr tyrosine phosphorylation, completely inhibited hypoxia-induced Flt1 tyrosine phosphorylation. These results clearly suggest that hypoxia-induced Kdr and Flt1 tyrosine phosphorylations are mediated by VEGFA and VEGFB, respectively, to a major level. However, the small but consistent effects of VEGFA downregulation on Flt1 tyrosine phosphorylation may infer that VEGFA, in addition to activating Kdr, also stimulates Flt1 in ischemic retina. Likewise, the attenuation of Kdr tyrosine phosphorylation by VEGFB downregulation suggests that VEGFB, in addition to Flt1 stimulation, also activates Kdr in ischemic retina. These observations further indicate that both VEGFA and VEGFB can activate either Kdr or Flt1, albeit to different levels. However, it is not clear whether the effects of VEGFA and VEGFB on Flt1 and Kdr activation, respectively, in the retina occur in ECs and/or other cell types such as pericytes, smooth muscle cells, and/or vascular stem/progenitor cells, influencing the development of new blood vessels.47,48 Although further studies are required to identify whether these VEGF molecules are involved in the recruitment of pericytes/vascular stem cells in the development of new blood vessels and the specific receptor(s) through which they influence these effects, the present observations clearly show that downregulation of either VEGFA or VEGFB or their receptors, Kdr and Flt1, significantly blocks pathological retinal angiogenesis.
VEGFB induced Src-PLD1-PKCγ-cPLA2 activation in HRMVECs in vitro, although less robustly than VEGFA. Because downregulation of Kdr or Flt1 attenuated Src-PLD1-PKCγ-cPLA2 activation in vitro in HRMVECs and in vivo in the mouse retina, and blockade of Src, PLD1, PKCγ, or cPLA2 levels completely abolished hypoxia-induced retinal angiogenesis,34,35 it is possible that both VEGFA and VEGFB are involved in hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation. Indeed, the finding that downregulation of either VEGFA or VEGFB suppressed hypoxia-induced Src-PLD1-PKCγ-cPLA2 activation suggests a role for both of these molecules in the stimulation of this signaling in the retina in vivo. Furthermore, siRNA-mediated depletion of either VEGFA or VEGFB levels also attenuated hypoxia-induced retinal angiogenesis. However, it was noted that whereas VEGFB activated the Src-PLD1-PKCγ-cPLA2 signaling to a modest level in HRMVECs, depletion of either VEGFA or VEGFB levels almost equally attenuated hypoxia-induced Src-PLD1-PKCγ-cPLA2 signaling in the mouse retina. These differences in VEGFB effects between HRMVECs and hypoxic mouse retina may be attributed to species differences or in vitro cell culture to in vivo disease model variations. Controversial observations were made previously in regard to the role of VEGFB in ischemic retinal angiogenesis. Although some studies showed that VEGFB plays role in ischemic retinal neovascularization,48 other reports indicated no such role.49,50 In this aspect, our findings using both HRMVECs and the mouse OIR model convincingly demonstrate that VEGFB, via the activation of Flt1 and Src-PLD1-PKCγ-cPLA2 signaling, plays a role in pathological retinal neovascularization. The present observations also reveal that VEGFA, via Kdr, leads to activation of Src-PLD1-PKCγ-cPLA2 signaling in influencing retinal neovascularization. Almost an equal inhibition of hypoxia-induced retinal neovascularization by depletion of VEGFA, VEGFB, or their receptors Kdr and Flt1 suggests the following possibilities: (1) these two molecules interact in the modulation of retinal neovascularization; or (2) these molecules may be mediating different aspects of retinal neovascularization, one in vessel sprouting and growth and the other in vessel survival and stability. Because Src-PLD1-PKCγ-cPLA2 signaling appears to be involved in the mediation of both VEGFA and VEGFB effects in mediating retinal neovascularization, drug development targeting this signaling may be promising in the treatment of this ischemic ocular lesion.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by a grant from the National Eye Institute, National Institutes of Health (EY014856) (G.N.R.).
National Institutes of Health
Authorship
Contributions: N.K.S. performed DNA synthesis, cell migration, tube formation, western blot analysis, immunoprecipitations, immunohistochemistry, and retinal angiogenesis. D.E.H. performed retinal angiogenesis and wrote the manuscript. V.K.S. performed western blot analysis. G.N.R. designed the experiments, interpreted the data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gadiparthi N. Rao, Department of Physiology, University of Tennessee Health Science Center, 894 Union Ave, Memphis, TN 38163; e-mail: rgadipar@uthsc.edu.

![Figure 3. Both Kdr and Flt1 mediate VEGFA-induced HRMVEC DNA synthesis, migration, and tube formation. (A-C) HRMVECs that were transfected with control, Kdr, or Flt1 siRNA (100 nM) and quiesced were treated with and without VEGFA (40 ng/mL) and either DNA synthesis (A), migration (B), or tube formation (C) was measured. DNA synthesis was measured by [3H]-thymidine incorporation. Cell migration was measured by modified Boyden chamber method. The representative HRMVEC migration and tube formation images are shown in panels B and C, respectively. The bar graphs represent the quantitative analysis of 3 independent experiments. The values are presented as mean ± SD. *P < .01 vs control siRNA; †P < .05 vs control siRNA + VEGFA; §P < .01 vs control siRNA + VEGFA.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/10/10.1182_blood-2012-03-419234/4/m_1911f3.jpeg?Expires=1769093760&Signature=BCripdzmsYRwC9ik0QWmzkJVdSGOGA1nhxX~ZFkLJhe~V49QDLg3Qgbvu6N2oM3x2sb2L3~P4eQGDix9Z5urOzOx9rpz~KsQne1LwsFv71Ljxt0WGU~LEWfdIGXs8EM~a5F4kEC947aLLJGg8WJjjwi5SQM7G7lmhPy136QSRbTM6ydVfPQF~vFflELq0gafLfACZlZXUMkrtet4WDTB1etBmksHdPqlK2xzXvAtHy4o7ke4OeROpx7XFFiZeY2PJaflUJ6mILd81XcanT6uW9Z4i-ORO4pVnvO0AxhuHbRnewOJxKcZdKhfZcHpKCpNRWaff8ER09JJQehCf4Qclg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)