To the editor:
Since the publication by Nguyen et al1 showing that Toso/FcμR is an inhibitor of Fas-mediated apoptosis, there has been debate regarding whether FcμR regulates apoptosis.2-4 An obvious problem is that these original studies used cell lines instead of normal primary T and B cells. To clarify the physiological role of FcμR in Fas-mediated apoptosis, we have now used FcμR-deficient mouse T and B cells. Our results clearly show that FcμR is not involved in Fas-mediated cell death in thymocytes or in spleen T and B cells before and after activation.
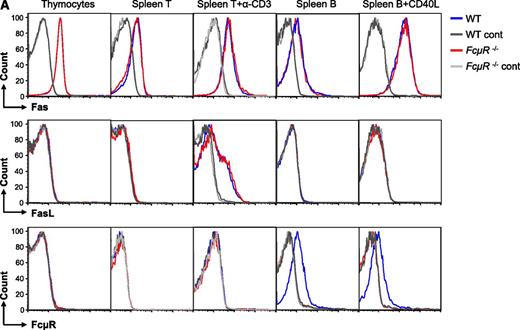
We first analyzed cell-surface expression of Fas, Fas ligand (FasL), and FcμR by wild-type (WT) and FcμR-deficient cells (Figure 1A). Thymocytes and spleen T and B cells before and after activation all expressed Fas, and WT and FcμR−/− cells had similar levels of expression (Figure 1A top panels). FasL was only expressed by activated T cells and again there was no difference in the expression levels between WT and FcμR−/− cells (Figure 1A middle panels). Consistent with our recent studies,5,6 FcμR was only expressed by B cells but not by thymocytes or T cells before and after activation (Figure 1A bottom panels).
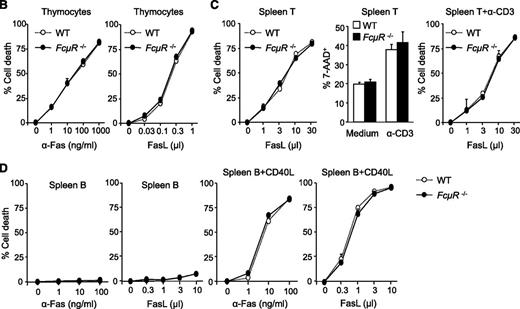
FcμR is not involved in Fas-mediated apoptosis in mouse T and B cells. (A) Expression of Fas, FasL, and FcμR by WT and FcμR−/− thymocytes and T and B cells before and after activation. To analyze Fas, FasL, and FcμR expression, cells were first incubated with a rat IgG2b anti-mouse CD16/CD32 antibody (clone 2.4G2; BD Biosciences) to block FcγR. For Fas expression, cells were stained with PE/Cy7 anti-CD95 (clone Jo2; BD Biosciences) or an isotype control (PE/Cy7 hamster IgG2, clone Ha4/8; BD Biosciences). For FasL expression, cells were stained with PE anti-CD178 (clone MFL3; Biolegend) or an isotype control (PE hamster IgG, clone HTK888; Biolegend). For FcμR expression, cells were first stained with the rat anti-mouse FcμR mAb (clone 4B5) or an isotype control (rat IgG2a; BD Biosciences), and then stained with PE anti-rat IgG2a (clone RG7/1.30; BD Biosciences). Purified spleen T and B cells were stimulated with plate-bound anti-CD3 (clone 2C11, 10 μg/mL) and CD40L for 44 hours, respectively. CD40L, CD40 ligand; Ig, immunoglobulin; mAb, monoclonal antibody; PE, phycoerythrin. (B) Normal Fas-mediated cell death in FcμR−/− thymocytes. Thymocytes were cultured with increasing concentrations of the Jo2 anti-Fas (left panel) or FasL in the presence of 0.5 μg/mL cycloheximide (right panel). (C) Normal Fas-mediated cell death in FcμR−/− spleen T cells before and after activation. Left panel, Spleen T cells were treated with increasing concentrations of FasL for 4 hours. Middle panel, Spleen T cells were cultured for 48 hours in medium alone or in the presence of plate-bound anti-CD3 (clone 2C11, 10 μg/mL) to induce AICD. Right panel, Cells were cultured in the presence of plate-bound anti-CD3 for 48 hours, and increasing concentrations of FasL were added for the last 4 hours. (D) Normal Fas-mediated cell death in FcμR−/− B cells. Purified spleen B cells (left 2 panels) or spleen B cells stimulated with CD40L for 44 hours (right 2 panels) were cultured for 4 hours in the presence of increasing concentrations of the Jo-2 anti-Fas or FasL. Cells were stained with 7-AAD, and the cell death was calculated by the following formula: (experimental cell death − spontaneous cell death)/(100 − spontaneous cell death).9,10 7-AAD, 7-Aminoactinomycin D.
FcμR is not involved in Fas-mediated apoptosis in mouse T and B cells. (A) Expression of Fas, FasL, and FcμR by WT and FcμR−/− thymocytes and T and B cells before and after activation. To analyze Fas, FasL, and FcμR expression, cells were first incubated with a rat IgG2b anti-mouse CD16/CD32 antibody (clone 2.4G2; BD Biosciences) to block FcγR. For Fas expression, cells were stained with PE/Cy7 anti-CD95 (clone Jo2; BD Biosciences) or an isotype control (PE/Cy7 hamster IgG2, clone Ha4/8; BD Biosciences). For FasL expression, cells were stained with PE anti-CD178 (clone MFL3; Biolegend) or an isotype control (PE hamster IgG, clone HTK888; Biolegend). For FcμR expression, cells were first stained with the rat anti-mouse FcμR mAb (clone 4B5) or an isotype control (rat IgG2a; BD Biosciences), and then stained with PE anti-rat IgG2a (clone RG7/1.30; BD Biosciences). Purified spleen T and B cells were stimulated with plate-bound anti-CD3 (clone 2C11, 10 μg/mL) and CD40L for 44 hours, respectively. CD40L, CD40 ligand; Ig, immunoglobulin; mAb, monoclonal antibody; PE, phycoerythrin. (B) Normal Fas-mediated cell death in FcμR−/− thymocytes. Thymocytes were cultured with increasing concentrations of the Jo2 anti-Fas (left panel) or FasL in the presence of 0.5 μg/mL cycloheximide (right panel). (C) Normal Fas-mediated cell death in FcμR−/− spleen T cells before and after activation. Left panel, Spleen T cells were treated with increasing concentrations of FasL for 4 hours. Middle panel, Spleen T cells were cultured for 48 hours in medium alone or in the presence of plate-bound anti-CD3 (clone 2C11, 10 μg/mL) to induce AICD. Right panel, Cells were cultured in the presence of plate-bound anti-CD3 for 48 hours, and increasing concentrations of FasL were added for the last 4 hours. (D) Normal Fas-mediated cell death in FcμR−/− B cells. Purified spleen B cells (left 2 panels) or spleen B cells stimulated with CD40L for 44 hours (right 2 panels) were cultured for 4 hours in the presence of increasing concentrations of the Jo-2 anti-Fas or FasL. Cells were stained with 7-AAD, and the cell death was calculated by the following formula: (experimental cell death − spontaneous cell death)/(100 − spontaneous cell death).9,10 7-AAD, 7-Aminoactinomycin D.
We next examined Fas-mediated cell death in thymocytes and T cells. As shown previously,7 thymocytes were readily sensitive to cell death induced by either anti-Fas (Jo-2) or FasL, and WT and FcμR−/− thymocytes showed similar Fas susceptibility (Figure 1B). In addition, WT and FcμR−/− spleen T cells showed the same sensitivity to FasL-induced cell death (Figure 1C left panel). Activated T cells are known to undergo an activation-induced cell death (AICD) mediated by Fas/FasL interaction,8 and WT and FcμR−/− T cells showed similar AICD after CD3 stimulation (Figure 1C middle panel). Treatment of anti-CD3–activated T cells with FasL further increased cell death and, again, WT and FcμR−/− T cells were equally sensitive (Figure 1C right panel). These results demonstrate that WT and FcμR−/− thymocytes and spleen T cells before and after activation have similar sensitivity to Fas-mediated cell death, which is also consistent with the lack of FcμR expression by these cells (Figure 1A).
We then analyzed Fas-mediated cell death in B cells (Figure 1D). Consistent with earlier studies,9,10 spleen B cells were resistant to anti-Fas– or FasL-induced cell death (Figure 1D left 2 panels) and became susceptible after CD40 ligation (Figure 1D right 2 panels). Despite the fact that FcμR was only expressed by WT but not FcμR−/− B cells (Figure 1A), these cells exhibited similar susceptibility to cell death induced by either the anti-Fas mAb or FasL (Figure 1D right two panels). These results demonstrate that FcμR−/− B cells do not show elevated sensitivity to Fas-mediated cell death.
Based on the above results, we conclude that FcμR is NOT an inhibitor of Fas-mediated cell death in normal mouse T and B cells. Our results cannot exclude the possibility that FcμR may have a role in Fas-mediated apoptosis in human B and T cells. However, given the controversial results from different groups,1-4 further studies are required to clarify the role of FcμR in human B and T cells.
Authorship
Acknowledgment: The authors thank Prof Peter Burrows for helpful comments.
Contribution: R.O. and H.M. performed the experiments; H.O. provided FcμR-deficient mice; and J.-Y.W. designed the experiments and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ji-Yang Wang, Laboratory for Immune Diversity, RIKEN Research Center for Allergy and Immunology, 1-7-22 Suhehiro-cho, Tsurumi, Yokohama 230-0045, Japan; e-mail: oh@rcai.riken.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal