Key Points
Control of Helios+/− Treg subset development is mediated through distinct cytokines and monocyte subpopulations.
CD16+ monocytes inhibit Helios+ Treg proliferation through IL-12, whereas CD16− monocytes suppress Helios− Treg development through TNF-α.
Abstract
Foxp3+ regulatory T cells (Tregs) play a pivotal role in control of autoimmunity and pathological immune responses. Helios, the Ikarus family transcription factor, binds to the Foxp3 promoter, stabilizing its expression, and is expressed in 70% of peripheral Tregs of healthy individuals. This frequency is altered during malignancy, infection, and autoimmunity, although the mechanisms that control proliferation and relative numbers of Helios+/− Tregs remain largely unknown. Using a T-cell–monocyte in vitro stimulation assay, we now show that proliferation of Helios+ Tregs is inhibited by CD16+ monocyte subset. Antibody blocking with anti–interleukin (IL)-12 reversed this inhibition, whereas addition of IL-12 suppressed Helios+ Treg expansion, indicating that CD16+ monocyte control of Helios+ Treg numbers is mediated through IL-12. In contrast, proliferation of Helios− Tregs, which express higher levels of tumor necrosis factor receptor II (TNFRII), was suppressed by TNF-α, whereas anti–TNF-α and anti-TNFRII reversed the inhibition. CD16− monocyte subset was mainly responsible for TNF-α–mediated control of Helios− Treg expansion. Altogether, these data suggest a differential role for monocyte subsets in control of Helios+/− Treg development that is mediated by distinct inflammatory cytokines. These data may have important implications for understanding the pathogenesis as well as control of chronic inflammatory and autoimmune diseases.
Introduction
T regulatory cells (Tregs) play a key role in immunologic homeostasis. As with other T lymphocytes, Tregs are produced in the thymus (natural) but can also be generated outside the thymus in the periphery (induced). Foxp3 is the critical transcription factor for specification and maintenance of Treg phenotype such that genetic deficiencies of Foxp3 are associated with development of severe autoimmune diseases.1 Among the transcription factors that interact with Foxp3,2 Helios, a member of the Ikaros zinc finger transcription factor family, was recently reported to bind to the Foxp3 promoter, stabilizing its expression and possibly increasing Treg suppressive function.3,4 This implies that Helios+ Tregs may have a more stable expression of Foxp3, and perhaps stabilized suppressive activity. Helios expression in Tregs was originally reported to be a marker to distinguish natural from induced Tregs,5 although conflicting results have since emerged.6,7 In mice and man, Helios+ Tregs represent about 70%, whereas Helios− Tregs constitute around 30% of Foxp3+ T cells in the periphery.5 However, these frequencies appear differently in malignancies,8-14 autoimmunity,15-19 and after infection.20,21 For example, Helios+ Treg frequencies are increased in the peripheral blood of cancer patients, and most carcinoma-infiltrating Tregs are Helios positive.8-12 In contrast, in premalignant tumors, such as nasal polyposis13 and respiratory papillomas,14 most infiltrating Tregs were shown to be Helios negative. Moreover, in type 2 diabetes mellitus, peripheral blood Helios− Treg numbers were decreased, although Helios+ Treg counts were normal,18 whereas in patients with myasthenia gravis, Helios+ Treg frequency was lower.16 Certain therapies also affect Helios+/− Treg frequencies,15,22 suggesting that Helios+/− Treg subsets may be associated with clinical response to therapy and could potentially be used to monitor treatment response. Understanding the mechanism of how and why Helios levels change in Tregs in various diseases may therefore provide valuable information regarding the pathophysiology of such disease and may even help predict treatment outcomes. However, mechanisms that control expansion of Helios+/− Treg subsets remain largely unknown, although a role for inflammatory cytokines has been invoked. Specifically, Helios− Treg levels were increased in patients with rheumatoid arthritis (RA) responding to anti-TNF antibody therapy.15 In addition, in a transgenic mouse expressing elevated levels of IL-6,23 Helios− Treg expansion was strongly inhibited, whereas Helios+ Treg development remained unaffected. These studies indicate that certain inflammatory cytokines can selectively target and regulate Helios+ or Helios− Treg expansion.
Monocytes, which are generally regarded as precursors of tissue macrophages and dendritic cells (DCs),24 can be phenotypically divided based on surface expression of CD14 and CD16 expression into CD14+CD16− and CD16+ cells. Both cell types have distinct functional activities and secrete different patterns of inflammatory cytokines after stimulation.25 We recently showed that CD16+ monocyte subset selectively inhibits Treg expansion via IL-12,26 although control of the Helios+/− Treg subset was not examined. In the present study, we explored the role of the CD16+ and CD16− cells and 2 cytokines, namely IL-12 and TNF-α, in the regulation of Helios+/− Treg development in healthy control volunteers. Our findings are consistent with a model in which Helios+/− Treg numbers are differentially regulated by CD16+ and CD16− monocytes through IL-12 and TNF-α, respectively.
Methods and materials
Human samples
All of the studies were approved by the Institutional Review Boards of the New York Blood Center. Fresh leukocyte-enriched peripheral blood samples were obtained without any identifiers from healthy volunteer donors of the New York Blood Center. The study was conducted in accordance with the Declaration of Helsinki.
Cell isolation and purification
Peripheral blood mononuclear cells (PBMCs) were separated from leukocyte-enriched blood samples by Ficoll (GE Healthcare, Port Washington, NY) density centrifugation and subjected to cell subsets purification by magnetic beads (all from Miltenyi Biotec, Aubum, CA). Total T cells and monocytes were purified using PAN T cell isolation kit and CD14 microbeads, respectively (purity >95% for both). CD16+ monocytes were purified from PBMCs using a CD16+ monocyte isolation kit by positive selection (purity >95%), and the negatively selected fraction was then incubated with CD14 microbeads to obtain the CD14+CD16− cells (purity >95%) according to the manufacturer’s instructions.
T-cell stimulation assays
Purified T cells were resuspended at a final concentration of 5 × 106/mL before staining with 1 μg/mL carboxyfluoresceindiacetatesuccinimidyl ester (CFSE; Invitrogen, Grand Island, NY) in phosphate-buffered saline solution for 5 minutes at room temperature, according to the manufacturer’s instructions. CFSE-labeled total T cells were resuspended in culture medium containing RPMI1640 (Invitrogen) supplemented with 5% Human AB serum (Valley Biomedical, Winchester, VA), 2 mM glutamine (Invitrogen), 100 U penicillin and streptomycin (Invitrogen), and 0.055 mM 2-mercaptoethanol (Invitrogen) and stimulated with 1 μg/mL soluble anti-CD3 antibody (1 μg/mL, clone HIT3α, BD Biosciences, San Jose, CA) in U-bottom 96-wells plate for 7 days.
For co-culture studies, CFSE-labeled purified T cells (1.25 × 105 cells/mL) were mixed with autologous purified total monocytes at a ratio of 2:1 in the presence of soluble anti-CD3 antibody for 7 days. Alternatively, purified CD14+CD16− cells and CFSE-labeled T cells (2:1 ratio) in the absence or presence of CD16+ monocyte (CD14+CD16−/CD16+ monocyte ratio of 2:1) were cultured for 7 days with anti-CD3.
Antibody blocking and cytokine addition assays
For the antibody blocking studies, anti–TNF-α (1 μg/mL), anti–IL-6 (1 μg/mL), anti–IL-10 (1 μg/mL), anti–IL-12 (2 μg/mL) antibody (BD Biosciences), anti-TNFRI (4 μg/mL), and anti-TNFRII (4 μg/mL) or isotype-matched controls (2 μg/mL; R&D Systems, Minneapolis, MN) were added at the start of the cultures to CFSE-labeled purified T cells (1.25 × 105 cells/mL) and then co-cultured with autologous total monocytes or subsets in the presence of soluble anti-CD3 antibody for 7 days.
For cytokine addition studies, TNF-α (5 ng/mL) and IL-12 (0.05 ng/mL) (R&D Systems) were included at the start of the cultures to CFSE-labeled purified T cells (1.25 × 105 cells/mL) co-cultured with autologous total monocyte or its subsets in the presence of soluble anti-CD3 antibody for 7 days.
Intracellular and surface expression analysis
At day 7 after co-cultures of T cells and monocytes, cells were harvested, fixed, and permeabilized with Foxp3 fixation/permeabilization solution (eBiosciences, San Jose, CA) and incubated with anti–CD4-PerCP (clone RPA-T4), anti-CD3 Alexa700 (clone UCHT1), anti–Foxp3-PE (clone PCH101) (eBiosciences), and anti–Helios-APC (clone 22F6; Biolegend, Inc, San Diego, CA) following the manufacturers’ instructions. All expression analysis was performed by flow cytometry using BD FACS Canto with Diva software (BD, Franklin Lakes, NJ). CD4+ T cells that had divided were defined as the CFSEloCD3+CD4+ population. Tregs were identified as Foxp3hiCD4+ T cells. The percentages of Helios+ or Helios− Tregs in divided CD4+ T cells were also analyzed. The TNFRII surface staining was performed by incubation with anti–TNFRII-biotin (clone hTNFR-M1; BD Biosciences) for 15 minutes, followed by incubation with streptavidin-PE-Cy7 (eBiosciences, San Diego, CA) for another 15 minutes. After this, the cells were subjected to intracellular staining for Foxp3 and Helios as described before.
Statistical analysis
Data are expressed as mean values ± SEM. Statistical significance of differences between groups was determined by the Mann-Whitney U test, and statistical significance of differences of paired data were determined by using the paired t-test or Wilcoxon signed-rank test. Statistical analyses were performed using PASW Statistics 18 software (IBM Inc., Armonk, NY).
Results
CD16+ monocytes inhibit Helios+ Treg expansion through IL-12
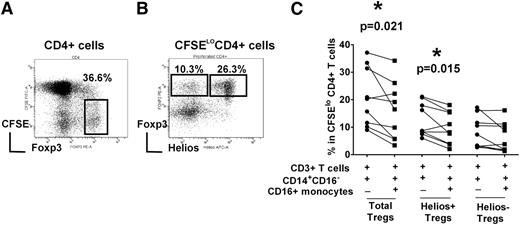
To study Treg proliferation, we used an in vitro T-cell stimulation co-culture system in which purified CFSE-labeled T cells were cultured with purified total monocyte fraction and stimulated with soluble anti-CD3 antibody for 7 days.26 As expected, Th cells (CD3+CD4+ cells) proliferated after stimulation with anti-CD3 antibody, as indicated by dilution of the CFSE dye. Cells expressing high levels of Foxp3, a hallmark of Treg population, were distinguishable as a distinct subset within the expanded CD4+ T cells (Figure 1A).26 Furthermore, based on Helios expression, Tregs could be further separated into Helios+ and Helios– subsets (Figure 1B and supplemental data for isotype control staining). To examine the role of monocyte subsets in mediating Helios+/− Treg expansion, the CD14+CD16− subset was purified and cultured with autologous T cells in the presence or absence of CD16+ monocytes. The addition of CD16+ monocyte significantly decreased total Treg proliferation (Figure 1C, P = .021), as we have reported previously.26 We found a statistically significant decrease in Helios+ Treg numbers (P = .015) but no significant change in Helios− Treg frequency (P > .05), indicating that CD16+ monocytes preferentially target Helios+ Tregs in their inhibitory activity (Figure 1C).
Helios+/− Treg proliferation is differentially controlled by monocyte subsets. Autologous CD3+ T cells, CD14+CD16- and CD16+ monocyte subsets were purified from PBMCs of healthy volunteer controls. T cells were CFSE-labeled and co-cultured with CD14+CD16− cells with or without CD16+ monocytes (as indicated by + and −) in the presence of anti-CD3 for 7 days. (A) The gating strategy to analyze the frequency of Foxp3hi in divided (CFSElo) CD4+ subset is shown. (B) Representative dot plot of Helios and Foxp3 expression gated on divided CFSElo CD4+ cells. Gating strategy for cells expressing Foxp3hiHelios+ and Foxp3hiHelios− is indicated. (C) The percentage of Foxp3hi in CFSElo CD4+ subset (“Total Tregs”) as well as Foxp3hiHelios+ (“Helios+ Tregs”) and Foxp3hiHelios− (“Helios− Tregs”) before and after addition of CD16+ monocytes is shown. The P value was calculated by paired t-test and indicates that addition of CD16+ cells decreases Treg development in healthy normal volunteers (P = .021), as per our previously published data,26 as well as Helios+ Treg development (P = .015), but has a less obvious (not statistically significantly) effect on Helios− Treg proliferation.
Helios+/− Treg proliferation is differentially controlled by monocyte subsets. Autologous CD3+ T cells, CD14+CD16- and CD16+ monocyte subsets were purified from PBMCs of healthy volunteer controls. T cells were CFSE-labeled and co-cultured with CD14+CD16− cells with or without CD16+ monocytes (as indicated by + and −) in the presence of anti-CD3 for 7 days. (A) The gating strategy to analyze the frequency of Foxp3hi in divided (CFSElo) CD4+ subset is shown. (B) Representative dot plot of Helios and Foxp3 expression gated on divided CFSElo CD4+ cells. Gating strategy for cells expressing Foxp3hiHelios+ and Foxp3hiHelios− is indicated. (C) The percentage of Foxp3hi in CFSElo CD4+ subset (“Total Tregs”) as well as Foxp3hiHelios+ (“Helios+ Tregs”) and Foxp3hiHelios− (“Helios− Tregs”) before and after addition of CD16+ monocytes is shown. The P value was calculated by paired t-test and indicates that addition of CD16+ cells decreases Treg development in healthy normal volunteers (P = .021), as per our previously published data,26 as well as Helios+ Treg development (P = .015), but has a less obvious (not statistically significantly) effect on Helios− Treg proliferation.
CD16+ monocyte−mediated inhibition of Helios+ Treg proliferation is through IL-12
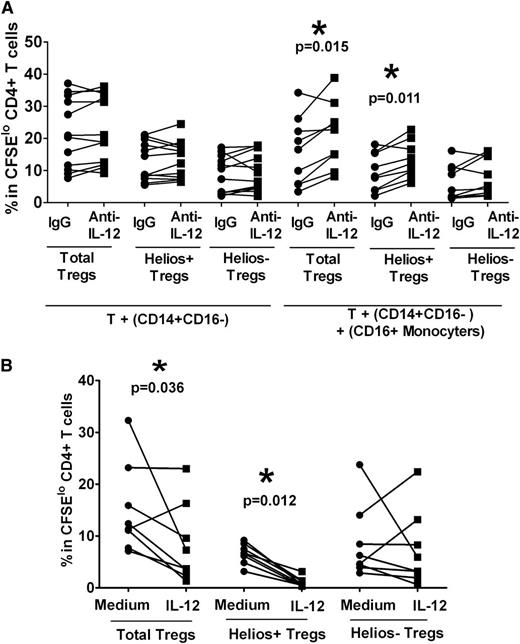
We have shown previously that CD16+ monocytes inhibit Treg expansion through IL-12,26 although their role in the control of Treg subset (Helios+/−) remains unknown. Antibody blocking with anti–IL-12 did not significantly affect Helios+ or Helios− Treg proliferation when purified CD14+CD16− monocyte were used (Figure 2A). Addition of CD16+ cells to co-cultures of T cells and CD14+CD16− in the presence of anti−IL-12 resulted in expansion of total Tregs (Figure 2A, P = .015, compared with immunoglobulin [Ig] G control) as previously reported.26 We found that the Helios+ Treg subset was also expanded after addition of CD16+ monocytes compared with the IgG control (Figure 2A, P = .011), their numbers increasing by approximately 50% (expansion without CD16+ monocytes relative to IgG control 106% ± 5% versus expansion with CD16+ monocytes relative to IgG control 167% ± 22%, P = .003), but Helios− Treg proliferation was not affected (Figure 2A, P > .05). Conversely, addition of IL-12 cytokine suppressed total Treg proliferation (Figure 2B, P = .036), robustly inhibiting Helios+ Treg expansion (Figure 2B, P = .012), but had a variable effect on Helios− Treg proliferation (Figure 2B, P > .05). These data indicate that CD16+ monocytes, through secretion of IL-12, inhibit Helios+ but not Helios− Treg expansion.
Helios+ Treg proliferation is inhibited by CD16+ monocytes through IL-12. (A) Purified T cells were co-cultured with purified autologous CD14+CD16− cells without or with CD16+ monocytes in the presence of neutralizing anti–IL-12 or isotype control (“IgG”) antibodies, and after 7 days of stimulation with anti-CD3, the frequencies of Foxp3hi (“Total Tregs”) as well as Foxp3hiHelios+ (“Helios+ Tregs”) and Foxp3hiHelios− (“Helios− Tregs”) in CFSEloCD4+ subset were analyzed. The P value was calculated by paired t-test comparing the effects of treatment with anti–IL-12 relative to IgG control. In the presence of CD16+ cells, IL-12 neutralization increased total Treg and Helios+ Treg subset proliferation, but not the proliferation of Helios− Treg, indicating that CD16+ monocytes inhibit Helios+ Treg development through IL-12. (B) IL-12 was added at the start of the co-cultures of T cells and monocytes, which were stimulated with anti-CD3 for 7 days. Treg (total, Helios+, and Helios−) proliferation was then compared in the absence (“Medium”) or presence (“IL-12”) of cytokine by paired t-test. The calculated P values indicate that total Treg and Helios+, but not Helios−, Treg proliferation, are inhibited by IL-12.
Helios+ Treg proliferation is inhibited by CD16+ monocytes through IL-12. (A) Purified T cells were co-cultured with purified autologous CD14+CD16− cells without or with CD16+ monocytes in the presence of neutralizing anti–IL-12 or isotype control (“IgG”) antibodies, and after 7 days of stimulation with anti-CD3, the frequencies of Foxp3hi (“Total Tregs”) as well as Foxp3hiHelios+ (“Helios+ Tregs”) and Foxp3hiHelios− (“Helios− Tregs”) in CFSEloCD4+ subset were analyzed. The P value was calculated by paired t-test comparing the effects of treatment with anti–IL-12 relative to IgG control. In the presence of CD16+ cells, IL-12 neutralization increased total Treg and Helios+ Treg subset proliferation, but not the proliferation of Helios− Treg, indicating that CD16+ monocytes inhibit Helios+ Treg development through IL-12. (B) IL-12 was added at the start of the co-cultures of T cells and monocytes, which were stimulated with anti-CD3 for 7 days. Treg (total, Helios+, and Helios−) proliferation was then compared in the absence (“Medium”) or presence (“IL-12”) of cytokine by paired t-test. The calculated P values indicate that total Treg and Helios+, but not Helios−, Treg proliferation, are inhibited by IL-12.
Helios+/− Treg proliferation is controlled by TNF-α
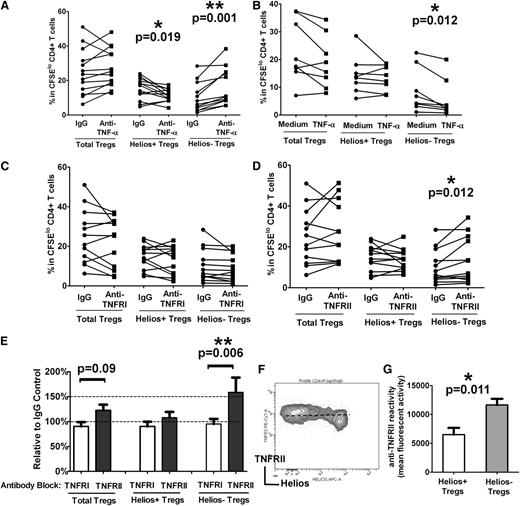
TNF-α effects the Treg compartment,27 albeit with conflicting reports on its inhibitory28 and stimulatory29,30 roles on Treg expansion and function. Helios− Treg levels were recently reported to be increased in patients with RA responding to anti–TNF-α antibody therapy.15 To begin to dissect the possible role of TNF-α in modulating Treg proliferative responses, we performed antibody blocking and cytokine addition experiments using our autologous T-cell stimulation assay containing total monocyte fractions. Compared with cultures treated with an isotype control, addition of a neutralizing anti–TNF-α antibody at the start of the co-cultures increased total Treg proliferation in 10 of 12 samples (Figure 3A “Total Tregs”), but the difference did not reach significance (P > .05). However, the frequency of Helios+ and Helios− Tregs was profoundly affected by TNF-α blockade (Figure 3A). Specifically, Helios− Treg numbers increased by 100% (expansion relative to IgG control 206% ± 23%) as a result of increased proliferation after anti–TNF-α treatment (Figure 3A, P = .001), whereas Helios+ Treg proliferation decreased (Figure 3A, P = .019). We also performed the reverse experiment in which TNF-α (5 μg/mL) was supplemented in the culture medium at the start of the T cell–monocyte cocultures. We found a significant decrease in Helios− Treg expansion (Figure 3B, P = .012) but no obvious alteration in total Tregs or Helios+ Treg proliferation (Figure 3B, P > .05). Altogether, these data indicate that TNF-α strongly inhibits Helios− Treg proliferation but to some extent is also essential for Helios+ Treg expansion.
TNF-α inhibits Helios− Treg proliferation through TNFRII. (A) Purified T cells were co-cultured with purified monocytes in the presence of neutralizing anti–TNF-α antibody or an isotype control (“IgG”) antibody, and after 7 days of stimulation with anti-CD3, the frequencies of Foxp3hi (“Total Tregs”) as well as Foxp3hiHelios+ (“Helios+ Tregs”) and Foxp3hiHelios− (“Helios− Tregs”) in theCFSElo CD4+ subset were analyzed. The P value was calculated by paired t-test comparing the effects of treatment with anti–TNF-α relative to IgG control and indicates that Helios+ Treg proliferation is reduced, whereas Helios− Treg proliferation is increased when TNF-α is neutralized through anti–TNF-α. (B) TNF-α was added at the start of the co-cultures of T cells and monocytes stimulated with anti-CD3 for 7 days. Treg (total, Helios+, and Helios−) proliferation was then compared in the absence (“Medium”) or presence (“TNF-α”) of cytokine by paired t-test. The calculated P values indicate that only Helios− Treg proliferation is targeted by TNF-α, causing a decrease in proliferation of this subset. (C) Co-cultures of T cells and monocytes were treated with anti-TNFRI or (D) anti-TNFRII and stimulated with anti-CD3 for 7 days. Treg (total, Helios+, and Helios−) proliferation was then compared with IgG control antibody by paired t-test. The calculated P values indicate that only blocking with anti-TNFRII affects proliferation of Helios− Tregs. (E) Percent increase in the numbers of Tregs (total, Helios+, and Helios−) in the presence of anti-TNFRI and anti-TNFRII relative to IgG control is shown for the studies presented in (C) and (D) and is consistent with a role for TNFRII, but not TNFRI, in Helios− Treg development. (F) Representative dot plot of TNFRII and Helios expression in divided Foxp3hi Tregs, showing surface expression levels of TNFRII on Helios+/− Tregs. (G) Relative mean fluorescence intensity analysis of TNFRII expression on Helios+/− Tregs is shown, indicating that Helios− Tregs express higher levels of TNFRII compared with Helios+ Tregs.
TNF-α inhibits Helios− Treg proliferation through TNFRII. (A) Purified T cells were co-cultured with purified monocytes in the presence of neutralizing anti–TNF-α antibody or an isotype control (“IgG”) antibody, and after 7 days of stimulation with anti-CD3, the frequencies of Foxp3hi (“Total Tregs”) as well as Foxp3hiHelios+ (“Helios+ Tregs”) and Foxp3hiHelios− (“Helios− Tregs”) in theCFSElo CD4+ subset were analyzed. The P value was calculated by paired t-test comparing the effects of treatment with anti–TNF-α relative to IgG control and indicates that Helios+ Treg proliferation is reduced, whereas Helios− Treg proliferation is increased when TNF-α is neutralized through anti–TNF-α. (B) TNF-α was added at the start of the co-cultures of T cells and monocytes stimulated with anti-CD3 for 7 days. Treg (total, Helios+, and Helios−) proliferation was then compared in the absence (“Medium”) or presence (“TNF-α”) of cytokine by paired t-test. The calculated P values indicate that only Helios− Treg proliferation is targeted by TNF-α, causing a decrease in proliferation of this subset. (C) Co-cultures of T cells and monocytes were treated with anti-TNFRI or (D) anti-TNFRII and stimulated with anti-CD3 for 7 days. Treg (total, Helios+, and Helios−) proliferation was then compared with IgG control antibody by paired t-test. The calculated P values indicate that only blocking with anti-TNFRII affects proliferation of Helios− Tregs. (E) Percent increase in the numbers of Tregs (total, Helios+, and Helios−) in the presence of anti-TNFRI and anti-TNFRII relative to IgG control is shown for the studies presented in (C) and (D) and is consistent with a role for TNFRII, but not TNFRI, in Helios− Treg development. (F) Representative dot plot of TNFRII and Helios expression in divided Foxp3hi Tregs, showing surface expression levels of TNFRII on Helios+/− Tregs. (G) Relative mean fluorescence intensity analysis of TNFRII expression on Helios+/− Tregs is shown, indicating that Helios− Tregs express higher levels of TNFRII compared with Helios+ Tregs.
TNFRII mediates TNF-α–dependent inhibition of Helios− Treg proliferation
Two distinct membrane receptors—TNFRI and TNFRII—bind to TNF-α.31 To determine the relative role of each of these two receptors in TNF-α–mediated inhibition of Helios− Treg expansion, we performed antibody-blocking studies using neutralizing antibodies to TNFRI and TNFRII in our T cell–monocyte cocultures. Neutralization with anti-TNFRI had no obvious effect on total Tregs, Helios+, or Helios− Treg proliferation (Figure 3C). However, inhibition with anti-TNFRII resulted in expansion of Helios− Tregs (Figure 3D, P = .012), increasing their numbers by more than 50% compared with cultures that were pretreated with isotype control (Figure 3E, P = .006). Unlike the effect of anti–TNF-α on Helios+ Tregs (Figure 3A), antibody blockade with anti-TNFRII did not decrease Helios+ Treg proliferation (Figure 3D-E). We found higher levels of TNFRII expression on Helios− compared with Helios+ Tregs (Figure 3F-G), which may explain why anti-TNFRII treatment preferentially targeted the Helios− Treg subset. Altogether, these data indicate that TNF-α is likely to mediate its inhibitory effect on Helios− Treg expansion through TNFRII, but not TNFRI.
TNF-α inhibition of Helios− Treg proliferation is mediated by CD16− monocytes
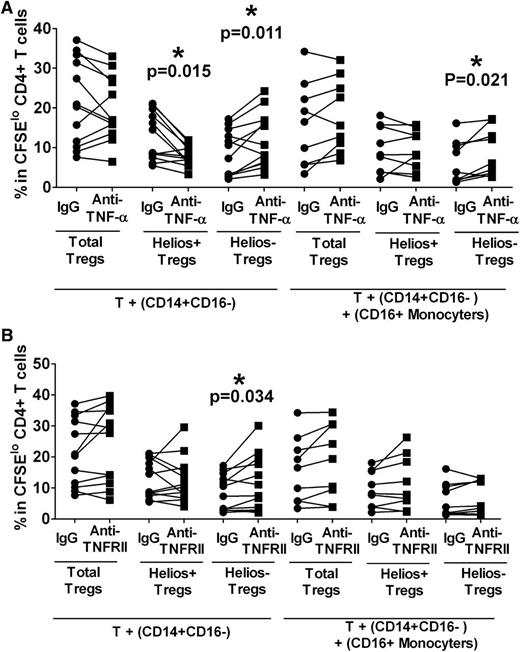
We next tested the role of monocyte subsets in mediating the inhibitory effect of TNF-α on Treg proliferation. CD14+CD16− subset was purified and cultured with autologous T cells in the presence or absence of CD16+ monocytes. Anti–TNF-α or an isotype control was added at the start of the cultures. After stimulation with anti-CD3, we found a robust increase in Helios− Treg expansion (Figure 4A, P = .011) with a concomitant decrease in Helios+ Treg proliferation (Figure 4A, P = .015) in anti-TNF-α–treated cocultures of T cells and CD14+CD16− cells lacking CD16+ monocytes. These data were similar to results obtained using total monocyte fraction (Figure 3A) and suggest that CD14+CD16− are the main monocyte subset responsible for mediating TNF-α control of Helios+/− Treg proliferation with little contribution from CD16+ monocytes. Consistent with the data using total monocytes (Figure 3B), we also found that in co-cultures with purified CD14+CD16− and T cells, addition of TNF-α cytokine inhibited Helios− Treg proliferation by almost half (data not shown, P = .015) but had a less significant impact on Helios+ Treg (data not shown, P = .08). In addition, blocking with TNFRII enhanced the Helios− Treg expansion (Figure 4B, P = .034) but did not appear to effect Helios+ Treg proliferation (Figure 4B), similar to data obtained with total monocyte fraction (Figure 3D). Altogether, these data suggest that CD14+CD16− monocytes, but not CD16+ monocytes, mediate TNF-α control of Helios+/− Treg proliferation with a more pronounced effect on the Helios− Treg subset.
CD16− monocytes control TNF-α–mediated Helios− Treg proliferation. Purified T cells were co-cultured with purified autologous CD14+CD16− cells without or with CD16+ monocytes in the presence of neutralizing (A) anti–TNF-α or (B) anti-TNFRII, and after 7 days of stimulation with anti-CD3, the frequencies of Foxp3hi (“Total Tregs”) as well as Foxp3hiHelios+ (“Helios+ Tregs”) and Foxp3hiHelios− (“Helios− Tregs”) in the CFSElo CD4+ subset were analyzed. The P value was calculated by paired t-test comparing the effects of treatment with neutralization antibody relative to IgG control. In co-cultures without CD16+ cells, anti–TNF-α treatment decreased Helios+ proliferation but increased Helios− Treg expansion, similar to data obtained when total monocytes were used. In the presence of CD16+ monocytes, Helios− Tregs were expanded relative to IgG control. (B) The same assays were performed as in (A) except that for TNF-α blockade, anti-TNFRII was used instead of anti–TNF-α. In co-cultures without CD16+ cells, anti-TNFRII treatment only affected Helios− Treg proliferation as indicated by P values, similar to data obtained when total monocytes (Figure 3A) were used.
CD16− monocytes control TNF-α–mediated Helios− Treg proliferation. Purified T cells were co-cultured with purified autologous CD14+CD16− cells without or with CD16+ monocytes in the presence of neutralizing (A) anti–TNF-α or (B) anti-TNFRII, and after 7 days of stimulation with anti-CD3, the frequencies of Foxp3hi (“Total Tregs”) as well as Foxp3hiHelios+ (“Helios+ Tregs”) and Foxp3hiHelios− (“Helios− Tregs”) in the CFSElo CD4+ subset were analyzed. The P value was calculated by paired t-test comparing the effects of treatment with neutralization antibody relative to IgG control. In co-cultures without CD16+ cells, anti–TNF-α treatment decreased Helios+ proliferation but increased Helios− Treg expansion, similar to data obtained when total monocytes were used. In the presence of CD16+ monocytes, Helios− Tregs were expanded relative to IgG control. (B) The same assays were performed as in (A) except that for TNF-α blockade, anti-TNFRII was used instead of anti–TNF-α. In co-cultures without CD16+ cells, anti-TNFRII treatment only affected Helios− Treg proliferation as indicated by P values, similar to data obtained when total monocytes (Figure 3A) were used.
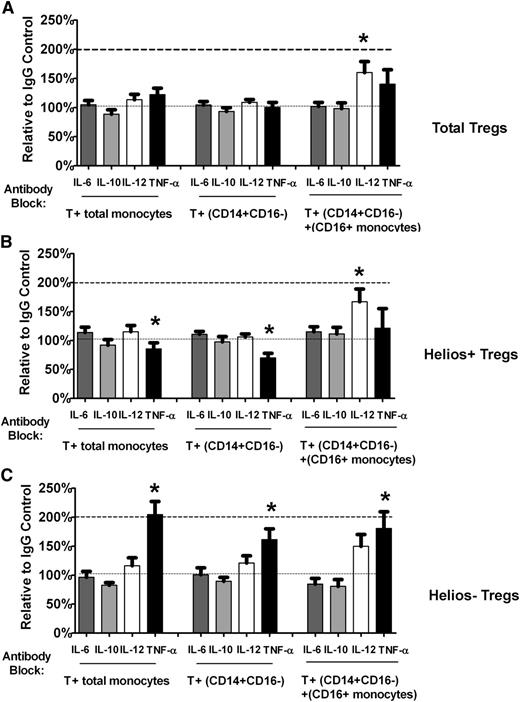
Lack of effect of antibody blocking with anti–IL-6 and anti–IL-10 on Helios+/− Treg proliferation
We also tested the effect of antibody blocking with anti–IL-6 and anti–IL-10. Neither of these antibodies had any significant effect on total Treg (Figure 5A), Helios+ (Figure 5B), or Helios− (Figure 5C) Treg numbers using either total monocytes or purified monocyte subsets, indicating that in normal healthy controls under our T cell–monocyte co-culture conditions, the effect of blockade of these cytokines on Treg total/subset expansion is minimal.
Helios+/− Treg numbers are not affected by antibody blocking with anti– IL-6 or anti–IL-10 in T cell–monocyte co-cultures. Relative change compared with IgG control in (A) total Treg (“Total Tregs”) as well as (B) Helios+ (“Helios+ Tregs”) and (C) Helios– (“Helios− Tregs”) Treg numbers in T cell co-cultured with total monocytes or monocyte subsets (CD14+CD16− cells without or with CD16+ monocytes) treated with neutralizing antibodies to IL-6 and IL-10 are shown. For comparison, the relative change in Treg numbers after treatment with anti–IL-12 and anti-TNF-α is also shown.
Helios+/− Treg numbers are not affected by antibody blocking with anti– IL-6 or anti–IL-10 in T cell–monocyte co-cultures. Relative change compared with IgG control in (A) total Treg (“Total Tregs”) as well as (B) Helios+ (“Helios+ Tregs”) and (C) Helios– (“Helios− Tregs”) Treg numbers in T cell co-cultured with total monocytes or monocyte subsets (CD14+CD16− cells without or with CD16+ monocytes) treated with neutralizing antibodies to IL-6 and IL-10 are shown. For comparison, the relative change in Treg numbers after treatment with anti–IL-12 and anti-TNF-α is also shown.
Discussion
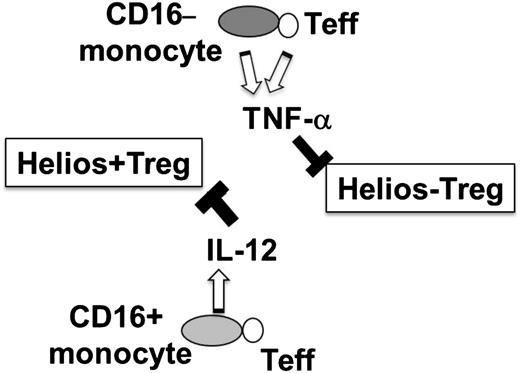
In the present study, we have found that Helios+ and Helios− Treg proliferation can be regulated by CD16+ and CD14+CD16− monocyte subsets through IL-12 and TNF-α, respectively (Figure 6). These findings support the notion that Tregs are a heterogenic cell population that can be further divided into subsets, each regulated by a specific monocyte subpopulation and different inflammatory cytokines. Our finding that the frequency of Helios+/− Tregs can vary depending on the cytokine environment raises the possibility that under disease conditions in which pro-inflammatory cytokines are increased, Helios+/− levels will be subject to alterations.
Hypothetical model of differential regulation of Helios+/− Treg development by monocyte subsets. Interaction of T effector (Teff) with CD16− monocyte subset results in expression of TNF-α, which in turn inhibits Helios− Treg development, whereas T cell–CD16+ monocyte interactions trigger IL-12 expression, which inhibits Helios+ Treg proliferative responses.
Hypothetical model of differential regulation of Helios+/− Treg development by monocyte subsets. Interaction of T effector (Teff) with CD16− monocyte subset results in expression of TNF-α, which in turn inhibits Helios− Treg development, whereas T cell–CD16+ monocyte interactions trigger IL-12 expression, which inhibits Helios+ Treg proliferative responses.
Few studies have addressed the role of IL-12 in control of Treg numbers,32-35 We have previously shown that CD16+ monocytes inhibit Treg proliferation via IL-12,26 which is also consistent with reports in mice showing that IL-12 inhibits Treg expansion, decreasing Foxp3 levels.32,33 Our current data indicate that IL-12 preferentially targets the Helios+ Treg subset. With regard to how TNF-α affects the Treg compartment, several studies have revealed a complex view,27 with conflicting reports on its inhibitory28 and stimulatory29,30 effect on Treg expansion and function. In the present study, we have shown that anti–TNF-α treatment has differential effects on Helios+/− Treg responses, inducing Helios− Treg proliferation while inhibiting Helios+ Treg expansion, which may explain previous contradictory reports of its effect on the Treg compartment. The underlying mechanism of why Helios+/− Treg subsets respond differently to TNF-α and IL-12 remains unclear, but clearly these cytokines signal through distinct pathways, leading to different gene transcription profiles. Specifically, IL-12 signaling results in the expression of Stat-4,36 whereas signaling through TNF-α activates NF-κB transcription,28 which in turn may down or upregulate Helios expression. Alternatively, the effects of IL-12 and TNF-α on Helios+/− Tregs may be indirect, through the action of other cytokines. For example, IL-12 inhibits IL-2, which may be critical for Helios+ but not Helios− Treg expansion,32 whereas TNF-α, through its ability to inhibit transforming growth factor-beta, may be required for Helios−, but not Helios+, Treg proliferation.37 Additional studies are clearly needed to decipher the specific roles of Helios+ and Helios− Tregs. Nevertheless, our finding that the two subsets are controlled by disparate cytokines implies that they may have different activities. TNF-α is generally considered a pro-inflammatory cytokine with wide and complex immunomodulatory effects, whereas IL-12 is considered a very specific and potent Th1 cytokine. It may be that some of the functional consequences of these two cytokines are mediated through inhibition of Helios+/− Tregs. Because Helios is an intracellular transcription factor, it is not possible to use Helios staining for isolation of live Helios+/− Tregs needed to delineate their functional activities. In this study, we have found that Helios− Tregs express higher levels of TNFRII compared with Helios+ Tregs after ani-CD3 stimulation. We may therefore be able to use TNFRII as a marker to sort the Helios+/− Treg populations for functional studies. In addition, through manipulating IL-12 and TNF-α levels simultaneously in our culture conditions, we can selectively enrich either Helios+ or Helios– Tregs for detailed characterization of their functional properties and to expand these cells for possible use in cellular therapy studies. Specifically, our data indicate that simultaneous addition of TNF-α/anti–IL-12 may be used to expand Helios+ Tregs, whereas the mixture of IL-12/anti–TNF-α may enrich Helios− Tregs.
Blood monocytes are recognized as a heterogeneous population with potentially diverse immune regulatory properties that remain to be fully characterized.38 In our previous study, we showed that CD16+ promotes Th1 responses, while concomitantly inhibiting Th17 and Treg development.26 In the present study, we have found that the two monocyte populations, CD16+ and CD16− cells, can regulate Helios+/− Treg by IL-12 and TNF, respectively. RA patients were recently shown to have altered Helios+ Treg numbers,15 which may be explained by previously described changes in their monocyte subsets.39 Detailed characterization of monocyte populations and their control of Treg development in autoimmune disorders may lead to better understanding of disease pathogenesis and for guiding treatment options.
Cytokine/anticytokine therapy has tremendous potential for treating a variety of diseases. Multiple anti–TNF-α drugs have been licensed,40 and anti–IL-12 antibody (ustekinumab) has been approved for treating plaque psoriasis.41 Similarly, TNF-α42 and IL-1243 cytokines have been used for treatment of various cancers. Our data showing that manipulations of IL-12 and TNF control Helios+/− Treg development, respectively, may help target their use in specific disease types. Furthermore, it may explain variability of response to treatment with these agents. For example, it was recently shown that in RA, only responders to anti–TNF-α therapy had a significant increase in Helios− Tregs,15 suggesting that response to therapy may be dictated by Helios+/− Treg numbers. We also found differences in Helios+/− Treg proliferation depending on the agents used to block TNF-α. Specifically, treatment with anti–TNF-α resulted in a decrease of Helios+ Treg frequency, whereas antibody blocking with anti-TNFRII did not affect this subset. Interestingly, patients’ treatment outcomes after anti–TNF-α therapy versus the use of a soluble form of TNFRII differ.44 Because TNFRII binds both TNF-α and TNF-β, it has been proposed that soluble TNFRII neutralizes both TNF-α and TNF-β.45 It may be that in our co-cultures, anti-TNFRII, similar to soluble TNFRII, inhibits not only TNF-α but also TNF-β pathways.46 The role of TNF-β, if any, on Treg development, including its effect on Helios+/− subsets, remains to be elucidated. Because TNF-α can signal through TNFRI and TNFRII,47 an alternative explanation for the differential effects of anti-TNFRII versus anti–TNF-α is that the latter is expected to prevent TNF-α from binding to its two receptors, thereby blocking signaling events, whereas the former antibody will only block signaling through TNFRII. Although it remains to be determined, blockade of signaling through both or just one TNFR may have different effects on Helios+/− Treg development. Our data showing that TNFRII blockade did not decrease Helios+ Treg expansion, whereas anti–TNF-α did, also suggests that inhibition with TNFRII antagonists as opposed to ant–TNF-α therapy may be advantageous for indications in which a reduction in Helios+ Treg frequency would not be desirable.
In summary, we have found that IL-12 and TNF-α have a differential effect on Helios+/− Treg proliferation in normal healthy individuals in ex vivo cultures. Future studies using our experimental system are ongoing to address the role of these two cytokines on Helios+/− Tregs in vivo in mice as well as in patients with immune disorders.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgment
This work was supported in part by the National Heart, Lung, and Blood Institute (National Institutes of Health) grant R01 HL096497-01 (K.Y.).
Authorship
Contribution: H.Z. conceived the idea, performed research, analyzed data, and drafted the manuscript; K.Y. designed, directed, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karina Yazdanbakhsh, Laboratory of Complement Biology, New York Blood Center, 310 E 67th Street, New York, NY 10065; e-mail: kyazdanbakhsh@nybloodcenter.org