Key Points
Macrophage Wnt signaling regulates wound angiogenesis and repair.
Abstract
The treatment of festering wounds is one of the most important aspects of medical care. Macrophages are important components of wound repair, both in fending off infection and in coordinating tissue repair. Here we show that macrophages use a Wnt-Calcineurin-Flt1 signaling pathway to suppress wound vasculature and delay repair. Conditional mutants deficient in both Wntless/GPR177, the secretory transporter of Wnt ligands, and CNB1, the essential component of the nuclear factor of activated T cells dephosporylation complex, displayed enhanced angiogenesis and accelerated repair. Furthermore, in myeloid-like cells, we show that noncanonical Wnt activates Flt1, a naturally occurring inhibitor of vascular endothelial growth factor-A–mediated angiogenesis, but only when calcineurin function is intact. Then, as expected, conditional deletion of Flt1 in macrophages resulted in enhanced wound angiogenesis and repair. These results are consistent with the published link between enhanced angiogenesis and enhanced repair, and establish novel therapeutic approaches for treatment of wounds.
Introduction
Recently a great deal of research has been devoted to understanding the underlying mechanisms behind wound repair. Many studies have carefully documented the cells involved in wound repair, their function, and the mediators by which they perform such functions.1-3
One process critical to wound repair is angiogenesis, the development of new blood vessels.4 One of the most potent proangiogenic molecules is vascular endothelial growth factor A (VEGF-A). Produced by a variety of cells including macrophages,5 VEGF-A can bind to Flk1 (VEGF-R2) on endothelial cells to induce migration and proliferation. Mice deficient in VEGF-A6,7 or Flk18 die early in development from a lack of blood vessel formation. Importantly, another VEGF-A receptor Flt1 (VEGFR1) binds VEGF-A, but has severely deficient signaling capacity. Therefore, Flt1, which can be spliced into a soluble Flt1 or membrane-tethered Flt1, can act as a suppressor of angiogenesis.9,10 Consistent with this, mice deficient in Flt1 die in utero of excessive angioblast proliferation.11,12
In uninjured skin, VEGF-A is expressed at basal levels, but in response to injury, VEGF-A levels rise.13 When VEGF-A is neutralized during wound repair, granulation tissue is diminished and wound repair is perturbed.14 Many of the known proangiogenic factors are products of cells that infiltrate during the inflammatory phase.4 Macrophages, for instance, are sources of both proangiogenic and antiangiogenic factors. Such factors can affect blood vessel development by modifying the extracellular matrix,15 affecting the growth factor milieu,16,17 and guiding vessel anastomosis.18 During wound repair, mice deficient in macrophages have abnormally overgrown vasculature that surprisingly results in enhanced repair rate.19
One of the mechanisms by which macrophages regulate vessel branching in development involves a noncanonical Wnt-Flt1 signaling pathway.17 Wnt signaling is one of the core pathways regulating developmental patterning.20 Wnt ligands are transported to the cell surface for secretion by Wntless/GPR177 (Wls).21,22 In canonical Wnt signaling, Wnt ligands (eg, Wnt3a) bind to a Frizzled-Lrp5 receptor complex that results in the stabilization and nuclear translocation of β-catenin. Noncanonical Wnt signaling (eg, Wnt5a) involves the many downstream Wnt signaling responses independent of β-catenin.20 One important noncanonical response is the enhancement of intracellular calcium, activation of calcineurin, and dephosporylation of nuclear factor of activated T cells (NFAT).23
Because macrophages are known to regulate developmental angiogenesis via noncanonical Wnt signaling,17 and because wound angiogenesis has been linked to macrophage presence,19 it is quite likely that macrophages are recapitulating their developmental role during wound repair. Here we show that wound macrophages use the Wnt-Flt1 pathway to regulate wound responses, and that calcineurin is the critical mediator.
Study design
In vitro experiments
RAW264.7 and EOC-2 cells (ATCC) were exposed to recombinant Wnt5a, RNA was isolated (RNeasy; Qiagen, Hilden, Germany), and either quantitative reverse-transcription polymerase chain reaction or enzyme-linked immunosorbent assay (Quantikine; R&D Systems, Minneapolis, MN) was performed. Aortic ring assay (ARA) was conducted as described.24
Dermal wounding and quantifications
Wounds were made with 2-mm dermal biopsy punches. For immunofluorescence quantification, wounds were sectioned, labeled with platelet endothelial cell adhesion molecule (PECAM-1; BD Biosciences, San Jose, CA), Iba1 (Wako, Richmond, VA), or Flt1 (R&D Systems), imaged using a Zeiss ApoTome or dissecting microscope and AxioCam camera (Zeiss, Oberkochen, Germany), and quantified using Image J (NIH, Bethesda, Maryland). PECAM intensity was normalized to the avascular epidermis of the respective section.
Animals
Breeding and genotyping of Wlsfl,25 cfms-icre,26 Flt1fl,27 and CNB1fl28 were performed as previously described. All experiments used littermates as controls. Mouse husbandry and experimentation was approved by the Cincinnati Children's Hospital Research Foundation Institutional Animal Care and Use Committee.
Results and discussion
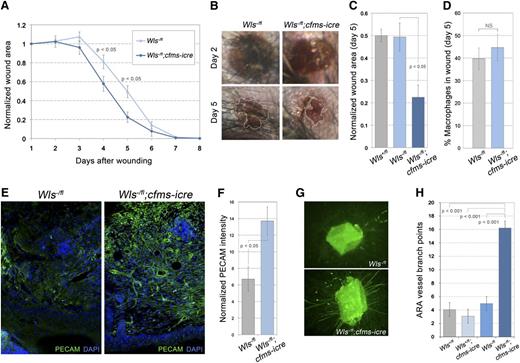
To investigate the role of macrophage Wnt signaling during wound repair, we compromised Wnt ligand secretion by inducing deletion of the conditional Wls allele25 with the transgene cfms-icre.26 In response to full thickness dermal wounding, animals lacking macrophage Wls (Wlsfl/-;cfms-icre) repaired faster than littermate controls (Wlsfl/-) (Figure 1A-C). Significant differences were detected at both 4 and 5 days after wounding (Figure 1A-C), but no difference was detected in the number of wound macrophages (Figure 1D). Importantly, cfms-icre expression was not responsible for the phenotype (supplemental Figure 1A). Because many articles have documented a correlation between wound repair and angiogenesis,29-36 vascular density was assessed using PECAM antibody labeling. The Wlsfl/-;cfms-icre animals displayed enhanced angiogenesis with more intact vascular structures (Figure 1E,F).
Macrophage Wnts suppress wound repair and angiogenesis. (A) Time-course of wound area in control and homozygous null Wls animals. (B) Images of wounds 2 and 5 days after initial injury (×20). (C) Quantification of wound area at day 5. (D) Percentage of F4/80-positive cells (macrophages) in control and mutant wounds. (E) Immunolabeling of wound sections for vasculature (PECAM) in control and null animals (×100). (F) Quantification of PECAM staining intensity per unit area in control and null wounds that was normalized to adjacent avascular epidermis. (G) Images of ARAs after 10 days of culture stained with isolectin-B4 (×50). (H) Quantification of ARA vessels in Wls control and mutant aortas. Statistical analysis performed was Student t test (A, D, F) and one-way analysis of variance with Tukey’s post-hoc test (C, H) using SPSS (IBM, Armonk, NY) software.
Macrophage Wnts suppress wound repair and angiogenesis. (A) Time-course of wound area in control and homozygous null Wls animals. (B) Images of wounds 2 and 5 days after initial injury (×20). (C) Quantification of wound area at day 5. (D) Percentage of F4/80-positive cells (macrophages) in control and mutant wounds. (E) Immunolabeling of wound sections for vasculature (PECAM) in control and null animals (×100). (F) Quantification of PECAM staining intensity per unit area in control and null wounds that was normalized to adjacent avascular epidermis. (G) Images of ARAs after 10 days of culture stained with isolectin-B4 (×50). (H) Quantification of ARA vessels in Wls control and mutant aortas. Statistical analysis performed was Student t test (A, D, F) and one-way analysis of variance with Tukey’s post-hoc test (C, H) using SPSS (IBM, Armonk, NY) software.
To determine whether the role for macrophage Wnts in dermal wound repair was a more general response, we implemented the ARA, an in vitro analysis of angiogenic wound responses.24 Vessel growth in the ARA requires endogenous macrophages.37 In response to aortic wounding, many more vessels were seen in Wlsfl/-;cfms-icre animals relative to controls (Figure 1G,H). Taken together, these data indicated that macrophage Wnt ligands normally suppress angiogenesis.
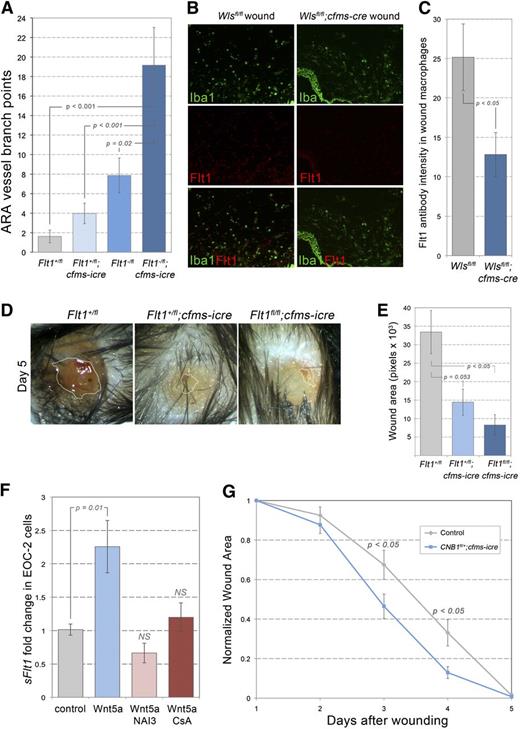
Myeloid Wnts have been shown to suppress retinal angiogenesis by inducing the secretion of Flt1.17 To test the role of macrophage Flt1 during wound repair, mice with a loxP-flanked Flt1 allele27 were crossed to the cfms-icre animals. ARA analysis revealed significantly increased angiogenesis in Fltfl/-;cfms-icre relative to controls (Figure 2A). Because macrophage Flt1 suppresses retinal and ARA angiogenesis, and because Wnt signaling upregulates Flt1,17 we quantified Flt1 immunolabeling in Wlsfl/fl;cfms-icre wound macrophages. Importantly, macrophages from Wlsfl/fl;cfms-icre wounds had diminished Flt1 labeling (Figure 2B,C). Furthermore, when Flt1fl/fl;cfms-icre mutant animals were exposed to full-thickness dermal wounds, they demonstrated enhanced repair (Figure 2D,E). It is important to note that these animals are deficient in both soluble Flt1 and membrane-tethered Flt1, and future work should serve to elucidate the relative role of these 2 splice variants.
Macrophage Flt1 and CNB1 in wound repair. (A) Quantification of ARA vessels in Flt1 control and mutant aortas. (B,C) Immunolabeling for Iba1 (wound macrophages) and Flt1 in control (Wlsfl/fl) and mutant (Wlsfl/fl;cfms-icre) wound sections (B) and quantification of Flt1 staining intensity (C) (×100). (D) Images of wounds in Flt1 control and mutant animals 5 days after initial injury (×20). (E) Quantification of wound size in Flt1 control and mutant animals. (F) Quantitative reverse-transcription polymerase chain reaction for soluble Flt1 from EOC-2 cells exposed for 24 hours to the factors indicated. (G) Time course of wound repair in animals deficient in CNB1. Statistical analysis was performed using one-way analysis of variance with Tukey’s post-hoc test (A, E, F) and Student t test (C, G) using SPSS (IBM, Armonk, NY) software.
Macrophage Flt1 and CNB1 in wound repair. (A) Quantification of ARA vessels in Flt1 control and mutant aortas. (B,C) Immunolabeling for Iba1 (wound macrophages) and Flt1 in control (Wlsfl/fl) and mutant (Wlsfl/fl;cfms-icre) wound sections (B) and quantification of Flt1 staining intensity (C) (×100). (D) Images of wounds in Flt1 control and mutant animals 5 days after initial injury (×20). (E) Quantification of wound size in Flt1 control and mutant animals. (F) Quantitative reverse-transcription polymerase chain reaction for soluble Flt1 from EOC-2 cells exposed for 24 hours to the factors indicated. (G) Time course of wound repair in animals deficient in CNB1. Statistical analysis was performed using one-way analysis of variance with Tukey’s post-hoc test (A, E, F) and Student t test (C, G) using SPSS (IBM, Armonk, NY) software.
To further define the Wnt-Flt1 signaling pathway, an in vitro culture system was used to assess whether certain inhibitors could prevent noncanonical Wnt5a from inducing Flt1. One of the best candidate mediators of this noncanonical Wnt response is Calcineurin-NFAT signaling. When myeloid EOC-2 cells were exposed to Wnt5a, soluble Flt1 expression was upregulated (Figure 2F). Importantly, this effect was not observed in the presence of Cyclopsorine A or NFAT activation inhibitor III (INCA-6), a potent inhibitor of Calcineurin-NFAT interactions but not an effector of other calcinuerin functions.38 Similar findings were observed in the myeloid-like RAW264.7 cells (supplemental Figure 1B,C). To determine whether the in vivo wound repair process also required calcineurin, animals were generated with a conditional deletion in CNB1,28 the subunit of the Calcineurin-NFAT complex that is required for signaling.28 Analysis of dermal wounds in these animals revealed enhanced wound repair in the absence of calcineurin signaling (Figure 2G), a response consistent with both the Wls and Flt1 mutant wound responses. Interestingly, ARA angiogenesis was enhanced in the presence of Cyclosporine A.39 Taken together, these data suggest that macrophages of the wound stroma use a Wnt-Calcineurin-NFAT-Flt1 pathway to suppress wound angiogenesis and slow wound repair.
One important caveat in the analysis presented here is the somewhat promiscuous activity of the cfms-icre transgene.26 The cfms-icre efficiency is nearly 100% in macrophages, but approximately 50% in granulocytes and lymphocytes.26 In wound repair, several lines of reasoning suggest macrophages are the principle effector: (1) responses are seen 3 to 4 days after injury when macrophages are abundant but lymphocytes are rare; (2) Flt1 protein levels were diminished in Wls-deficient macrophages; (3) endogenous immune cells in the ARA are predominantly macrophages37 ; and (4) similar wound vascular responses are observed in PU.1−/− mice that have relatively normal lymphocyte populations.40
In the wound, it seems counterintuitive that natural mechanisms would exist to suppress angiogenesis and slow repair rates. One hypothesis is that increasing angiogenesis may increase repair rates, although it might also make the wound weaker and more susceptible to a second injury during repair. Interestingly, wounds in patients treated with cyclosporine were significantly weaker.41 Therefore, it is possible that the Wnt-Calcinuerin-Flt1 pathway identified here is used by macrophages to suppress wound angiogenesis and consequently increase the transient strength of the repairing wound. However, in a context in which wounds can be protected during repair, therapeutic targeting of this pathway may elucidate novel mechanism by which wound repair rates could be increased.
The online version of this article contains a data supplement.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Paul Speeg for his technical assistance and Gerald R. Crabtree for the CNB1fl mice.
This work was supported by grants from the National Institutes of Health (R01CA131270) (J.P. and R.A.L) and (T32GM063483-08S1) (J.A.S).
Authorship
Contribution: J.A.S and R.A.L designed the experiments and wrote the manuscript. J.A.S., S.R., and K.B. performed the experiments. A.C.A. and R.F.N. provided technical assistance and advice. J.W.P. and N.F. developed critical reagents.
Conflict-of-interest disclosure: N.F. is an employee of Genentech Corporation. The remaining authors declare no competing financial interests.
Correspondence: Richard A. Lang, Division of Pediatric Ophthalmology, Cincinnati Children's Hospital Medical Center, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: richard.lang@cchmc.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal