Key Points
Isolation of BOECs from multiple patients with VWD is feasible, and the study of BOECs helps explain the pathogenic complexity of VWD.
Abnormalities in WPB biogenesis and exocytosis and defects in VWF string formation correlate with the phenotypic features of patients with VWD.
Abstract
Patients with von Willebrand disease (VWD) are often heterozygous for a missense mutation in the von Willebrand factor (VWF) gene. Investigating the pathogenic features of VWF mutations in cells directly derived from patients has been challenging. Here, we have used blood outgrowth endothelial cells (BOECs) isolated from human peripheral blood to analyze the storage and secretion of VWF. BOECs showed full endothelial characteristics and responded to Weibel-Palade body (WPB) secretagogues except desmopressin. We examined BOECs derived from a single subject heterozygous for a type 2N mutation (p.Arg854Gln) and from 4 patients with type 1 VWD who were, respectively, heterozygous for p.Ser1285Pro, p.Leu1307Pro, p.Tyr1584Cys, and p.Cys2693Tyr. Compared with normal BOECs, BOECs heterozygous for p.Ser1285Pro, p.Leu1307Pro, or p.Cys2693Tyr showed morphologically abnormal WPB and retention of VWF in the endoplasmic reticulum, whereas BOECs heterozygous for p.Arg854Gln or p.Tyr1584Cys showed normal WPB. The agonist-induced exocytosis of WPB from BOECs and formation of VWF strings on BOECs heterozygous for p.Ser1285Pro, p.Leu1307Pro, or p.Cys2693Tyr, but not for p.Arg854Gln or p.Tyr1584Cys, were reduced. In conclusion, VWD phenotype can be recapitulated in BOECs, and thus BOECs provide a feasible bona fide cell model to study the pathogenic effects of VWF mutations.
Introduction
Von Willebrand disease (VWD) is the most common inherited human bleeding disorder. It is mainly caused by mutations in the von Willebrand factor (VWF) gene that encodes von Willebrand factor (VWF), a large multimeric, hemostatic protein.1 VWF supports primary hemostasis by recruiting platelets to the subendothelial matrix or endothelial cell surface on vascular perturbation or injury. Furthermore, VWF binds coagulation factor VIII (FVIII) to protect it from premature proteolysis in the circulation.2,3 In endothelial cells, VWF is stored in equimolar amounts with VWF propeptide (VWFpp) in Weibel-Palade bodies (WPBs) for basal and regulated secretion.4,5 On exocytosis of WPBs, VWF is released and forms ultralong, hyperadhesive strings that stay attached to endothelial cells, whereas VWFpp rapidly diffuses into the blood. VWF strings bind platelets from flowing blood to initiate clot formation, and then the strings are rapidly cleaved into smaller VWF multimers by ADAMTS-13 (a disintegrin and metalloproteinase with a thrombospondin type 1 motif, member 13) to avoid vascular occlusion.6 Defects in the formation or function of VWF strings may lead to a bleeding tendency, as seen in VWD.
VWD has been classified into 3 categories: type 1 and type 3 with quantitative deficiency of VWF and type 2 with functional defects.1 Type 1 VWD is the most common type (up to 75% of all VWD cases), with reduced VWF levels caused by multiple factors including inefficient synthesis, storage, or secretion; endoplasmic reticulum (ER)–related intracellular degradation; or faster clearance from the circulation.7,8 The mutations identified in type 1 VWD are heterogeneous and located throughout the whole gene.9 Although several VWF mutations identified in type 1 VWD have been extensively studied in heterologous cell systems,7 interpreting the phenotypic defects of such mutations is not always easy. One of the limitations is the fact that in most cell model systems, such as COS cells, VWF is not intracellularly stored.10 Only few cell lines, such as human embryonic kidney 293 (HEK293) cells, form pseudo-WPB and are useful for analyzing the structure–function relation of VWF. Studies in these cell lines have provided insights into the effects of VWF mutations on the storage and secretion of VWF.11-17 In contrast, the use of nonendothelial cell systems has obvious limitations: The possible gross overexpression of recombinant VWF on transfection may influence interpretation of the data, and the exocytotic machinery is probably different from that of endothelial cells. Mimicking the heterozygous state of VWF mutations by cotransfection of wild-type and mutant VWF is potentially also problematic.
Human umbilical vein endothelial cells (HUVECs) have been derived from patients with VWD and characterized many years ago.18-22 However, HUVECs cannot be widely used for such studies because the access to HUVECs with specific VWF mutations is extremely limited.
Blood outgrowth endothelial cells (BOECs) may provide an alternative for HUVECs. BOECs can be derived from circulating endothelial progenitor cells on culture of human peripheral or cord blood mononuclear cells.23,24 In culture, BOECs have all the endothelial characteristics, including the expression of endothelial cell markers and the capability of forming capillary-like structures in Matrigel.24 It has been suggested that BOECs provide a useful system to study the release of WPB and the formation of VWF strings.25 In addition, morphology of WPB in the BOECs derived from a single patient with VWD has been characterized in a recent study.17 The aim of the current study was to further explore the feasibility of using BOECs as an endothelial cell model to study the pathogenic nature of VWF mutations at the molecular cellular level. To this end, we isolated BOECs from 5 healthy donors and 5 patients carrying VWF mutations and then characterized these cells. Furthermore, we tested the response of BOECs to different WPB secretagogues and analyzed VWF storage in and regulated secretion from VWD BOECs.
Patients, materials, and methods
Patients
Patients with VWD who are heterozygous for VWF mutations p.Ser1285Pro, p.Leu1307Pro, p.Tyr1584Cys, and p.Cys2693Tyr were enrolled in this study (Table 1). All 4 patients were initially diagnosed with type 1 VWD. A single asymptomatic participant was heterozygous for the type 2N VWF mutation p.Arg854Gln. Plasma was prepared and tested using standard procedures for FVIII and VWF evaluation, including FVIII:C, VWF:Ag, VWF:RCo, VWF:CB, and VWF multimer profile. The study protocol was approved by the Leiden University Medical Center ethics review board. Informed consent was obtained from all participants in accordance with the Declaration of Helsinki.
Characteristics of investigated subjects
| Subject . | Sex . | Blood group . | Nucleotide change . | Amino acid change . | VWF:Ag, IU/dL . | VWF:RCo, IU/dL . | VWF:RCo/ VWF:Ag . | FVIII:C, IU/dL . | VWF:CB, IU/dL . | Multimer pattern . | VWD type* . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P6F1III1† | Female | O/O | c.2561G>A | p.Arg854Gln | 73 | 114 | 1.56 | 59 | Not tested | Normal | 2N |
| P6F3II1§ | Female | O/O | c.3853T>C | p.Ser1285Pro | 16 | 3 | 0.19 | 27 | 8 | Abnormal‡ | 1 |
| P6F13II1§ | Female | O/O | c.3920T>C | p.Leu1307Pro | 16 | 7 | 0.44 | 33 | 6 | Abnormal | 2A |
| P6F12II1§ | Female | O/O | c.4751A>G | p.Tyr1584Cys | 48 | 36 | 0.75 | 64 | 52 | Normal | 1 |
| P6F16II1§ | Female | O/O | c.8078G>A | p.Cys2693Tyr | 46 | 64 | 1.39 | 69 | 42 | Normal | 1 |
| Subject . | Sex . | Blood group . | Nucleotide change . | Amino acid change . | VWF:Ag, IU/dL . | VWF:RCo, IU/dL . | VWF:RCo/ VWF:Ag . | FVIII:C, IU/dL . | VWF:CB, IU/dL . | Multimer pattern . | VWD type* . |
|---|---|---|---|---|---|---|---|---|---|---|---|
| P6F1III1† | Female | O/O | c.2561G>A | p.Arg854Gln | 73 | 114 | 1.56 | 59 | Not tested | Normal | 2N |
| P6F3II1§ | Female | O/O | c.3853T>C | p.Ser1285Pro | 16 | 3 | 0.19 | 27 | 8 | Abnormal‡ | 1 |
| P6F13II1§ | Female | O/O | c.3920T>C | p.Leu1307Pro | 16 | 7 | 0.44 | 33 | 6 | Abnormal | 2A |
| P6F12II1§ | Female | O/O | c.4751A>G | p.Tyr1584Cys | 48 | 36 | 0.75 | 64 | 52 | Normal | 1 |
| P6F16II1§ | Female | O/O | c.8078G>A | p.Cys2693Tyr | 46 | 64 | 1.39 | 69 | 42 | Normal | 1 |
BOECs isolation and culture
BOECs were isolated and cultured as previously described,23 with modifications. Eighty milliliters of venous blood from each participant were collected in S-Monovette tubes (S-Monovette 10 mL, coagulation 9 NC, containing 0.1 volume 0.106 M trisodium citrate; Sarstedt, Nümbrecht, Germany). The citrated blood was diluted 1:1 with Ca2+- and Mg2+-free HBSS (Invitrogen, Carlsbad, California). Buffy coat mononuclear cells were isolated by gradient centrifugation over Ficoll-Paque PLUS (GE Healthcare, Diegem, Belgium) at 800 g for 30 minutes and washed twice at 700 g for 10 minutes. Cell pellets were resuspended in EGM-2 medium (Lonza, Breda, The Netherlands) supplemented with 20% fetal bovine serum, an EGM-2 BulletKit (final concentration in the medium: 0.03% hydrocortisone, 0.33% hFGF-B, 0.08% vascular endothelial growth factor, 0.08% R3-IGF-1, 0.08% hEGF, 0.08% ascorbic acid, 0.08% heparin, and 0.08% GA-1000; Lonza) and 250 ng/mL amphotericin (Invitrogen) and seeded onto 6-well tissue culture plates precoated with rat tail collagen type 1 (BD Biosciences, Bedford, Massachusetts). Culture medium was refreshed daily until the first colony appeared. The medium was then refreshed every 2 days. Cells were used at passages 3 to 8 in all experiments unless stated otherwise.
FACS analysis
BOECs were washed once with phosphate-buffered saline containing 1% bovine serum albumin and 0.01% sodium azide and were incubated with the primary antibody or isotype control for 30 minutes at room temperature in the dark. We used primary mouse monoclonal antibodies (all purchased from BD Biosciences unless stated otherwise) against human CD14 conjugated to fluorescein isothiocyanate, human CD45 conjugated to peridinin-chlorophyll proteins, human CD31 conjugated to phycoerythrin, human CD34, and human CD133 conjugated to allophycocyanin. A secondary antibody conjugated to Alexa Fluor 647 (Invitrogen) was used to label endothelial protein C receptor (EPCR). In separate tubes, cells were incubated with isotype controls. Samples were fixed in 1% paraformaldehyde and analyzed by flow cytometry (fluorescence-activated cell sorter [FACS] LSRII, BD Biosciences) within 24 hours. Data were analyzed using FACSDiVa software (BD Biosciences).
Matrigel assay and microscopic imaging
Matrigel assays were performed according to the manufacturer’s instructions. Matrigel (BD Biosciences) was thawed overnight on ice. Ninety-six-well tissue culture plates were coated with 30 μL/well Matrigel for 30 minutes at 37°C. BOECs were suspended in complete EGM-2 medium and seeded for 4 to 6 hours at a cell density of 5000 to 20 000 cells/well. Images were taken by Leica DM IL LED inverted microscopy equipped with the Leica DFC295 digital camera (Leica Microsystems).
Confocal immunofluorescence microscopy
BOECs were grown on coverslips coated with collagen type 1. Confluent cells were fixed, permeabilized, and stained essentially as previously described.11 To visualize VWF strings, confluent cells were stimulated as indicated before fixation. Monoclonal antibody CLB-RAg3526 and polyclonal antibody rabbit antihuman VWF (DAKO, Glostrup, Denmark) were used to visualize VWF. VWF strings per field were semiquantified by visual inspection. Monoclonal antibody CD62P (Clone AC1.2, BD Biosciences), polyclonal antibody rabbit antihuman β-catenin (Santa Cruz Biotechnology, Santa Cruz, California), and polyclonal antibody rabbit antihuman protein disulfide isomerase (PDI) antibody A66 (obtained from Prof. I. Braakman, Department of Chemistry, Utrecht University, The Netherlands) were used to visualize P-selectin, β-catenin, and the ER marker PDI, respectively. Alexa 488- and Alexa 594-conjugated secondary antibodies were purchased from Invitrogen. Samples were analyzed by Leica SL confocal laser scanning microscopy with a 63×/1.40 numerical aperture (NA) oil objective (Leica Microsystems).
Stimulation of Weibel-Palade body exocytosis
BOECs were grown on collagen type 1–coated coverslips or in collagen type 1–coated 12-well tissue culture plates. Confluent cells were stimulated for 1 hour with 160 nM phorbol-12-myristate-13-acetate (PMA), 100 μM histamine, 0.05 to 1 μM desmopressin acetate 3-water (DDAVP) plus 100 μM isobutylmethylxanthine (IBMX), or 10 μM epinephrine plus 100 μM IBMX in serum-free medium (OPTIMEM1 medium [Invitrogen], 10 mM N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid (HEPES), and 0.2% bovine serum albumin at pH 7.4). DDAVP was purchased from Ferring Pharmaceuticals (Hoofddorp, The Netherlands). PMA, histamine, epinephrine, and IBMX were purchased from Sigma-Aldrich (St. Louis, Missouri).
Quantification of VWF, VWFpp, and proVWF
VWF antigen11 and VWFpp27 were measured essentially as described before, with minor modifications. Polyclonal antibody rabbit antihuman VWF and polyclonal antibody goat antirabbit IgG coupled to peroxidase (DAKO) were used to measure VWF antigen. Monoclonal antibody mouse antihuman VWFpp CLB-Pro 35 and CLB-Pro 14.3 coupled to peroxidase (Sanquin, Amsterdam, The Netherlands) were used to measure VWFpp. VWF:Ag and VWFpp were measured using normal pooled human plasma as the reference, and results were expressed in units. To measure the uncleaved proVWF in the cell lysate, 96-well enzyme-linked immunosorbent assay (ELISA) plates were precoated with polyclonal antibody rabbit antihuman VWF (DAKO), and the proVWF was detected by CLB-Pro 14.3 coupled to peroxidase (Sanquin). To validate this assay, the normal pooled plasma (NPP) was used as a negative control in which no proVWF was detected.
To quantify basal secretion of VWF, confluent cells in 12 wells were incubated at 37°C in EGM-2 medium (containing 2% fetal bovine serum and supplemented with a cocktail of growth factors and antibiotics, as described above). On the basis of previous work by Giblin et al,5 the conditioned media were collected after incubation for 5 hours. VWF:Ag secreted into the conditioned media in 5 hours was defined as basal secretion of VWF. Cells were lysed overnight at 4°C with serum-free medium containing 0.1% Triton-X100 and the protease inhibitor cocktail Complete with EDTA (Roche Diagnostics, Mannheim, Germany). Cell lysates were vortexed for 10 seconds before centrifugation. The supernatants of conditioned media and cell lysates were collected after centrifugation at 14 000 rpm for 5 minutes. VWF:Ag in the medium and lysate was measured by ELISA.
To quantify the release of VWF or VWFpp during exocytosis of WPB, the conditioned media and lysates were collected after stimulation, as described earlier. All the supernatants were supplemented with phenylmethylsulfonyl fluoride (Roche) at a final concentration of 100 μM and then analyzed immediately or snap-frozen. The percentage of secreted VWF:Ag or VWFpp during stimulation was defined as regulated secretion and was expressed as a fraction of total amount of antigen (% of total = absolute antigen in the medium/[absolute antigen in the medium + absolute antigen in the cell lysate] × 100).
Multimer analysis
Frozen plasma was thawed at 37°C and centrifuged for 5 minutes at 14 000 rpm. The supernatants were collected for VWF multimer analysis. BOECs were cultured until confluent and then incubated with EGM-2 medium (containing 2% serum) for 24 hours. The supernatants of conditioned media and lysates of BOECs were collected as described earlier. Each sample containing 0.8 to 1.0 mU VWF:Ag was used for gel analysis. VWF multimers were separated by nonreducing 1.6% agarose gel electrophoresis with sodium dodecyl sulfate and visualized by Western blotting, as described.28
Graphing and statistical analysis
Data and statistical analysis were performed with GraphPad Prism for Windows (version 5.01). Statistical analyses were performed using the Student’s unpaired t-test or 1-way ANOVA with the Bonferroni post test where applicable; P < .05 was considered statistically significant.
Results
Phenotypic and genotypic characterization of patients with VWD
The 4 patients with VWD are heterozygous for VWF mutations p.Ser1285Pro, p.Leu1307Pro, p.Tyr1584Cys, and p.Cys2693Tyr, respectively. All the patients are women and have blood group O (Table 1). They were historically diagnosed as type 1 VWD because of low plasma levels of VWF and nearly normal VWF multimer patterns.29 A detailed analysis of VWF multimers identified marginal decrease of the largest VWF multimers in the patients heterozygous for VWF mutations p.Ser1285Pro and p.Leu1307Pro.30 According to the 2006 classification criteria, p.Ser1285Pro fits the diagnosis of type 1, whereas p.Leu1307Pro might be reclassified as type 2A.1,30 The asymptomatic subject heterozygous for type 2N mutation p.Arg854Gln showed a reduced binding ability to FVIII but a normal level of VWF (Table 1).
Isolation and characteristics of BOECs
BOECs were derived from peripheral blood mononuclear cells. After a 2- to 4-week culture, colonies with cobblestone-like cells appeared (Figure 1A). These cells were late-outgrowth endothelial cells, referred to here as BOECs. Immunofluorescence microscopy showed that VWF was stored by BOECs in elongated organelles (Figure 1B) that displayed internal striations under electron microscopy (data not shown).25 Furthermore, these organelles also stored the WPB-specific membrane protein P-selectin (Figure 1B). These data indicate that VWF was stored in authentic WPB.
Phenotypic and functional analysis of BOECs. (A) Cobblestone-like morphology of BOECs under bright field microscopy. (Scale bar, 100 μm.) (B) BOECs store VWF (shown in red) and P-selectin (shown in green) in WPB. Yellow indicates the costorage of VWF and P-selectin in WPB (elongated organelles). Image was taken by Leica SL confocal laser scanning microscopy with a 63×/1.40 NA oil objective. (Scale bar, 10 μm.) (C) BOECs formed capillary-like structures in Matrigel. (Scale bar, 200 μm). (D-M) FACS analysis of BOECs derived from a single healthy donor. The representative data demonstrate that BOECs were negative for leukocyte cell makers CD14 (D, isotype; E, CD14) and CD45 (F, isotype; G, CD45) but positive for endothelial cell markers CD31 (endothelial cell marker CD31; H, isotype; I, CD31) and EPCR (J, isotype; M, EPCR). In addition, BOECs completely lost expression of the stem cell maker CD133 (J, isotype; K, CD133), although the majority of cells still express the progenitor cell marker CD34 (J, isotype; L, CD34). Shown are representative data from at least 4 independent experiments using BOECs derived from a single healthy donor. Cells derived from other healthy donors and the patients showed similar results.
Phenotypic and functional analysis of BOECs. (A) Cobblestone-like morphology of BOECs under bright field microscopy. (Scale bar, 100 μm.) (B) BOECs store VWF (shown in red) and P-selectin (shown in green) in WPB. Yellow indicates the costorage of VWF and P-selectin in WPB (elongated organelles). Image was taken by Leica SL confocal laser scanning microscopy with a 63×/1.40 NA oil objective. (Scale bar, 10 μm.) (C) BOECs formed capillary-like structures in Matrigel. (Scale bar, 200 μm). (D-M) FACS analysis of BOECs derived from a single healthy donor. The representative data demonstrate that BOECs were negative for leukocyte cell makers CD14 (D, isotype; E, CD14) and CD45 (F, isotype; G, CD45) but positive for endothelial cell markers CD31 (endothelial cell marker CD31; H, isotype; I, CD31) and EPCR (J, isotype; M, EPCR). In addition, BOECs completely lost expression of the stem cell maker CD133 (J, isotype; K, CD133), although the majority of cells still express the progenitor cell marker CD34 (J, isotype; L, CD34). Shown are representative data from at least 4 independent experiments using BOECs derived from a single healthy donor. Cells derived from other healthy donors and the patients showed similar results.
Functional analysis showed that BOECs were able to form capillary-like structures in Matrigel (Figure 1C). FACS analysis (Figure 1D-M) showed that BOECs were positive for endothelial cell marker CD31 and EPCR and negative for leukocyte markers CD14 and CD45. In addition, BOECs were negative for stem cell marker CD133. This indicates that BOECs have differentiated to mature endothelial cells. Furthermore, 70% to 80% of the BOECs were positive for CD34, similar to in a previous report.31 These representative data (Figure 1) were generated from the BOECs of a single healthy donor; cells derived from other participants showed similar results.
The histamine receptor H1 mediates histamine-induced secretion of VWF from endothelial cells.32 Using real-time polymerase chain reaction, we found that in BOECs, H1 receptor was indeed highly expressed, whereas the expression of H2, H3, and H4 receptors was nearly undetectable (supplemental Figure 1).
The yield of BOEC colonies was donor-dependent. So far, we have isolated BOECs from more than 20 patients, including several with VWD type 1, 2A, and 2N (some data unpublished), and only failed to isolate BOECs from a single healthy donor and a single patient with VWD who was heterozygous for p.Cys1149Arg. Several attempts to establish BOECs from these 2 participants were unsuccessful, although BOECs were obtained from 2 healthy donors in each of 4 different attempts. Importantly, except for the BOECs derived from the patient heterozygous for p.Tyr1584Cys, the other 9 participants' BOECs reported in this study were obtained from the first isolation. The success in isolating BOECs did not appear to be related to age or sex of donors (data not shown). However, we have not yet succeeded in isolating BOECs from patients with type 3 VWD; it is therefore not clear whether the variability in isolating BOECs is related to the endogenous VWF level.
Storage of VWF in BOECs derived from patients with VWD
VWF drives the biogenesis of WPB. Mutations in VWF may disturb the formation and function of WPB. VWF was stored normally in elongated WPB in BOECs derived from healthy donors and from patients with VWF mutations p.Arg854Gln and p.Tyr1584Cys (Figure 2A-C). Mainly short/round WPB were observed in BOECs derived from patients with VWF mutations p.Ser1285Pro, p.Leu1307Pro, and p.Cys2693Tyr (Figure 2D-F). Abundant reticular VWF staining was observed in BOECs heterozygous for the latter 3 VWF mutations, indicating retention of VWF in the ER. Further analysis showed that the diffuse VWF staining indeed colocalized with the ER marker PDI (Figure 2G). The retention of VWF in the ER was also indicated by the presence of more uncleaved proVWF (percentage of total VWF) in the cell lysate of p.Cys2693Tyr (1.82-fold higher than that of normal BOECs; P < .01). Compared with p.Ser1285Pro or p.Leu1307Pro, storage of p.Cys2693Tyr was heterogeneous in BOECs, with 10% to 30% cells containing more elongated WPB (Figure 2F′). This suggests that the latter mutation has a milder defective effect on the storage of VWF. In p.Cys2693Tyr BOECs, the mutant allele was expressed at a lower level: 39% of total mRNA originates from the mutant allele 2693Tyr and 61% from wild-type allele 2693Cys (see the TaqMAMA assay for genotyping in the supplemental data). This suggests that the heterogeneity of WPB formation in BOECs of p.Cys2693Tyr may be the result of varied expression of the mutated allele in different cells.
Storage of VWF in BOECs. BOECs derived from a healthy donor or patients with mutations as indicated were grown on collagen type 1–precoated coverslips until confluent. (A-F′) Cells were fixed and stained for VWF. The punctate staining indicates storage of VWF in WPB, whereas the diffuse staining in (D-F) indicates VWF retained within the ER. (G) Representative image showing that the diffuse VWF staining observed in BOECs derived from the patient heterozygous for p.Cys2693Tyr was colocalized with the PDI. BOECs were fixed and stained for VWF (middle, green) and the ER marker PDI (right, red). Merge of VWF and PDI staining is also shown (left). Note that the majority of WPB observed in BOECs derived from the patients heterozygous for VWF p.Ser1285Pro, p.Leu1307Pro, and p.Cys2693Tyr were short or round instead of elongated, as seen in (A). (F′) Cell derived from the patient heterozygous for p.Cys2693Tyr with WPB of normal morphology. (Scale bars, 5 μm.) All images were taken by Leica SL confocal laser scanning microscopy with a 63×/1.40 NA oil objective.
Storage of VWF in BOECs. BOECs derived from a healthy donor or patients with mutations as indicated were grown on collagen type 1–precoated coverslips until confluent. (A-F′) Cells were fixed and stained for VWF. The punctate staining indicates storage of VWF in WPB, whereas the diffuse staining in (D-F) indicates VWF retained within the ER. (G) Representative image showing that the diffuse VWF staining observed in BOECs derived from the patient heterozygous for p.Cys2693Tyr was colocalized with the PDI. BOECs were fixed and stained for VWF (middle, green) and the ER marker PDI (right, red). Merge of VWF and PDI staining is also shown (left). Note that the majority of WPB observed in BOECs derived from the patients heterozygous for VWF p.Ser1285Pro, p.Leu1307Pro, and p.Cys2693Tyr were short or round instead of elongated, as seen in (A). (F′) Cell derived from the patient heterozygous for p.Cys2693Tyr with WPB of normal morphology. (Scale bars, 5 μm.) All images were taken by Leica SL confocal laser scanning microscopy with a 63×/1.40 NA oil objective.
Production and basal secretion of VWF from BOECs
Production and basal secretion of VWF were examined for BOECs (Table 2). Compared with that of healthy donors, total VWF production was much lower for BOECs with VWF mutations p.Ser1285Pro and p.Leu1307Pro, although it was similar for BOECs with p.Arg854Gln, p.Tyr1584Cys, or p.Cys2693Tyr. Basal secretion of VWF was in the normal range for BOECs with VWF mutation p.Tyr1584Cys and was slightly lower for BOECs with VWF mutations p.Arg854Gln and p.Cys2693Tyr. For BOECs with VWF mutations p.Ser1285Pro or p.Leu1307Pro, VWF basal secretion was drastically decreased (3 to 5 mU vs 20 to 66 mU).
Production and basal secretion of VWF from BOECs
| Subject . | Amino acid change . | Total VWF production (mU) . | VWF secreted in medium (mU) . |
|---|---|---|---|
| Healthy donor 1 | — | 175.5 ± 0.8 | 61.7 ± 0.7 |
| Healthy donor 2 | — | 98.2 ± 10.2 | 66.2 ± 5.9 |
| Healthy donor 3 | — | 41.5 ± 1.0 | 20.5 ± 1.3 |
| Healthy donor 4 | — | 128.1 ± 6.9 | 42.8 ± 1.6 |
| Healthy donor 5 | — | 181.8 ± 4.6 | 48.8 ± 3.5 |
| P6F1III1 | p.Arg854Gln | 82.2 ± 1.2 | 18.7 ± 0.4 |
| P6F3II1 | p.Ser1285Pro | 16.4 ± 1.1 | 5.4 ± 0.2 |
| P6F13II1 | p.Leu1307Pro | 5.7 ± 0.5 | 3.0 ± 0.3 |
| P6F12II1 | p.Tyr1584Cys | 155.5 ± 35.1 | 45.4 ± 1.2 |
| P6F16II1 | p.Cys2693Tyr | 121.5 ± 0.0 | 16.0 ± 0.1 |
| Subject . | Amino acid change . | Total VWF production (mU) . | VWF secreted in medium (mU) . |
|---|---|---|---|
| Healthy donor 1 | — | 175.5 ± 0.8 | 61.7 ± 0.7 |
| Healthy donor 2 | — | 98.2 ± 10.2 | 66.2 ± 5.9 |
| Healthy donor 3 | — | 41.5 ± 1.0 | 20.5 ± 1.3 |
| Healthy donor 4 | — | 128.1 ± 6.9 | 42.8 ± 1.6 |
| Healthy donor 5 | — | 181.8 ± 4.6 | 48.8 ± 3.5 |
| P6F1III1 | p.Arg854Gln | 82.2 ± 1.2 | 18.7 ± 0.4 |
| P6F3II1 | p.Ser1285Pro | 16.4 ± 1.1 | 5.4 ± 0.2 |
| P6F13II1 | p.Leu1307Pro | 5.7 ± 0.5 | 3.0 ± 0.3 |
| P6F12II1 | p.Tyr1584Cys | 155.5 ± 35.1 | 45.4 ± 1.2 |
| P6F16II1 | p.Cys2693Tyr | 121.5 ± 0.0 | 16.0 ± 0.1 |
VWF:Ag was determined for all BOECs at passage 6. Cells were grown until confluent in 12-well plates (approximately 1 × 105 cells per well) and incubated for 5 hours with EGM-2 medium (containing 2% serum). Total VWF production = VWF:Ag in conditioned medium (basal secretion) + VWF:Ag in the lysate. Each value represents the mean ± standard error of the mean of duplicate or triplicate measurements.
Distribution of VWF multimers from BOECs
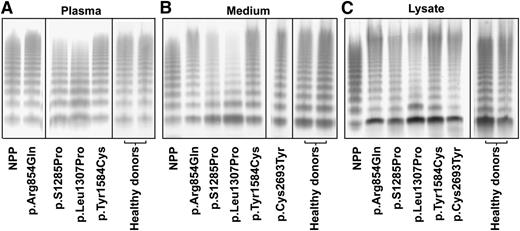
To correlate the phenotype of the BOECs with that of the patients, we also compared the VWF multimers from both BOECs and patients’ plasma (Figure 3). In agreement with our previous reports,29,30 plasma VWF from the patients with p.S1285Pro and p.Leu1307Pro showed a slightly abnormal multimer pattern with a marginal loss of the largest multimers, whereas plasma VWF from the patients with p.Arg854Gln, p.Tyr1584Cys, and p.Cys2693Tyr29 showed a normal multimer pattern (Figure 3A). The multimer patterns of plasma VWF from all healthy donors were normal. Indeed, VWF secreted from BOECs derived from healthy individuals and mutation carriers with p.Arg854Gln, p.Tyr1584Cys, and p.Cys2693Tyr showed a normal multimer pattern (Figure 3B). In the culture media of BOECs derived from the patients with p.S1285Pro and p.Leu1307Pro mutations, the dimer band of VWF was more prominent than that in the media of BOECs with the other mutants, and there was relatively more of the low–molecular weight multimers and less of the high–molecular weight multimers (Figure 3B). In the medium of the BOECs, higher–molecular weight multimers were present compared with NPP. This is explained by the insufficient, if any, proteolysis by ADAMTS-13 and the absence of clearance in the experimental conditions of the BOECs culture. The VWF multimer patterns in cell lysates were similar to the corresponding multimer patterns in medium (Figure 3B-C).
Multimer analysis of VWF. Multimers are shown for VWF in the plasma from indicated patients (A), in the culture medium (B), or in the cell lysates (C) of BOECs. The lanes separated by the black lines are representative results from the same gels or from different experiments. Normal pooled plasma (lane NPP) was used as reference. The plasma of the patient heterozygous for p.Cys2693Tyr was unfortunately no longer available, but the multimer pattern for this patient has been reported before as normal.29 VWF multimer pattern of all healthy donors was normal; only 2 representative patterns are shown. The multimer patterns of VWF were analyzed by sodium dodecyl sulfate–agarose gel electrophoresis and Western blot under nonreducing conditions.
Multimer analysis of VWF. Multimers are shown for VWF in the plasma from indicated patients (A), in the culture medium (B), or in the cell lysates (C) of BOECs. The lanes separated by the black lines are representative results from the same gels or from different experiments. Normal pooled plasma (lane NPP) was used as reference. The plasma of the patient heterozygous for p.Cys2693Tyr was unfortunately no longer available, but the multimer pattern for this patient has been reported before as normal.29 VWF multimer pattern of all healthy donors was normal; only 2 representative patterns are shown. The multimer patterns of VWF were analyzed by sodium dodecyl sulfate–agarose gel electrophoresis and Western blot under nonreducing conditions.
Regulated secretion of VWF and VWFpp
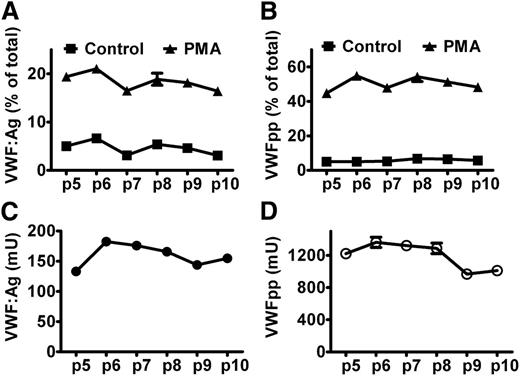
VWF and VWFpp are stored together in WPB for both basal and regulated secretion. To quantify exocytosis of WPB, we determined the secretion of both VWF and VWFpp. We first examined the variations of VWF and VWFpp secretion and production from BOECs between culture passages and clones. As shown in Figure 4 for BOECs from a healthy donor, and in supplemental Figure 2 for BOECs from the patient heterozygous for p.Tyr1584Cys, the regulated secretion of VWFpp, the relative secretion induced by PMA (ie, the fraction of total amount of VWFpp), was rather stable within the first 10 culture passages. The regulated secretion of VWF from BOECs heterozygous for p.Tyr1584Cys varied and did not reflect the extent of exocytosis very well (supplemental Figure 2A). The absolute production of VWFpp was relatively constant within the first 8 culture passages for both BOECs. Although the absolute production of VWFpp could vary to a large extent, the regulated secretion of VWFpp was rather constant between different clones of BOECs (supplemental Figure 3).
Regulated secretion of VWF and VWFpp from BOECs. BOECs derived from a healthy donor were cultured in 12-well plates until confluent. After rinsing with HBSS, cells were incubated for 60 minutes without (Control) or with (PMA) 160 nM PMA at 37°C. The regulated secretion of VWF (A) and VWFpp (B) was determined for BOECs at given passages. (C,D) Y-axis indicates the total production (medium plus lysate) of VWF or VWFpp by approximate 1 × 105 cells. Mean ± SEM indicate variation between triplicate measurements.
Regulated secretion of VWF and VWFpp from BOECs. BOECs derived from a healthy donor were cultured in 12-well plates until confluent. After rinsing with HBSS, cells were incubated for 60 minutes without (Control) or with (PMA) 160 nM PMA at 37°C. The regulated secretion of VWF (A) and VWFpp (B) was determined for BOECs at given passages. (C,D) Y-axis indicates the total production (medium plus lysate) of VWF or VWFpp by approximate 1 × 105 cells. Mean ± SEM indicate variation between triplicate measurements.
The total production of VWFpp expressed in units seemed 5 to 9 times higher than the total production of VWF:Ag (Figure 4C-D and supplemental Figure 2C-D). This can be explained as follows: We used NPP as a reference to measure VWFpp and VWF:Ag. In plasma, VWFpp and VWF:Ag are expressed in units rather than in molars, and by definition, 1 mL of plasma contains 1 unit VWFpp and VWF:Ag. Although VWFpp and VWF:Ag are secreted in equimolar amounts, the molar concentrations of the 2 antigens in normal plasma differ greatly because of the differences in half-lives of VWFpp and mature VWF (1 U/mL VWFpp corresponds to 5 to 6 nM and 1 U/mL VWF:Ag to 31 nM).33,34 As there is no clearance in cell culture, the measured total production of the 2 antigens will be equal in molars, meaning that the measured VWFpp when expressed in units will be 5 to 6 times higher than VWF:Ag. Our data are in line with this, although the observed ratio seemed slightly higher than the expected ratio, which may be explained by the additional loss of mature VWF during sample preparations (large multimers or strings attached to cell membrane or plastic tubes) and different types of ELISA. In the regulated secretion, the relative secretion of VWFpp was 2-fold higher than that of VWF:Ag (Figure 4A-B and supplemental Figure 2A-B), which can be explained by the attachment of part of VWF (eg, in the form of strings) to the cell membrane after induced exocytosis of WPB, whereas VWFpp diffuses immediately into the medium.35 Taken together, these results suggest that it is more reliable to determine VWF and VWFpp production at early culture passages of BOECs (as done in Table 2) and that measuring VWFpp is better suited than determining VWF:Ag as a measure of WPB exocytosis. Therefore, we determined the percentage of secreted VWFpp to quantify exocytosis of BOECs.
We examined the exocytosis of WPB from BOECs by induction with different agonists. For BOECs derived from healthy donors (n = 5), 40% to 60% VWFpp was released to the medium on stimulation with 160 nM PMA or 100 μM histamine for 60 minutes, whereas 30% to 50% VWFpp was released on stimulation with 10 μM epinephrine for 60 minutes (Figure 5A). BOECs derived from the patient with p.Arg854Gln showed a completely normal response to all the 3 agonists, whereas the responses of BOECs derived from the patients with p.Ser1285Pro, p.Leu1307Pro, or p.Cys2693Tyr were reduced (Figure 5B-D). The BOECs of p.Tyr1584Cys showed a trend of lower response to PMA and histamine, but this difference was not statistically significant. The relatively milder impairment in WPB exocytosis of BOECs with p.Cys2693Tyr compared with that of BOECs with p.Leu1307Pro is consistent with the responses of the 2 patients to DDAVP infusion (Table 3). The relative increase in VWF after DDAVP infusion is larger for the patient with p.Leu1307Pro (3× increase for p.Leu1307Pro vs 2× increase for p.Cys2693Tyr). This is probably because of the low baseline level of plasma VWF (under steady state) because of the faster clearance of VWF variant p.Leu1307Pro in the patient.36 In line with these findings, we predict that the patient with p.Ser1285Pro will show a poor response to DDAVP infusion, whereas the other 2 individuals (carrying p.Arg854Gln and p.Tyr1584Cys, respectively) will show a very good response.
Exocytosis of WPB of BOECs. BOECs were cultured in 12-well plates until confluent and then rinsed and incubated for 60 minutes at 37°C with release medium in the absence (Control) or presence (PMA) of 160 nM PMA, 100 μM histamine (Histamine), or 10 μM epinephrine plus 100 μM IBMX (Epinephrine). Each bar represents the percentage of secreted VWFpp as a fraction of total VWFpp (medium plus lysate) by 3 different passages of BOECs (between passages 4 and 8). (A) Regulated secretion of VWFpp from BOECs of a single representative healthy donor. Mean ± SD indicates variation of VWFpp secretion between 3 passages of BOECs. (B-D) Regulated secretion of VWFpp induced by PMA, histamine, or epinephrine was compared between BOECs derived from healthy donors (mean, 5 donors) and 5 participants heterozygous for a given VWF mutation. Mean ± SD indicates variation of VWFpp secretion between 3 passages of a single clone of BOECs (clone A for healthy donor 2 and clone A for donor 5, as presented in supplemental Figure 3). The bars for healthy donors in (B-D) were generated by pooling data from 5 healthy donors. In this case, the error bars indicate SD between the 5 healthy donors. The different responses to the indicated stimuli between patients and healthy donors were statistically analyzed using 1-way ANOVA with the Bonferroni post test. **P < .01; ***P < .001; ns, difference is statistically not significant.
Exocytosis of WPB of BOECs. BOECs were cultured in 12-well plates until confluent and then rinsed and incubated for 60 minutes at 37°C with release medium in the absence (Control) or presence (PMA) of 160 nM PMA, 100 μM histamine (Histamine), or 10 μM epinephrine plus 100 μM IBMX (Epinephrine). Each bar represents the percentage of secreted VWFpp as a fraction of total VWFpp (medium plus lysate) by 3 different passages of BOECs (between passages 4 and 8). (A) Regulated secretion of VWFpp from BOECs of a single representative healthy donor. Mean ± SD indicates variation of VWFpp secretion between 3 passages of BOECs. (B-D) Regulated secretion of VWFpp induced by PMA, histamine, or epinephrine was compared between BOECs derived from healthy donors (mean, 5 donors) and 5 participants heterozygous for a given VWF mutation. Mean ± SD indicates variation of VWFpp secretion between 3 passages of a single clone of BOECs (clone A for healthy donor 2 and clone A for donor 5, as presented in supplemental Figure 3). The bars for healthy donors in (B-D) were generated by pooling data from 5 healthy donors. In this case, the error bars indicate SD between the 5 healthy donors. The different responses to the indicated stimuli between patients and healthy donors were statistically analyzed using 1-way ANOVA with the Bonferroni post test. **P < .01; ***P < .001; ns, difference is statistically not significant.
DDAVP response of VWF:Ag levels
| Subject . | Amino acid change . | DDAVP administration . | VWF:Ag, IU/dL . |
|---|---|---|---|
| P6F13II1 | p.Leu1307Pro | Before | 14 |
| 1 h | 48 | ||
| P6F16II1 | p.Cys2693Tyr | Before | 48 |
| 1 h | 90 | ||
| 2 h | 101 | ||
| 4 h | 85 |
| Subject . | Amino acid change . | DDAVP administration . | VWF:Ag, IU/dL . |
|---|---|---|---|
| P6F13II1 | p.Leu1307Pro | Before | 14 |
| 1 h | 48 | ||
| P6F16II1 | p.Cys2693Tyr | Before | 48 |
| 1 h | 90 | ||
| 2 h | 101 | ||
| 4 h | 85 |
VWF string formation on the cell surface of BOECs
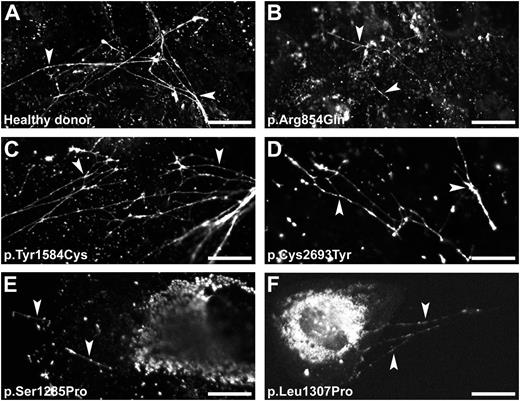
VWF strings readily appeared on the cell surface on stimulation with histamine (Figure 6A). BOECs derived from the patients with p.Arg854Gln, p.Tyr1584Cys, or p.Cys2693Tyr released VWF strings that were similar to those released from healthy BOECs (Figure 6B-D). BOECs with p.Cys2693Tyr showed fewer VWF strings. Only a few short VWF strings were observed for BOECs derived from the patients with mutation p.Ser1285Pro or p.Leu1307Pro (Figure 6E-F), indicating that at least part of the abnormal WPB was indeed able to secrete their contents. This observation is also consistent with the limited increase of VWF secretion after stimulation (Figure 5C). Compared with BOECs derived from other participants, the BOECs derived from these 2 patients showed considerable amounts of VWF remaining within the cells after stimulation. This indicates that large amounts of VWF were not stored in WPB, as shown in Figure 2. Epinephrine also induced formation of VWF strings (supplemental Figure 4). Similar to previous observations in HUVECs,26 epinephrine induced clustering of remaining WPB to the perinuclear region of the cell. Stimulation with PMA (data not shown) of BOECs derived from the corresponding participants showed similar results to that presented in Figure 6.
VWF string formation on exocytosis of WPB. BOECs derived from a healthy donor (A) or from the patients heterozygous for a VWF mutation (B-F) were stimulated for 60 minutes with 100 μM histamine at 37°C. Cells were fixed and stained for VWF to visualize the VWF string-like structures (arrowheads). (Scale bars, 20 μm.) All images were taken by Leica SL confocal laser scanning microscopy with a 63×/1.40 NA oil objective.
VWF string formation on exocytosis of WPB. BOECs derived from a healthy donor (A) or from the patients heterozygous for a VWF mutation (B-F) were stimulated for 60 minutes with 100 μM histamine at 37°C. Cells were fixed and stained for VWF to visualize the VWF string-like structures (arrowheads). (Scale bars, 20 μm.) All images were taken by Leica SL confocal laser scanning microscopy with a 63×/1.40 NA oil objective.
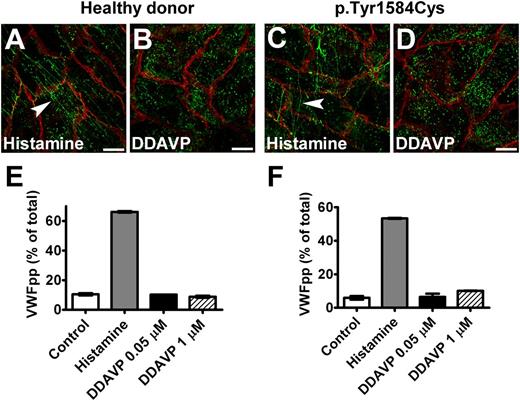
Because DDAVP is assumed to induce exocytosis of WPB via the vasopressin V2 receptors in endothelial cells,37,38 we tested whether DDAVP response can be used in BOECs. We observed that BOECs did not respond to DDAVP treatment (Figure 7). However, when BOECs were transduced to induce expression of V2 receptor, they did release VWF on stimulation with DDAVP (supplemental Figure 5). These results indicate that BOECs probably do not express V2 receptors.
Response of BOECs to DDAVP. BOECs derived from a healthy donor (A,B) or the patient heterozygous for p.Tyr1584Cys (C,D) showed VWF strings (indicated by arrowheads) after incubation with 100 μM histamine for 1 hour (A,C), but no strings were seen after stimulation with 1 μM DDAVP (DDAVP) for 2 hours (B,D). Cells were fixed and stained for VWF (green) and β-catenin (red). (Scale bars, 20 μm.) All images were taken by Leica SL confocal laser scanning microscopy with a 63×/1.40 NA oil objective. (E,F) Cells derived from a healthy donor (donor 1) (E) and from the patient heterozygous for p.Tyr1584Cys (F) were cultured in 12-well plates at passage 6. Confluent cell layers were incubated for 60 minutes at 37°C in the absence (Control) or presence (Histamine) of 100 μM histamine or DDAVP (DDAVP) at indicated final concentrations. There was no response to DDAVP. Mean ± SEM indicates variation between triplicate measurements.
Response of BOECs to DDAVP. BOECs derived from a healthy donor (A,B) or the patient heterozygous for p.Tyr1584Cys (C,D) showed VWF strings (indicated by arrowheads) after incubation with 100 μM histamine for 1 hour (A,C), but no strings were seen after stimulation with 1 μM DDAVP (DDAVP) for 2 hours (B,D). Cells were fixed and stained for VWF (green) and β-catenin (red). (Scale bars, 20 μm.) All images were taken by Leica SL confocal laser scanning microscopy with a 63×/1.40 NA oil objective. (E,F) Cells derived from a healthy donor (donor 1) (E) and from the patient heterozygous for p.Tyr1584Cys (F) were cultured in 12-well plates at passage 6. Confluent cell layers were incubated for 60 minutes at 37°C in the absence (Control) or presence (Histamine) of 100 μM histamine or DDAVP (DDAVP) at indicated final concentrations. There was no response to DDAVP. Mean ± SEM indicates variation between triplicate measurements.
Discussion
In this study, we examined whether human peripheral blood-derived BOECs are useful for the investigation of VWF mutations in their natural environment, in particular with respect to intracellular storage and regulated secretion of VWF. Five healthy donors and 5 individuals carrying VWF mutations were recruited in this study. We also showed that isolation and culturing of BOECs from multiple patients with VWD was feasible and demonstrated that BOECs were authentic endothelial cells (Figure 1). Using such BOECs, we demonstrated that reduced VWF production (or increased intracellular degradation), defective biogenesis, and exocytosis of WPB most likely contribute to the quantitative deficiency of VWF. Furthermore, the defective formation of VWF strings may deteriorate the bleeding tendency in those patients (summarized in Table 4). These findings indicate that a comprehensive analysis of patient-derived BOECs is insightful to understand the pathogenic complexity of VWD.
Summary of VWF storage and secretion for BOECs with VWD variants*
| Mutation . | VWD type . | Total VWF production . | Secretion . | ER retention . | WPB formation . | VWF string formation . | Secreted VWF multimers . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Basal . | Stimulated . | |||||||||
| PMA . | His . | Epi . | ||||||||
| p.Arg854Gln | 2N | Normal/↓ | Normal/↓ | Normal | Normal | Normal | No | Normal | Normal | Normal |
| p.Ser1285Pro | 1 | ↓↓↓ | ↓↓↓ | ↓↓ | ↓↓↓ | ↓↓↓ | ↑↑↑ | Abnormal | Abnormal | Abnormal |
| p.Leu1307Pro | 1 or 2A | ↓↓↓ | ↓↓↓ | ↓ | ↓↓↓ | ↓↓ | ↑↑↑ | Abnormal | Abnormal | Abnormal |
| p.Tyr1584Cys | 1 | Normal | Normal | Normal | Normal | Normal | No | Normal | Normal | Normal |
| p.Cys2693Tyr | 1 | Normal | Normal/↓ | ↓ | ↓↓ | ↓ | ↑ | Abnormal† | Normal‡ | Normal |
| Mutation . | VWD type . | Total VWF production . | Secretion . | ER retention . | WPB formation . | VWF string formation . | Secreted VWF multimers . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| Basal . | Stimulated . | |||||||||
| PMA . | His . | Epi . | ||||||||
| p.Arg854Gln | 2N | Normal/↓ | Normal/↓ | Normal | Normal | Normal | No | Normal | Normal | Normal |
| p.Ser1285Pro | 1 | ↓↓↓ | ↓↓↓ | ↓↓ | ↓↓↓ | ↓↓↓ | ↑↑↑ | Abnormal | Abnormal | Abnormal |
| p.Leu1307Pro | 1 or 2A | ↓↓↓ | ↓↓↓ | ↓ | ↓↓↓ | ↓↓ | ↑↑↑ | Abnormal | Abnormal | Abnormal |
| p.Tyr1584Cys | 1 | Normal | Normal | Normal | Normal | Normal | No | Normal | Normal | Normal |
| p.Cys2693Tyr | 1 | Normal | Normal/↓ | ↓ | ↓↓ | ↓ | ↑ | Abnormal† | Normal‡ | Normal |
PMA, phorbol-12-myristate-13-acetate; His, histamine; and Epi, epinephrine (combined with isobutylmethylxanthine).
As compared with BOECs derived from healthy donors.
Heterogeneous population of WPB; some WPB are morphologically normal (elongated).
Morphologically normal but reduced in quantity.
We chose to explore the pathogenic mechanisms of VWD by using BOECs because of their advantages over other cell systems. Ectopic expression of VWF in some heterologous cell systems has advanced our understanding of the pathogenic nature of VWF mutations, including defects in the assembly of VWF multimers, increased retention and/or degradation of VWF in the ER, and impairment in VWF storage.11,12,28,39-41 However, confirming the pathogenic nature of type 1 VWD mutations by expression studies is inherently difficult. First, ectopic (over)expression may lead to different posttranslational modifications compared with endothelial cells. Second, most patients with type 1 VWD are heterozygous for VWF mutations, and it is difficult to exactly mimic the heterozygous state in cotransfection experiments. With the use of BOECs, those drawbacks inherent to heterologous expression can be eliminated.
The advantage of using BOECs over other primary endothelial cells is the much easier access to patients with different VWF mutations. HUVECs have only been extracted from the cords of sporadic patients with VWD,18-22 and other endothelial sources are even more difficult to access. In contrast, BOECs can, in principle, be obtained from the peripheral blood of almost any consenting patient. In practice, the isolation approach described here is simple and can be routinely applied with a high rate of success.
One of the main findings in this study is that the abnormalities in the biogenesis of WPB in the BOECs derived from patients that are mainly characterized by a quantitative deficiency in VWF (mutations p.Ser1285Pro, p.Leu1307Pro, and p.Cys2693Tyr). In line with previous findings,11,12,42 impaired storage of WPB was probably caused by the increased retention of VWF in the ER, rather than by a decrease in production of VWF. Indeed, BOECs with p.Arg854Gln stored VWF properly, even though the total VWF production was much lower than that of BOECs with p.Cys2693Tyr in which abnormal WPB dominated. Furthermore, compared with p.Ser1285Pro and p.Leu1307Pro, p.Cys2693Tyr showed less ER retention and better WPB formation in BOECs (Figure 2), which is consistent with the extent of VWF deficiency in those patients (Table 1). The main source of plasma VWF is the vascular endothelial cell in which VWF is stored in WPB.4 Alterations in biogenesis of WPB may impair secretion of VWF.11,12,42 Compared with BOECs derived from the 2 patients with mild type 1 VWD (p.Tyr1584Cys and p.Cys2693Tyr), the basal secretion of VWF was strongly reduced for BOECs derived from the 2 patients with moderate type 1/2A VWD (p.Ser1285Pro and p.Leu1307Pro; Table 2). Accordingly, WPB formation was indeed more severely impaired in BOECs derived from the latter 2 patients (Figure 2).
The strikingly low production of VWF in BOECs derived from the patients heterozygous for p.Ser1285Pro and p.Leu1307Pro suggests that a dominant-negative effect of these 2 mutations led to gross intracellular degradation of VWF, as described for VWF mutation p.Cys1149Arg.39 This intracellular degradation of VWF is probably induced by retention of VWF in the ER (Figure 2).39,43 The slightly lower basal secretion of the type 2N mutation p.Arg854Gln and the lower plasma VWF in the patient is likely a result of low production of VWF, rather than defective WPB biogenesis. Taken together, the severity of plasma VWF deficiency in the patients with VWD was well recapitulated in the BOECs by analyzing VWF basal secretion (Tables 1 and 2). For p.Tyr1584Cys, the normal basal secretion of VWF from BOECs but lower plasma level in the patient may be explained by possible faster clearance from the circulation or by variation in basal secretion from BOECs. The variation in VWF basal secretion and production between culture passages and clones of BOECs could be minimized by using BOECs at the same culture passage as we did in this study. This variability might also be overcome by isolating BOECs from a larger number of healthy controls, but for specific mutations this will not be feasible, as the number of available patients carrying specific mutations is usually small. Furthermore, quantitative analysis of the relative secretion of VWFpp (percentage of secretion) seems promising to circumvent the limitations caused by cell culture conditions and to accurately characterize VWD mutations (Figure 4, supplemental Figure 2, and supplemental Figure 3).
Another important finding is the identification of defects in VWF string formation. On (patho)physiological stimulation, the activated vascular endothelial cells release VWF to form string-like structures under shear stress. Those strings may be pivotal to the thrombus plug formation, as they are hyperadhesive to platelets.6 In contrast, defective formation of VWF strings may contribute to the bleeding tendency seen in VWD. Consistent with this hypothesis, we found that formation of VWF strings was severely impaired for BOECs derived from the patients heterozygous for p.Ser1285Pro and p.Leu1307Pro (Figure 6). p.Cys2693Tyr also impaired VWF string formation, but to a lesser extent. We postulated that abnormal storage of VWF led to diminished exocytosis of WPB and, consequently, to less VWF string formation. The extent of defects in VWF string formation was independent of WPB secretagogues but appeared well-correlated with the phenotypic severity of the patients from whom BOECs were derived.
In conclusion, BOECs provide an easily accessible, bona fide cell model to analyze the properties of VWF mutations in vitro. BOECs were particularly useful to study aspects of intracellular storage, regulated secretion, and string formation of VWF. They also provided an optimal model to investigate possible dominant-negative effects that may be present in the heterozygous state. Furthermore, this endothelial cell model can be used to examine the response to several WPB secretagogues because the relative secretion of VWFpp was quite constant between cell passages and clones.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr C.T. Esmon (Howard Hughes Medical Institute, Chevy Chase, Maryland) for the monoclonal antibody against EPCR (unlabeled), Richard Dirven from the Department of Thrombosis and Hemostasis, Leiden University Medical Center, for optimizing VWFpp assay and help with ELSA and TaqMAMA assays, Professor Anton-Jan van Zonneveld from the Department of Nephrology, Leiden University Medical Center, for helpful discussion, and Annelies van der Laan from the Department of Molecular Cell Biology, Leiden University Medical Center, for expert technical assistance.
This work was financially supported by a grant from the China Scholarship Council (2007U21083) and by a grant from the Netherlands Organisation for Scientific Research (91209006).
Authorship
Contribution: J.-W.W., J.E., and P.H.R. designed the research, analyzed and interpreted the data, and wrote the manuscript; J.-W.W., E.A.M.B., M.C.P., and H.S. performed experiments; J.V., K.M.V., and K.M. designed the research, provided reagents, interpreted data, and reviewed the manuscript; and H.C.d.B. provided helpful discussion and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jeroen Eikenboom, Einthoven Laboratory for Experimental Vascular Medicine, Department of Thrombosis and Hemostasis, C7-Q, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, The Netherlands; e-mail: h.c.j.eikenboom@LUMC.nl; and Jiong-Wei Wang, Einthoven Laboratory for Experimental Vascular Medicine, Department of Thrombosis and Hemostasis, D2-P, Leiden University Medical Center, PO Box 9600, 2300 RC Leiden, The Netherlands; e-mail: j.w.h.wang@lumc.nl.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal