Abstract
In the human genome, 43 different genes are found that encode proteins belonging to the family of the POK (poxvirus and zinc finger and Krüppel)/ZBTB (zinc finger and broad complex, tramtrack, and bric à brac) factors. Generally considered transcriptional repressors, several of these genes play fundamental roles in cell lineage fate decision in various tissues, programming specific tasks throughout the life of the organism. Here, we focus on functions of leukemia/lymphoma-related factor/POK erythroid myeloid ontogenic factor, which is probably one of the most exciting and yet enigmatic members of the POK/ZBTB family.
Introduction
The Drosophila broad complex, tramtrack, and bric à brac factors (BTB) are all zinc finger (ZF) transcriptional repressors characterized by BTB, a unique N-terminal domain. These genes play fundamental roles during Drosophila development, including metamorphosis, central nervous system organization,1,2 ommatidial cell development,3 ovary morphogenesis,4 homeotic transformation of the bristle pattern of tarsal segments,5 and wing and limb formation.4,6
This family of genes has expanded over time to comprise, in mammals, a group of 43 different BTB/poxvirus and zinc finger (POZ)-ZF (BTB alias POZ-ZF) transcription factors playing key functions in a spectrum of diverse biological processes such as cell cycle progression, DNA damage responses, apoptosis, cell fate determination, and a multitude of developmental processes.7 Accordingly, dysfunction of vertebrate POZ-ZF proteins such as promyelocytic leukemia ZF (PLZF), B-cell lymphoma 6 (BCL6), hypermethylated in cancer 1, ZBTB7, and Fanconi anemia ZF (FAZF/PLZP) has been linked to developmental disorders and tumorigenesis.7
Here we focus our attention on leukemia/lymphoma-related factor (LRF)/Pokemon (POK [POZ and Krüppel] erythroid myeloid ontogenic factor), one of the most intriguing members of the BTB/POZ-ZF family, which has reached prominence in view of its pleiotropic role in the control of critical processes within the hemopoietic compartment and beyond.
LRF: protein structure, interactions, and modifications
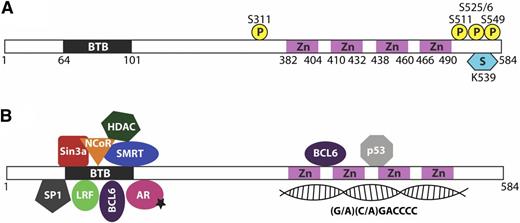
The transcription factor LRF (also known as Pokemon, osteoclast-derived ZF, FBI-1, and ZBTB7A) is characterized by a peculiar protein structure shared by 43 POK proteins in humans.8,9 This family of proteins contains a POZ/BTB domain at the N terminus and multiple Krüppel-type ZFs at the C terminus (Figure 1A). Although the POZ/BTB domain is involved in protein–protein interactions such as homo- and possibly heterodimerization and multimerization of POK family members (Figure 1B), the ZF region mediates sequence-specific binding to DNA elements (Figure 1B). Finally, the poorly conserved hinge region between the POZ and ZF domains, as well as the C terminus at the end of the ZFs domains, are often the targets of posttranslational modifications responsible for the regulation of protein function (Figure 1A).10-18
LRF protein structure, modifications, and interactions. (A) LRF protein domains and posttranslational modifications. (B) LRF protein interactions.
LRF protein structure, modifications, and interactions. (A) LRF protein domains and posttranslational modifications. (B) LRF protein interactions.
Multiple POK proteins, LRF included, have been shown to act as transcriptional repressors by directly binding specific consensus sequences on DNA and interacting with corepressors such as NCoR, SMRT, and Sin3a via the POZ domain at the N terminus.19,20 For its part, LRF preferentially binds to the GC-rich sequence [(G/A)(C/A)GACCCC], as has been revealed by cyclic amplification and selection of target analysis,21 gel-shift assay,22 and chromatin immunoprecipitation sequencing.23 Ultimately, this binding leads to the recruitment of histone deacetylases to gene promoters and results in a closed chromatin conformation that is refractory to transcription. Recently, however, the inventory of LRF/Pokemon interactors has been expanded to include other transcription factors such as tumor protein p53 (TP53), androgen receptor, specificity protein 1, BCL6, sex determining region Y-box 9 (SOX9), and growth factor independent 1, thereby suggesting an indirect transcriptional repressive activity of LRF on specific subclasses of genes (Figure 1B).10-18 These findings in turn highlight LRF as a key node for the transcriptional regulation of fundamental pathways involved in cell cycle control, apoptosis, and cell fate decision.
LRF functions in hematopoietic cell lineages
Erythroid
The role for POK family proteins such as LRF, BCL6, and PLZF in tumorigenesis was initially inferred from genetic studies in human hemopoietic malignancies and was finally confirmed through the study of genetically engineered knockout and transgenic mouse models.21,24-29
An additional and striking observation derived from these mouse models, however, was the realization of the central role played by POK family proteins in a variety of developmental processes. For instance, Bcl6-deficient mice lack formation of germinal centers (GCs) and display a profound Th2-type inflammatory response, demonstrating the key role played by BCL6 in the regulation of lymphocyte differentiation.24 In addition, mice lacking Plzf expression display defects in limb morphogenesis and germline stem cell maintenance resulting from alterations in cell proliferation, apoptosis, and self-renewal.9,30
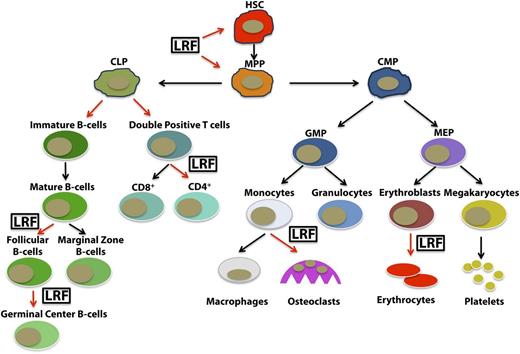
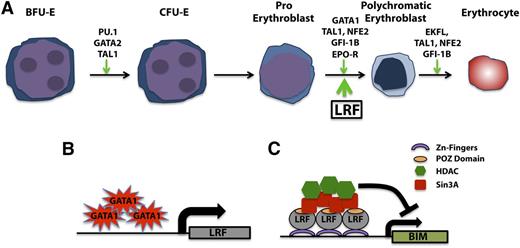
Studies regarding the developmental functions of LRF have likewise brought unique insight into the cellular pathways regulated by this protein, especially in the hematopoietic cell lineages (Figure 2).31,32 Lrf−/− embryos exhibit an embryonic lethality around 16.5 days postcoitum as a consequence of extensive anemia.31 Pandolfi and colleagues defined a strong apoptotic induction of late-stage erythroblasts as the primary cause of lethality in Lrf-null embryos.31 This cell death response occurs despite an intact erythropoietin signaling pathway and in an Arf/p53-independent manner and is associated with strong upregulation of the proapoptotic factor BCL2 interacting mediator of cell death (Bim).32 Importantly, these studies identified LRF as a new direct transcriptional target of GATA1 that is essential to the transcriptional repression of the proapoptotic factor Bim, thus defining a novel transcriptional cascade for the suppression of apoptosis during erythroid cell fate decision (Figure 3).32
LRF in the hematopoietic cell lineages. LRF regulates hematopoiesis by playing specific roles in different cell lineages.
LRF in the hematopoietic cell lineages. LRF regulates hematopoiesis by playing specific roles in different cell lineages.
LRF in the erythrocytes lineage. (A) LRF promotes erythrocyte differentiation. (B) GATA1-dependent LRF upregulation drives a potent antiapoptotic activity during the late stage of erythroblast differentiation through (C) BIM transcriptional repression.
LRF in the erythrocytes lineage. (A) LRF promotes erythrocyte differentiation. (B) GATA1-dependent LRF upregulation drives a potent antiapoptotic activity during the late stage of erythroblast differentiation through (C) BIM transcriptional repression.
It is worth noting that erythroid Krüppel-like factor (Eklf, also known as Klf1) upregulates Lrf expression in mouse fetal liver, where erythropoiesis takes place at the late embryonic stage.33 Furthermore, Cantor and colleagues recently showed that Gata1 and Lrf cooccupy regulatory elements of key erythroid genes,34 suggesting that Gata1 not only trans-activates the Lrf gene32 but also functions with LRF on erythroid genes regulation.34
Myeloid
Although LRF is abundantly expressed in normal and malignant myeloid cells, hematopoietic-specific Lrf conditional knockout mice (LrfFlox/Flox;Mx1-Cre) did not exhibit a gross defect in the numbers of mature myeloid lineage cells in peripheral blood or bone marrow; however, mature myeloid cells (Gr-1+CD11b+c-Kit−) were barely detectable in Lrf−/− fetal livers.35 Furthermore, a slight but significant reduction in the numbers of granulocyte-macrophage progenitors was observed in Lrf−/− fetal liver and in LrfFlox/Flox;Mx1-Cre bone marrow.31 It remains to be clarified whether Lrf intrinsically functions during myeloid development in a developmental stage–specific manner or does so in a non-cell-autonomous fashion (eg, niche effects).
Lymphoid
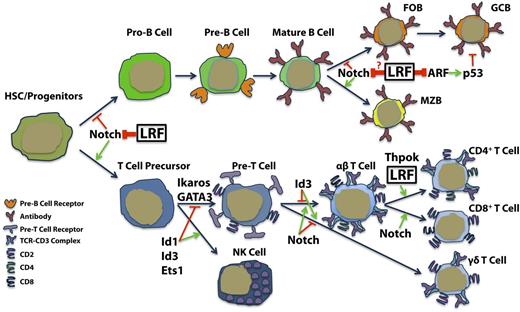
Inactivation of LRF dictates dramatic consequences in the lymphoid compartment. Cre-lox mediated Lrf inactivation at hematopoietic stem cell (HSC)/progenitor stages in adult mice (LrfFlox/Flox;Mx1-Cre) leads to development and accumulation of CD4/8 double-positive T-cells in the bone marrow at the expense of B lymphopoiesis.31 The number of pro-B, pre-B, and immature B-cells is drastically reduced, but prepro-B-cells, which resemble normal thymic CD4/8 double-negative T-cells, accumulate.31 Interestingly, LrfFlox/Flox;Mx1-Cre mice phenocopied mice overexpressing the intracellular domain of Notch1, in that ectopic CD4/CD8 double-positive cell development was induced at the expense of B-cell development in the bone marrow.36 Furthermore, treatment of LrfFlox/Flox;Mx1-Cre mice with γ secretase inhibitors, which block Notch signaling, did rescue aberrant lymphoid development, suggesting that LRF antagonizes the Notch pathway at the HSC/progenitor level.31
Our understanding of the role of LRF in peripheral T-cell differentiation continues to evolve. Observations of the thymus have shown LRF to be suppressed in CD4/8 double-positive T cells there and re-expressed in CD4 or CD8 single-positive T cells via as-yet-unknown mechanisms, although LRF deficiency was not observed to grossly affect CD4/CD8 T-cell differentiation.31 Further light was shed on these findings when a new and unexpected role for LRF in peripheral T-cell function was recently revealed.37
Previous reports had suggested that Thpok (T-helper-inducing POZ/Kruppel like factor; also known as ZBTB7B) functions as a master regulator of CD4+ T-cells, as in its absence, CD4+CD8+ double-positive T-cells preferentially differentiate into CD8+ T− cells in the thymus.38,39 It was therefore presumed that Thpok was essential not only for CD4+ T-cell differentiation but also for T helper (Th) cell functions. Carpenter et al37 demonstrated, however, that Thpok is dispensable for many features of Th cell differentiation and that Lrf promotes Th cell gene expression in Thpok-deficient cells. In this study, analysis of Lrf/Thpok double-knockout T cells revealed that mutant cells fail to express Th cell genes or undergo Th cell differentiation in vivo, suggesting that these 2 transcription factors critically regulate Th cell gene expression in a cooperative fashion (Figure 4). However, the precise mechanisms by which LRF and THPOK maintain Th cell genes transcription remain elusive.
LRF roles in lymphoid differentiation. LRF promotes B-cell lineage by repressing Notch activity in early lymphoid precursors, whereas LRF regulates mature B-cell lineage fate and GC formation through distinct mechanisms.
LRF roles in lymphoid differentiation. LRF promotes B-cell lineage by repressing Notch activity in early lymphoid precursors, whereas LRF regulates mature B-cell lineage fate and GC formation through distinct mechanisms.
Finally, LRF is also highly expressed in GC B-cells and non-Hodgkin lymphoma tissues,21,40 implying that LRF may also function in GC formation and lymphomagenesis, as does BCL6. Indeed, GC-B cells are dramatically reduced in B-cell-specific Lrf conditional knockout mice (LrfFlox/Flox;Mb1-Cre) after immunization with T-cell-dependent antigens.40 Although Bcl6 knockout mice exhibit complete loss of GC formation,24 a few GC B-cells were observed, and overall GC structures remain intact in LrfFlox/Flox;Mb1-Cre mice. The tumor suppressor p19Arf, a transcriptional target of LRF,21 was significantly upregulated in LRF-deficient GC B-cells, and de-repression of the p19Arf gene accounts for, at least in part, the impaired proliferation and increased apoptosis seen in LRF-deficient GC B-cells.40 Notably, despite its critical role in lymphoid lineage fate determination at the HSC/progenitor stages, LRF was found to be dispensable to the maintenance of immature B cells in the bone marrow of LrfFlox/Flox;Mb1-Cre mice. These findings were also consistent with the fact that treatment with γ secretase inhibitor restored normal B-cell development in LrfFlox/Flox;Mx1-Cre mice.31 LrfFlox/Flox;Mb1-Cre mice also exhibit an increase in marginal zone B (MZB) cell numbers and a concomitant decrease in follicular B (FOB) cell numbers, suggesting that LRF-mediated signals favor FOB fate at the branching point for the FOB vs MZB fate decision and regulate the balance between FOB and MZB development (Figure 4).40
HSCs: LRF’s role in cell fate decision goes beyond cell autonomy
Cell fate determination by positional cues is a fundamental mechanism in animal and plant development. Physiologically, a non-cell-autonomous trait occurs whenever a cell population instructs other cells, via direct contact or secreted molecules, how to develop or behave.
The stem cell niche represents a classic example of cell fate determination dictated by a non-cell-autonomous mechanism. A stem cell niche is defined as an environment in which stem cells are not only hosted but also supported in their self-renewal41 and are instructed to differentiate into specific cell lineages. In mammals, stem cell niches have been described in many different organs: HSC niches in the bone marrow,42 the epithelial stem cell niche in the skin,43 the intestinal stem cell niche,44 neural stem cell niches in the brain,45 and the germ-line stem cell niche, which was identified in mouse testis.46
HSCs and their bone marrow niche together make up one of the most studied microenvironment systems. It is widely believed that the regulation of the balance between HSC self-renewal and differentiation is mainly dependent on the cross talk between cell-autonomous pathways intrinsically wired into HSCs and cell nonautonomous signaling through the interaction of HSCs, with the different types of cells forming the bone marrow niche (Figure 5).
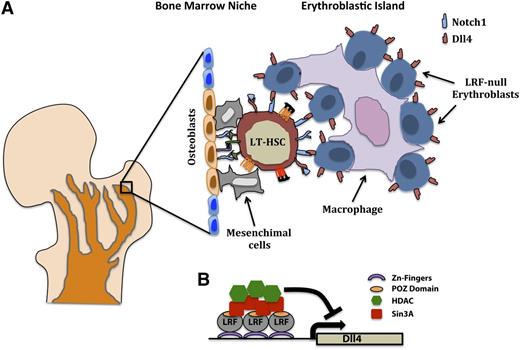
Cell nonautonomous function of LRF in LT-HSCs differentiation. (A) Cell nonautonomous inhibition of the Notch pathway and block of LT-HSCs differentiation through (B) LRF-dependent downregulation of Dll4 in the erythroblastic islands.
Cell nonautonomous function of LRF in LT-HSCs differentiation. (A) Cell nonautonomous inhibition of the Notch pathway and block of LT-HSCs differentiation through (B) LRF-dependent downregulation of Dll4 in the erythroblastic islands.
Regardless of their physical localization in the bone marrow, bone marrow niche components such as endothelial cells, mesenchymal cells, mesenchymal stem cells, osteoblasts, and osteoblast progenitors provide membrane-bound and secreted factors that promote the quiescence, self-renewal, migration, and differentiation of HSCs (Figure 5). Accordingly, the cellular composition of the bone marrow niche and its biological function is under intense scrutiny, as the specific contributions of different cellular residents to these processes are not yet completely delineated.
In a recent manuscript published in Blood,35 Maeda’s group provided the first evidence that differentiated hematopoietic cells, the progeny of HSCs, have the capacity to in turn regulate the fate and function of their parents (ie, undifferentiated HSCs) in a hematopoietic cell-autonomous but not HSC-autonomous circuit (Figure 5). In this study, LrfFlox/Flox;Mx1-Cre conditional mice exhibited a substantial decline in the numbers of long-term hematopoietic stem cells (LT-HSCs) and Flt3+ lymphoid biased multipotential progenitors, with a concomitant expansion of T-cell precursors. Importantly, this defect was observed to be primarily Notch1-dependent, as Notch1 loss rescued HSC defects seen in LrfFlox/Flox;Mx1-Cre mice, suggesting that Notchhigh+ CD34− LT-HSCs are hypersensitive to Notch signaling caused by LRF deficiency and that the signal is transmitted mainly through Notch1 in the most primitive LT-HSCs.35
Δ-like 4 (Dll4) is a nonredundant Notch1 ligand in the thymus47 that is expressed primarily in endothelial cells and thymic epithelial cells, but not (or at very low levels, if any) in hematopoietic cells.35,48-50 Strikingly, clusters of Dll4-positive erythroblasts were evident in the bone marrow of LrfFlox/Flox;Mx1-Cre mice35 (Figure 5). Furthermore, in vivo anti-Dll4 treatment restored HSC numbers in LrfFlox/Flox;Mx1-Cre mice.35 These results prompted the authors to investigate the erythroblasts in the bone marrow as a potential source of the non-cell-autonomous T-cell instructive signal responsible for the LT-HSCs phenotype.
To prove this in vivo, they generated LrfFlox/Flox;ErGFP-Cre mice in which GFP/Cre fusion gene expression and its consequent Lrf inactivation were limited to the erythroblasts. Accordingly, they observed that LrfFlox/Flox;ErGFP-Cre mice strikingly phenocopied the LrfFlox/Flox;Mx1-Cre mice, showing a significant reduction in the bone marrow of the total number of LT-HSCs concomitant with aberrant T-cell differentiation and the block of B-cell development (Figure 5).35
Osteoclasts
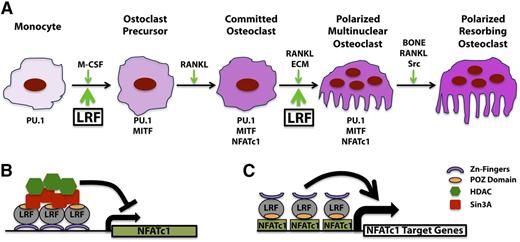
Osteoclast precursors derive from hematopoietic cells (monocytes/macrophages) that are prompted to differentiate into osteoclasts upon contact with osteoblasts or stromal cells.55 During a screening focused on cloning new factors involved in the osteoclast cell lineage specification, Kukita and colleagues identified a new transcription factor that they called osteoclast-derived ZFs, the ortholog of human LRF in the rat.56 Osteoclast differentiation is a multistep process starting with the differentiation of a monocytic cell to an osteoclast precursor, proceeding toward a committed osteoclast, and finishing with the fusion of mononuclear-committed osteoclasts into a polarized and resorbing multinucleated osteoclast (Figure 6). Spleen focus forming virus proviral integration oncogene and microphtalmia-associated transcription factor family members, as well as macrophage colony-stimulating factor, are key factors responsible for the initiation of the osteoclastogenesis; however, it is only after the induction of RANK (receptor activator of NF-κB) signals that the monocytes could transform into early osteoclast precursors.57-62 Once activated by the RANK signal, the osteoclast precursors become committed and engage the differentiation/polarization step, which requires NFATc1 (nuclear factor of activated T cells cytoplasmic 1), Rous sarcoma tyrosine kinase pp660 activation and αvβ3-integrin expression. Polarization permits the firm attachment of the mature osteoclasts to the bone cells, a fundamental prerequisite necessary to initiate their function (Figure 6).55
LRF role in osteoclastogenesis. (A-B) LRF transcriptional inhibition of Nfatc1 during the early stage of osteoclast differentiation. (A-C) LRF is an essential cofactor for NFATc1 transcriptional activity during osteoclast terminal differentiation.
LRF role in osteoclastogenesis. (A-B) LRF transcriptional inhibition of Nfatc1 during the early stage of osteoclast differentiation. (A-C) LRF is an essential cofactor for NFATc1 transcriptional activity during osteoclast terminal differentiation.
In a follow-up publication, Kukita and colleagues finely described the function of LRF in osteoclastogenesis.63 Taking advantage of a previous analysis of a transgenic mouse model overexpressing LRF in osteoclast progenitors under the Ctsk gene promoter, these authors linked the high levels of LRF to fragile bones and significantly lower bone mineral content and bone mineral density, as well as to a marked increase in the number of osteoclasts, but not osteoblasts.63 Mechanistically, Kukita and colleagues defined LRF as a positively regulated downstream target gene of RANKL signaling that is essential to the elevation of levels of NFATc1. Interestingly, they also showed that LRF decreases p21 expression in precursor cells but increases expression of p21 and p27 in osteoclasts, suggesting different roles for LRF in proliferating osteoclast precursors and mature osteoclasts.63
In a recent publication, however, Takayanagi’s group has unveiled new and important insights into the function of LRF in osteoclastogenesis.64 Conditional Lrf gene deletion during the early or late stage of osteoclast development in 2 different engineered mouse models (LrfFlox/Flox;Mx1-Cre and LrfFlox/Flox;Ctsk-Cre) has revealed a biphasic role for LRF during osteoclastogenesis. In particular, LRF is found to repress osteoclast differentiation by acting as a transcriptional repressor of the Nfatc1 gene in the early phase of osteoclasts development while also acting as a coactivator of NFATc1 in controlling the genes required for the resorbing activity in terminally differentiated osteoclasts (Figure 6).64
Although further studies will be necessary to reconcile the apparent contradiction between the results described by Takayanagi’s group and the working model proposed by Kukita and colleagues, both works contribute valuable detail to the picture of LRF as a stage-specific gene playing distinct roles in osteoclast lineage commitment.
LRF and the Occam’s razor theory
The famous dictum known as Occam’s razor warns that “Pluralitas non est ponenda sine necessitate” (ie, “Plurality should not be posited without necessity”). What we know of LRF, however, would seem to surprisingly contradict this generally sound advice. Although it would have been tempting to hypothesize that LRF could regulate cellular differentiation in such pleiotropic manner through a unifying mechanism, this turned out not to be the case.
Research has instead revealed an unexpected plurality of roles, pathways, and functions for this transcription factor, both within and beyond the hemopoietic compartment, so that now we must contend with a complex model in which the protein controls differentiation by partnering with different key players in an utterly tissue- and context-dependent manner.
In the hemopoietic compartment it is obvious that LRF exerts its activity through very different mechanisms: BIM transcriptional repression in erythrocyte terminal differentiation, Notch repression in the early lymphoid cell fate decision, ARF transcriptional repression for the GC B-cell differentiation, NFATc1 transcriptional repression during the early steps of osteoclast cell lineage, and an increase of NFATc1 transcriptional activity for osteoclast terminal differentiation.
Furthermore, recent publications have also described this gene as profoundly involved in chondrocyte, adipocyte, and oligodendrocyte cell lineages through the regulation of completely independent pathways.
Chondrogenesis also can be considered the earliest phase of skeletal development. In skeletal cells, SOX9, together with 2 other SOX family members, L-SOX5 and SOX6, favor chondrogenesis over osteogenesis through the negative regulation of RUNX2,65-67 a potent osteogenic inducer, and by driving cartilage formation through the transcription of collagen types 2, 9, and 11 (Col2a1, Col9a1, Col11a1); Aggrecan; and the cartilage oligomeric matrix protein (COMP) genes.68-71 The COMP promoter is composed of a 30-bp negative regulator element and a 51-bp positive regulatory element. Although the 51-bp positive regulatory element contains 3 putative binding sites for SOX9, the 30-pb negative regulator element contains a binding site for LRF.68,72 To correctly establish the chondrocyte differentiation program, the LRF-SIN3a-histone deacetylase 1 repressor complex must dissociate from the COMP promoter to permit the binding of the SOX9/p300/CBP activator complex to the positive regulatory element in the COMP promoter, which induces the transcription of the COMP gene.68,72 Accordingly, micromasses of C3H10T1/2 (mesenchymal-like stem cells) overexpressing LRF are unable to differentiate toward chondrocytes when treated with bone morphogenetic protein 2,68,72 thereby defining LRF as a potent negative regulator of chondrocyte lineage fate decision, more than a simple transcriptional regulator of specific chondrocyte genes.
Although clearly distinct from chondrocytes, adipocytes are nevertheless another cell lineage derived from multipotent mesenchymal stem cells and mesenchymal precursors that reside both in the bone marrow as well as in the adipose tissues of the body. Recent observations of in vitro adipocyte differentiation from primary mesenchyme cells as well as from the 3T3L1 cell line have revealed much of the detail of this stepwise sequential process. On reaching confluence, cells arrest their cell cycle at the G1/S phase boundary. Under treatment with dexamethasone, indomethacin, and insulin, the cells then complete 2 cycles of cell division known as mitotic clonal expansion, and finally, after a second growth arrest, preadipocytes undergo terminal differentiation and acquire all the characteristics of mature adipocytes.73
The genetic determinants of this process are likewise being unraveled. In a screening focused on identifying new genes critically involved in human and mouse adipogenesis, Laudes and colleagues74 identified LRF as the second most upregulated gene during mitotic clonal expansion, being second only to fatty acid binding protein 4 (FABP-4). Accordingly, 3T3L1 cells that overexpress LRF also exhibit accelerated lipid accumulation and early terminal differentiation capacity.74,75 Although the molecular mechanism through which LRF promotes adipocyte differentiation remains a matter of debate, it has been proposed that LRF would exert its central role through the concomitant transcriptional repression of cyclin A and E2F-4.75
Finally, the Allen Brain Atlas describes a widespread distribution of LRF mRNA in embryonic mouse central nervous system, suggesting a potential role for LRF in neural and/or glial development. Accordingly, Armstrong’s group76 has recently demonstrated that knockout mouse models characterized by specific Lrf gene loss conditionally in the oligodendrocyte progenitors (OPs) at P7 are characterized by a strong increase in the number of NG2+ OPs and decreases in CC1+ mature oligodendrocytes at P28.76 This imbalance is mainly a result of the inhibition of OP differentiation into oligodendrocytes without any evident effect on OP proliferation or oligodendrocyte cell death. These in vitro results are again intriguing and are in line with the pleiotropic role of LRF in the control of cell fate determination, whereas the molecular mechanisms underlying this process have yet to be defined.
LRF/Pokemon oncogene or oncosuppressor?
A flurry of recent studies have conclusively proved the thesis that tumor suppressor genes and proto-oncogenes act as key factors regulating cell fate decision and tissue differentiation not only during embryonic development but also in adult tissues. Accordingly, the reactivation of such pathways as HEDGEHOG, WNT, NOTCH, SOX, and TGF-β is now seen as a turning point in tumor development, progression, and drug resistance in a range of malignancies.
Multiple members of the POK protein family align perfectly with this scenario, as they have been described as key regulators of important developmental processes and have shown striking positive or negative roles in neoplastic transformation.
Plzf-null mice, for instance, display severe defects in invariant natural killer T-cell differentiation, as well as in limb development and germ stem cell maintenance.77-81 Taking advantage of Plzf knockout mouse models, it has been also demonstrated, however, that PLZF acts as a tumor suppressor gene in the context of acute promyelocytic leukemia,28 whereas transgenic models have defined the causality of PLZF gene chromosomal translocation t(11;17) in human acute promyelocytic leukemia.29 Accordingly, transduction of cell lines with a PLZF construct results in consistent G1-phase cell cycle arrest resulting from PLZF-mediated transcriptional repression of the proto-oncogene c-myc.27
A second compelling example of POK family involvement in cancer pathogenesis is represented by BCL6. Both in vitro and in vivo mouse model studies have demonstrated the essential role of BCL6 in B-cell GC establishment and/or maintenance,24,82 as well as the ability of BCL6 to act as a potent oncogene in non-Hodgkin’s lymphoma through its ability to repress the expression of tumor suppressors and DNA-damage-sensing genes such as TP53 and ATR.83,84
POK proteins are therefore key developmental regulators and important players in the pathogenesis of human cancer that are found to act either as oncogenes or oncosuppressors, and LRF is not an exception.
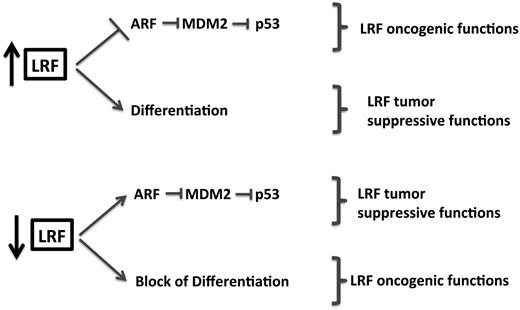
LRF may play an even more complex and multifaceted role in tumorigenesis than has been described for other POK family members because of its critical role in a plethora of different lineage fate decisions and terminal cell differentiation. Transgenic mice overexpressing LRF in the immature B- and T-cell compartments (lckEμ-Lrf) offered the first in vivo demonstration of a proto-oncogenic role for this gene.21 lckEμ-Lrf transgenic mice develop an aggressive and fatal T-cell lymphoblastic lymphoma/leukemia, strongly suggesting that LRF, similar to BCL6, may drive human lymphoma when ectopically overexpressed, as in non-Hodgkin lymphoma and nodular lymphocyte-predominant Hodgkin lymphoma21,85 (Julie Teruya-Feldstein, T.M., and P.P.P., manuscript in preparation). Furthermore, primary mouse embryonic fibroblasts generated from Lrf-null mouse embryos were found to be refractory to transformation by multiple classical oncogene combinations such as E1A+Ras or Myc+Ras.21 A remarkable feature of cultured Lrf-null mouse embryonic fibroblasts is their premature growth arrest and senescence on passaging when compared with wild-type cells. This premature senescence response is associated with a marked overexpression of the p19Arf tumor suppressor and subsequent Trp53 activation.
Accordingly, the promoters of both human and mouse Arf genes contain functional binding sites for, and are strictly regulated by, LRF. Thus, LRF is able to regulate expression of a key gene involved in p53 pathway activity, suggesting a molecular basis for the oncogenic role of the protein in lymphoma development.
Finally, shRNA-mediated LRF knockdown results in toxicity to a subset of B-cell lymphoma cell lines.37 Given that most lymphoma cell lines harbor the mutation in the ARF-p53 pathway, it is likely that LRF could also exert its oncogenic function through p53-independent mechanisms. Notably, it has recently been reported that LRF is overexpressed in non–small cell lung carcinomas in breast and ovarian cancer,86-90 reinforcing the idea of LRF as a proto-oncogene with multiple oncogenic activities.
Intriguingly, however, the ability of LRF to promote terminal differentiation in so many different cell lineages by antagonizing a number of oncogenic pathways such as SOX9, NOTCH, E2F4, or Cyclin A, together with a novel and key function of LRF in the control of genomic stability (Liu Xue Song et al, manuscript in preparation), suggest a range of potential oncosuppressive functions for this protein in specific cell systems and tumor types.91-96 Accordingly, for instance, downregulation of LRF in primary advanced prostate cancer accompanied by heterozygous genetic loss of the LRF gene in castration-resistant metastatic advanced prostate cancer have been recently described in subgroups of patients and directly linked to tumor progression and androgen deprivation resistance (G.W. et al, manuscript submitted December 2012; A.L. et al, manuscript submitted December 2012). In line with a possible context-dependent oncosuppressive function of LRF, although LRF is ectopically overexpressed in human non-Hodgkin lymphoma and nodular lymphocyte-predominant Hodgkin lymphoma,21 Reed-Sternberg cells in classical Hodgkin lymphoma show low to absent LRF expression (Julie Teruya-Feldstein, T.M., and P.P.P., manuscript in preparation).
In such a tumor-suppressive role, loss of LRF could therefore bestow to the cancer cell a progenitor/stem cell–like state by causing a block in terminal cellular differentiation (Figure 7).
LRF in tumorigenesis. Examples of context-dependent and dose-dependent oncogenic or oncosuppressive functions of LRF.
LRF in tumorigenesis. Examples of context-dependent and dose-dependent oncogenic or oncosuppressive functions of LRF.
Conclusions
What is truly remarkable and rather unique regarding LRF is the realization that this transcription factor is essential for the proper cell fate decision of virtually every tissue in which it is expressed, both positively and negatively, through its ability to modulate a number of different pathways.
It is a matter of fact, as supported by numerous in vitro and in vivo studies, that LRF controls fundamental pathways in erythroid, lymphoid, myeloid, osteoclast, adipocytic, chondrocytic, and glial cell lineage fate decisions by partnering with or opposing distinct and context-dependent transcriptional players. These findings in turn suggest unexpected implications regarding the tissue- and context-dependent oncogenic or oncosuppressive roles of this enigmatic and critical transcription factor.
Acknowledgments
The authors thank Thomas Garvey for insightful editing.
A.L. was supported in part by a fellowship from the Istituto Toscano Tumori. This work was supported by grants from the National Institutes of Health, National Institute of Allergy and Infectious Diseases (R01 AI084905 04 to T.M.) and National Cancer Institute (R01 CA102142-7 to P.P.P.).
Authorship
Contribution: A.L., J.G., G.W., T.M., and P.P.P. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Takahiro Maeda, Brigham and Women's Hospital, 1 Blackfan Circle, 02215 Boston, MA; e-mail: tmaeda@partners.org; and Pier Paolo Pandolfi, Beth Israel Deaconess Medical Center, 3 Blackfan Circle, 02215 Boston, MA; e-mail: ppandolf@bidmc.harvard.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal