Key Points
Lack of yolk-sac hematopoiesis in the Ldb1−/− mouse results from a decreased number of hemangioblasts and a differentiation block.
Identification of genes and pathways regulated by Ldb1 in the hemangioblast reveals potential targets for early developmental manipulation.
Abstract
The first site exhibiting hematopoietic activity in mammalian development is the yolk-sac blood island, which originates from the hemangioblast. Here we performed differentiation assays, as well as genome-wide molecular and functional studies in blast colony-forming cells to gain insight into the function of the essential Ldb1 factor in early primitive hematopoietic development. We show that the previously reported lack of yolk-sac hematopoiesis and vascular development in Ldb1−/− mouse result from a decreased number of hemangioblasts and a block in their ability to differentiate into erythroid and endothelial progenitor cells. Transcriptome analysis and correlation with the genome-wide binding pattern of Ldb1 in hemangioblasts revealed a number of direct-target genes and pathways misregulated in the absence of Ldb1. The regulation of essential developmental factors by Ldb1 defines it as an upstream transcriptional regulator of hematopoietic/endothelial development. We show the complex interplay that exists between transcription factors and signaling pathways during the very early stages of hematopoietic/endothelial development and the specific signaling occurring in hemangioblasts in contrast to more advanced hematopoietic developmental stages. Finally, by revealing novel genes and pathways not previously associated with early development, our study provides novel candidate targets to manipulate the differentiation of hematopoietic and/or endothelial cells.
Introduction
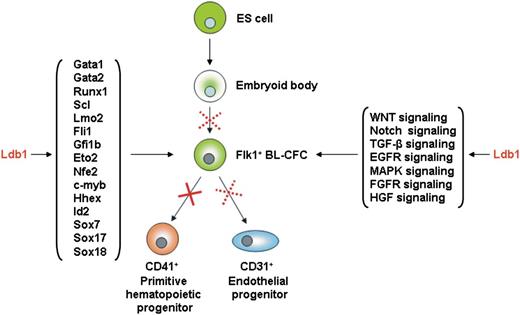
Primitive erythroid and endothelial progenitors emerge almost simultaneously in mesodermal blood islands from a common hemangioblast progenitor1 characterized by the expression of Flk1, the receptor for vascular endothelial growth factor (VEGF) (supplemental Figure 1). The other mesodermal-derived lineage expressing Flk1 is the cardiovascular progenitor cell, which is distinguishable from the hemangioblast by platelet-derived growth factor (PDGF) receptor expression. The hemangioblast has been studied in mouse, chick, and zebrafish embryos2 and its existence is supported by the observation that hematopoietic and endothelial progenitors share the expression of common genes.3-7
Deletion of Flk1 leads to embryonic lethality between 8.5 and 9.5 dpc, possibly because of the absence of yolk-sac blood islands.8,9 The hemangioblast stage can be more specifically studied in vitro through the differentiation of embryonic stem (ES) cells into embryoid bodies (EBs), widely considered to be a relevant model of embryonic development. Within EBs, blast colony-forming cells (BL-CFCs) can generate blast colonies able to differentiate into hematopoietic and endothelial progenitors. They are therefore considered the in vitro equivalent of the hemangioblast.10
Recently, Li et al demonstrated that Ldb1 is also required during primitive hematopoiesis in the mouse for the development of megakaryocytes and the maintenance of long-term hematopoietic stem cells.11,12 Ldb1 deletion is embryonic-lethal after 9.5 dpc, with severe developmental defects, including small size, anterior truncation, absence of heart/foregut structures, and impairment of yolk-sac hematopoiesis and vascular development.13 However, no functional studies or molecular mechanisms are known describing Ldb1 function in early hemangioblasts. Ldb1 molecular function has been well-studied in definitive erythropoiesis, where it plays an essential role by forming large transcriptional complexes with Gata1, Lmo2, Scl, E12/E47, HEB, Lyl1, E2-2, Cbfa2t3 (Eto2), Mtgr1, Cdk9, and Lmo4,14 binding E-box/GATA motifs genome-wide.15
In this study, we generated Ldb1−/− ES cells and performed differentiation assays and genome-wide molecular and functional studies in BL-CFCs to unravel the function of Ldb1 in early primitive hematopoietic development. We showed that Ldb1 deletion actually results in 2 separate defects: a decreased number of hemangioblasts and their inability to differentiate further down the hematopoietic and endothelial lineages. To gain insight into the mechanism underlying these defects, we determined the direct and indirect targets of Ldb1 in these progenitor cells (in contrast to more advanced hematopoietic developmental stages). The genome-wide binding pattern of Ldb1 revealed that it is directly bound to a number of gene loci involved in mammalian hematopoietic development. Their expression is misregulated in the absence of Ldb1, placing it as one of the most upstream factors controlling hematopoietic development. Furthermore our study reveals novel genes and pathways that are potentially important for early hematopoietic/endothelial development and shows the complex interplay between transcription factors and signaling pathways in this very early primitive development stage, providing novel candidate targets that can be used to manipulate the differentiation of hematopoietic and/or endothelial cells.
Methods
Ldb1−/− ES cell line generation/genotyping
This study was approved by Erasmus MC, Article 14.
Both Ldb1 alleles were inactivated using the same construct (supplemental Methods section, supplemental Figure 2A). After targeting the first allele, a single ES cell clone was transiently transfected with a Cre recombinase–expressing vector to delete exons 1/2 and the pMC-neo cassette, followed by targeting of the second allele. Southern blotting confirmed homologous recombination and deletion. Ldb1 protein levels were determined in nuclear extracts from Ldb1+/+ and Ldb1−/− ES cells with anti-Ldb1 N-18 (Cat. sc-11198, Santa Cruz Technologies, Santa Cruz, CA).
ES cell differentiation
Ldb1+/+ and Ldb1−/− ES cells were grown in suspension at 10 000 cells/mL on nonadherent dishes in Iscove’s modified Dulbecco’s medium (without leukemia inhibitory factor) with 15% fetal calf serum (FCS), 1% P/S, 1% L-glutamine (Gibco, Cat.25030-08), 0.05 µg/mL transferin (Roche, Cat.652-202), 0.05 µg/mL ascorbic acid (Sigma, Cat.A-4544), and 3 µl/mL monothioglycerol (Sigma, Cat.M-6145) for day 4 EBs and 1.8 µl/mL for day 6/day 8 EBs. Five percent protein-free hybridoma medium II (Gibco, Cat.12040-077) was added for day 6/day 8 EBs.
Blast colony-forming cell assay
Day 4 EBs were disrupted with trypsin–ethylenediamine tetraacetic acid and cells were transferred in methycellulose-based media with 10% FCS, 1% P/S, 1% L-glutamine, 0.25 µg/mL transferin, 0.25 µg/mL ascorbic acid, 2 µl/mL monothioglycerol, 0.01 µg/mL mIL6 (R&D Systems, Cat.406-ML), and 0.005 µg/mL hVEGF (R&D Systems, Cat.293-VE) for 3 days. Colonies were scored according to morphology and number under an inverted microscope.
Colony-forming cell assay
Ldb1+/+ and Ldb1−/− day 6 EBs were disrupted with 2.5% collagenase. Cells were transferred in methycellulose-based media with 10% FCS, 1% P/S, 1% L-glutamine, 0.25 µg/mL transferin, 0.25 µg/mL ascorbic acid, 2 µl/mL monothioglycerol, 5% protein-free hybridoma medium II, 0.01 µg/mL mIL6, 0.001 µg/mL IL3 (R&D Systems, Cat.403-ML), 0.005 µg/mL hIL11 (R&D Systems, Cat.418-ML), 0.003 µg/mL GM-CSF (R&D Systems, Cat.415-ML), 4 U/mL EPO (R&D Systems, Cat.959-ME), 0.005 µg/mL TPO (R&D Systems, Cat.488-TO), and 0.1 µg/mL SCF (R&D Systems, Cat.455-MC). Red primitive erythroid colonies and white macrophage colonies (composed of round cells growing in clumps) were identified microscopically after 6 days according to morphology and color.
Flow cytometry
Day 4 EB single-cell suspensions were labeled with Flk1-PE clone Avas 12α1 (BD Pharmigen, Cat.555308) in 1% bovine serum albumin/phosphate-buffered saline. Dead cells were excluded by Hoechst 33258 (Molecular Probes). Flk1+ cells were sorted from day 4 EBs on FACSAria using the Diva 5.1 software (BD Pharmingen). Day 6/8 EB single-cell suspensions were labeled in 1% bovine serum albumin/phosphate-buffered saline with CD41-PE clone MWReg30 (Santa Cruz, Cat.sc-19963) or CD31-FITC clone MEC 13.3 (BD Pharmigen, Cat.553372). Dead cells were excluded with 7-AAD (Invitrogen). Flow cytometry (FACS) was performed on FACScan (Becton Dickinson) and analyzed with Cell Quest.
Flk1+ blast colony-forming cell isolation
Day 4 EBs were disrupted with dissociation buffer (StemPro Accutase, Invitrogen). Dead cells were removed by Lymphoprep separation. Magnetic-assisted cell sorting was carried out with autoMACS Pro Separator (Miltenyi Biotec) using Anti-PE MicroBeads (Miltenyi Biotec) and the Flk1-PE clone Avas 12α1 (BD Pharmigen) antibody. Purity was assessed by FACS.
Chromatin immunoprecipitation sequencing
Gene expression profiling
RNA was isolated from Ldb1+/+ and Ldb1−/− Flk1+ cells with the QIAGEN RNeasy Mini Kit, and integrity was checked on the Agilent 2100 Bioanalyzer.
Microarray.
RNA was converted to biotin-labeled cRNA, hybridized on the Mouse Genome 430 2.0 Array, and analyzed with the Affymetrix GeneChip Scanner 3000 according to the manufacturer’s protocol.
RNA sequencing.
RNA sequencing was performed on Illumina HiSeq 2000 platform according to the manufacturer instructions.
For ChIP/RNA sequencing and microarray bioinformatic analysis see the supplemental material online.
ChIP/RNA sequencing and microarray datasets described in this study have been deposited in the NCBI Gene Expression Omnibus (Accession Number: GSE43044).
Results
No primitive erythropoiesis in the Ldb1−/− mouse
Generation of the Ldb1−/− mouse is presented in supplemental Figure 2A-D. The first striking observation in the embryos is the complete absence of any blood or vasculature from the extra-embryonic yolk-sac, which also fails to completely surround the whole embryo, showing that erythropoiesis and vascular development are severely impaired in the absence of Ldb1 (Figure 1A, in agreement with Li et al).11 Ldb1+/− mice were viable, able to breed, and did not exhibit any of the phenotypic characteristics of the Ldb1−/− mice (not shown11 ).
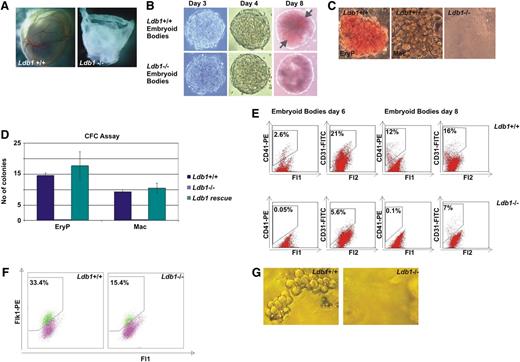
Ldb1 deletion leads to defects in primitive hematopoiesis and hemangioblast development. (A) At 9.5 dpc, blood and a vascular network in the Ldb1−/− embryo yolk-sac is completely absent. (B) Ldb1+/+ and Ldb1−/− ES cells were differentiated into EBs. After 3 and 4 days, Ldb1+/+ and Ldb1−/− EBs look similar. After 8 days, Ldb1−/− EBs lack erythroid clusters (gray arrows). (C) CFC assay with cell suspensions from Ldb1+/+ and Ldb1−/− day 6 EBs. Primitive erythroid and macrophage colonies were able to grow from Ldb1+/+ but not Ldb1−/− cultures. (D) CFC assay with cell suspensions from Ldb1+/+ and Ldb1−/− day 6 EBs as well as Ldb1−/− EBs at day 6 expressing exogenous Ldb1. The colonies that formed were counted as primitive erythroid or macrophage colonies according to morphology and color. The figure shows that although Ldb1 absence impairs the formation of primitive erythroid and macrophage colonies, this phenotype can be rescued with Ldb1 expression. (E) FACS analysis of day 6 and day 8 Ldb1+/+ and Ldb1−/− EBs. CD41+ primitive erythroid progenitors are not present in the Ldb1−/− EBs that contain less than half the number of CD31+ endothelial cells compared with the Ldb1+/+ EBs. Cell suspensions were stained with either CD41-PE or CD31-FITC. CD41-PE+ cells were detected through the Fl2 channel and CD31-FITC+ cells were detected through the Fl1 channel. (F) FACS analysis of day 4 EBs. Ldb1−/− EBs contain approximately 50% fewer Flk1+ BL-CFCs than Ldb1+/+ EBs. (G) Ldb1+/+ Flk1+ BL-CFCs give fully-grown blast colonies but Ldb1−/− Flk1+ BL-CFCs do not.
Ldb1 deletion leads to defects in primitive hematopoiesis and hemangioblast development. (A) At 9.5 dpc, blood and a vascular network in the Ldb1−/− embryo yolk-sac is completely absent. (B) Ldb1+/+ and Ldb1−/− ES cells were differentiated into EBs. After 3 and 4 days, Ldb1+/+ and Ldb1−/− EBs look similar. After 8 days, Ldb1−/− EBs lack erythroid clusters (gray arrows). (C) CFC assay with cell suspensions from Ldb1+/+ and Ldb1−/− day 6 EBs. Primitive erythroid and macrophage colonies were able to grow from Ldb1+/+ but not Ldb1−/− cultures. (D) CFC assay with cell suspensions from Ldb1+/+ and Ldb1−/− day 6 EBs as well as Ldb1−/− EBs at day 6 expressing exogenous Ldb1. The colonies that formed were counted as primitive erythroid or macrophage colonies according to morphology and color. The figure shows that although Ldb1 absence impairs the formation of primitive erythroid and macrophage colonies, this phenotype can be rescued with Ldb1 expression. (E) FACS analysis of day 6 and day 8 Ldb1+/+ and Ldb1−/− EBs. CD41+ primitive erythroid progenitors are not present in the Ldb1−/− EBs that contain less than half the number of CD31+ endothelial cells compared with the Ldb1+/+ EBs. Cell suspensions were stained with either CD41-PE or CD31-FITC. CD41-PE+ cells were detected through the Fl2 channel and CD31-FITC+ cells were detected through the Fl1 channel. (F) FACS analysis of day 4 EBs. Ldb1−/− EBs contain approximately 50% fewer Flk1+ BL-CFCs than Ldb1+/+ EBs. (G) Ldb1+/+ Flk1+ BL-CFCs give fully-grown blast colonies but Ldb1−/− Flk1+ BL-CFCs do not.
Ldb1−/− EBs contain no erythroid and fewer endothelial progenitor cells
We additionally generated an Ldb1−/− ES cell line to carry out differentiation and molecular and functional studies in hemangioblast-equivalent cells. The second Ldb1 locus allele in Ldb1+/− ES cells was deleted (supplemental Figure 2E) and the absence of Ldb1 protein was confirmed by Western blotting and immunofluoresence (supplemental Figure 2F-G). The morphology and growth of Ldb1−/− ES cells appeared similar to normal ES cells (not shown and Li et al).11,17 We then tested their differentiation potential into EBs. Because Ldb1+/− mice did not exhibit any phenotypic differences compared with Ldb1+/+ mice, they were not used for further investigations. Ldb1+/+ and Ldb1−/− EBs did not show any phenotypic differences at days 3 and 4 and appeared similar in size and structure (Figure 1B). However, after 8 days we observed a striking absence of erythroid clusters within the Ldb1−/− EBs (Figure 1B), correlating with the hematopoietic defect observed in the knock-out mouse. Of note, Ldb1−/− EBs survived at least until day 10 without apparent apoptosis but were more adherent than the wild-type ones. After 10 days the majority was found attached to the bottom of the culture dish (not shown). CFC assays were performed to test the potential of Ldb1−/− EBs to give rise to primitive erythroid and macrophage colonies. Both bright-red primitive erythroid colonies and white macrophage colonies, present in Ldb1+/+ day 6 EB cultures, were absent in the Ldb1−/− cultures. This defect was fully rescued by exogenous reexpression of Ldb1 (Figure 1C-D), indicating that those effects are specifically caused by the absence of Ldb1. In addition, the presence of primitive erythroid progenitors was examined by flow cytometry using CD41 (integrin alpha-IIb) as marker.18 Ldb1+/+ EBs contained 2.6% and 12% of CD41+ cells at days 6 and 8, respectively, whereas Ldb1−/− EBs did not contain any CD41+ erythroid progenitor cells in accordance with the absence of primitive erythroid colonies in Ldb1−/− CFC cultures (Figure 1E). The presence of endothelial progenitors in day 6 and day 8 EBs was also examined using CD31 (PECAM-1) as a marker, because Ldb1−/− embryos exhibit a severe defect in vascular development.19 Days 6 and 8 Ldb1+/+ EBs contained 21% and 16% CD31+ cells, respectively. These cells were also found to be present in Ldb1−/− EBs at the same time points but decreased to less than half when compared with the wild-type ones (Figure 1E).
Ldb1−/− EBs do not give rise to blast colonies
We then investigated whether BL-CFCs, the in vitro hemangioblast equivalent, were present in the Ldb1−/− EBs. Day 4 BL-CFCs were isolated using the VEGF receptor Flk1 as a marker (supplemental Figure 1). Flk1+ BL-CFCs were present in both Ldb1+/+ and Ldb1−/− EBs; however, a striking difference was observed because Ldb1−/− EBs repeatedly contained half the number of Flk1+ cells (Figure 1F). In three independent experiments, BL-CFC assays also showed that blast colonies did form in the wild-type but not in the knockout cultures (Figure 1G). In conclusion, BL-CFCs are present in Ldb1−/− EBs but fail to generate fully-grown blast colonies. Ldb1−/− Flk1+ cells eventually die in culture (not shown).
Transcriptome analysis identifies the genes and associated signaling pathways active in BL-CFCs
To gain insight into the gene expression network and signaling pathways involved in the maintenance of the hemangioblast in vitro–equivalent cells, transcriptome analysis of Flk1+ BL-CFCs by RNA sequencing showed 12 468 expressed genes. Pathway enrichment analysis of the expressed genes revealed the signaling pathways potentially active in these progenitor cells (Table 1, Column A).
Signaling pathways enriched in Ldb1+/+ and Ldb1−/− derived Flk1+ cells
| . | Column A . | Column B . | Column C . | Column D . | Column E . |
|---|---|---|---|---|---|
| Pathways . | Signaling pathways enriched with genes expressed in Flk1+ cells . | Signaling pathways enriched with genes expressed in Flk1+ cells (and bound by Ldb1) . | Signaling pathways perturbed in Ldb1−/− Flk1+ cells . | Signaling pathways perturbed in Ldb1−/− Flk1+ cells (and bound by Ldb1) (Set1*) . | Signaling pathways perturbed in Ldb1−/− Flk1+ cells (and not bound by Ldb1) (Set3) . |
| Focal adhesion | ✓ | ✓ | ✓ | ✓ | ✓ |
| Adherens junction | ✓ | ✓ | ✓ | ✓ | ✓ |
| Angiogenesis | ✓ | ✓ | ✓ | ✓ | ✓ |
| Colorectal cancer | ✓ | ✓ | ✓ | ✓ | ✓ |
| Hedgehog signaling pathway | ✓ | ✓ | ✓ | ✓ | ✓ |
| ErbB signaling pathway | ✓ | ✓ | ✓ | ✓ | ✓ |
| Insulin signaling pathway | ✓ | ✓ | ✓ | ✓ | ✓ |
| Integrin signaling pathway | ✓ | ✓ | ✓ | ✓ | ✓ |
| Leukocyte transendothelial migration | ✓ | ✓ | ✓ | ✓ | ✓ |
| Pathways in cancer | ✓ | ✓ | ✓ | ✓ | ✓ |
| MAPK signaling pathway | ✓ | ✓ | ✓ | ✓ | ✓ |
| Regulation of actin cytoskeleton | ✓ | ✓ | ✓ | ✓ | ✓ |
| WNT signaling pathway | ✓ | ✓ | ✓ | ✓ | ✓ |
| Acute myeloid leukemia | ✓ | ✓ | ✓ | ✓ | |
| Basal cell carcinoma | ✓ | ✓ | ✓ | ||
| Interleukin signaling pathway | ✓* | ✓* | ✓* | ✓ | |
| Melanoma | ✓ | ✓ | ✓ | ✓ | |
| T-cell–receptor signaling pathway | ✓ | ✓ | ✓ | ✓ | |
| VEGF signaling pathway | ✓ | ✓ | ✓ | ✓ | |
| Apoptosis | ✓ | ✓ | ✓ | ✓ | |
| Axon guidance | ✓ | ✓ | ✓ | ✓ | |
| Chronic myeloid leukemia | ✓ | ✓ | ✓ | ✓ | |
| PDGF signaling pathway | ✓ | ✓ | ✓ | ✓ | |
| Small-cell lung cancer | ✓ | ✓ | ✓ | ✓ | |
| EGF signaling pathway | ✓* | ✓ | ✓ | ✓ | |
| Endocytosis | ✓ | ✓ | ✓ | ✓ | |
| Glioma | ✓ | ✓ | ✓ | ✓ | |
| Neurotrophin signaling pathway | ✓ | ✓ | ✓ | ✓ | |
| TGF-β signaling pathway | ✓ | ✓ | ✓ | ✓ | |
| Prostate cancer | ✓ | ✓ | ✓ | ||
| Pancreatic cancer | ✓ | ✓ | ✓ | ||
| uCalpain and friends in Cell spread | ✓ | ✓ | |||
| Bladder cancer | ✓ | ✓ | ✓ | ||
| Dopamine receptor–mediated signaling pathway | ✓* | ✓ | ✓ | ||
| Tight junction | ✓ | ✓ | ✓ | ||
| Oxidative stress response | ✓ | ✓ | ✓ | ||
| Melanogenesis | ✓ | ✓ | |||
| B-cell activation | ✓ | ✓ | |||
| Telomeres, telomerase, cellular aging, and immortality | ✓ | ✓ | |||
| Cell cycle | ✓ | ✓ | ✓ | ||
| mTOR signaling pathway | ✓ | ✓ | ✓ | ||
| Ras pathway | ✓ | ✓ | ✓ | ||
| Glutathione metabolism | ✓ | ✓ | |||
| Control of gene expression by vitamin D receptor | ✓* | ✓ | |||
| Aminoacyl-tRNA biosynthesis | ✓ | ✓ | |||
| Amyotrophic lateral sclerosis | ✓ | ✓ | |||
| B-cell–receptor signaling pathway | ✓ | ✓ | |||
| Basal transcription factors | ✓ | ✓ | |||
| Endometrial cancer | ✓ | ✓ | |||
| Glyoxylate and dicarboxylate metabolism | ✓ | ✓ | |||
| Heme biosynthesis | ✓ | ✓ | |||
| Huntington disease | ✓ | ✓ | |||
| Interferon-γ signaling pathway | ✓ | ✓ | |||
| JAK/STAT signaling pathway | ✓ | ✓ | |||
| Lysosome | ✓ | ✓ | |||
| Transcription regulation by bZIP transcription factor | ✓ | ✓ | |||
| Adipocytokine signaling pathway | ✓ | ✓ | |||
| Alzheimer disease | ✓ | ✓ | |||
| Ubiquitin mediated proteolysis | ✓ | ✓ | |||
| Non–small-cell lung cancer | ✓ | ✓ | |||
| Nucleotide excision repair | ✓ | ✓ | |||
| Oxidative phosphorylation | ✓ | ✓ | |||
| p38 MAPK signaling pathway | ✓ | ✓ | |||
| p53 signaling pathway | ✓ | ✓ | |||
| Parkinson disease | ✓ | ✓ | |||
| Proteasome | ✓ | ✓ | |||
| Renal cell carcinoma | ✓ | ✓ | |||
| Ribosome | ✓ | ✓ | |||
| RNA degradation | ✓ | ✓ | |||
| Spliceosome | ✓ | ✓ | |||
| Apoptotic signaling in response to DNA damage | ✓ | ||||
| Arginine and proline metabolism | ✓ | ||||
| Base excision repair | ✓ | ||||
| Biosynthesis of unsaturated fatty acids | ✓ | ||||
| Chemokine signaling pathway | ✓ | ||||
| Chondroitin sulfate biosynthesis | ✓ | ||||
| Circadian clock system | ✓ | ||||
| Citrate cycle (TCA cycle) | ✓ | ||||
| CXCR4 signaling pathway | ✓ | ||||
| Cysteine and methionine metabolism | ✓ | ||||
| DNA replication | ✓ | ||||
| Dorsoventral axis formation | ✓ | ||||
| Endothelin signaling pathway | ✓ | ||||
| Fatty acid metabolism | ✓ | ||||
| Fc γ R–mediated phagocytosis | ✓ | ||||
| fMLP-induced chemokine gene expression in HMC-1 cells | ✓ | ||||
| Fructose and mannose metabolism | ✓ | ||||
| Gap junction | ✓ | ||||
| General transcription regulation | ✓ | ||||
| Glycerophospholipid metabolism | ✓ | ||||
| Glycosylphosphatidylinositol-anchor biosynthesis | ✓ | ||||
| Gonadotropin-releasing hormone signaling pathway | ✓ | ||||
| HIV-I Nef: negative effector of Fas and tumor necrosis factor | ✓ | ||||
| Homologous recombination | ✓ | ||||
| Hypoxia response via HIF activation | ✓ | ||||
| Influence of Ras and Rho proteins on G1 to S transition | ✓ | ||||
| Inositol phosphate metabolism | ✓ | ||||
| Keratan sulfate biosynthesis | ✓ | ||||
| Limonene and pinene degradation | ✓ | ||||
| Links between Pyk2 and MAP kinases | ✓ | ||||
| Long-term depression | ✓ | ||||
| Long-term potentiation | ✓ | ||||
| Lysine degradation | ✓ | ||||
| Mismatch repair | ✓ | ||||
| N-glycan biosynthesis | ✓ | ||||
| Notch signaling pathway | ✓ | ||||
| One carbon pool by folate | ✓ | ||||
| Oocyte meiosis | ✓ | ||||
| Other glycan degradation | ✓ | ||||
| Phosphatidylinositol signaling system | ✓ | ||||
| Phospholipids as signaling intermediaries | ✓ | ||||
| Progesterone-mediated oocyte maturation | ✓ | ||||
| Propanoate metabolism | ✓ | ||||
| Purine metabolism | ✓ | ||||
| Pyrimidine metabolism | ✓ | ||||
| Pyruvate metabolism | ✓ | ||||
| Ras-signaling pathway | ✓ | ||||
| Regulation of eIF4e and p7 S6 kinase | ✓ | ||||
| RNA polymerase | ✓ | ||||
| Role of BRCA1, BRCA2, and ATR in cancer susceptibility | ✓ | ||||
| Role of mitochondria in apoptotic signaling | ✓ | ||||
| Selenoamino acid metabolism | ✓ | ||||
| Signaling of hepatocyte growth factor receptor | ✓ | ||||
| Skeletal muscle hypertrophy is regulated via AKT/mTOR pathway | ✓ | ||||
| SNARE interactions in vesicular transport | ✓ | ||||
| Steroid biosynthesis | ✓ | ||||
| Amino sugar and nucleotide sugar metabolism | ✓ | ||||
| Valine, leucine, and isoleucine degradation | ✓ | ||||
| Thyroid cancer | ✓ |
| . | Column A . | Column B . | Column C . | Column D . | Column E . |
|---|---|---|---|---|---|
| Pathways . | Signaling pathways enriched with genes expressed in Flk1+ cells . | Signaling pathways enriched with genes expressed in Flk1+ cells (and bound by Ldb1) . | Signaling pathways perturbed in Ldb1−/− Flk1+ cells . | Signaling pathways perturbed in Ldb1−/− Flk1+ cells (and bound by Ldb1) (Set1*) . | Signaling pathways perturbed in Ldb1−/− Flk1+ cells (and not bound by Ldb1) (Set3) . |
| Focal adhesion | ✓ | ✓ | ✓ | ✓ | ✓ |
| Adherens junction | ✓ | ✓ | ✓ | ✓ | ✓ |
| Angiogenesis | ✓ | ✓ | ✓ | ✓ | ✓ |
| Colorectal cancer | ✓ | ✓ | ✓ | ✓ | ✓ |
| Hedgehog signaling pathway | ✓ | ✓ | ✓ | ✓ | ✓ |
| ErbB signaling pathway | ✓ | ✓ | ✓ | ✓ | ✓ |
| Insulin signaling pathway | ✓ | ✓ | ✓ | ✓ | ✓ |
| Integrin signaling pathway | ✓ | ✓ | ✓ | ✓ | ✓ |
| Leukocyte transendothelial migration | ✓ | ✓ | ✓ | ✓ | ✓ |
| Pathways in cancer | ✓ | ✓ | ✓ | ✓ | ✓ |
| MAPK signaling pathway | ✓ | ✓ | ✓ | ✓ | ✓ |
| Regulation of actin cytoskeleton | ✓ | ✓ | ✓ | ✓ | ✓ |
| WNT signaling pathway | ✓ | ✓ | ✓ | ✓ | ✓ |
| Acute myeloid leukemia | ✓ | ✓ | ✓ | ✓ | |
| Basal cell carcinoma | ✓ | ✓ | ✓ | ||
| Interleukin signaling pathway | ✓* | ✓* | ✓* | ✓ | |
| Melanoma | ✓ | ✓ | ✓ | ✓ | |
| T-cell–receptor signaling pathway | ✓ | ✓ | ✓ | ✓ | |
| VEGF signaling pathway | ✓ | ✓ | ✓ | ✓ | |
| Apoptosis | ✓ | ✓ | ✓ | ✓ | |
| Axon guidance | ✓ | ✓ | ✓ | ✓ | |
| Chronic myeloid leukemia | ✓ | ✓ | ✓ | ✓ | |
| PDGF signaling pathway | ✓ | ✓ | ✓ | ✓ | |
| Small-cell lung cancer | ✓ | ✓ | ✓ | ✓ | |
| EGF signaling pathway | ✓* | ✓ | ✓ | ✓ | |
| Endocytosis | ✓ | ✓ | ✓ | ✓ | |
| Glioma | ✓ | ✓ | ✓ | ✓ | |
| Neurotrophin signaling pathway | ✓ | ✓ | ✓ | ✓ | |
| TGF-β signaling pathway | ✓ | ✓ | ✓ | ✓ | |
| Prostate cancer | ✓ | ✓ | ✓ | ||
| Pancreatic cancer | ✓ | ✓ | ✓ | ||
| uCalpain and friends in Cell spread | ✓ | ✓ | |||
| Bladder cancer | ✓ | ✓ | ✓ | ||
| Dopamine receptor–mediated signaling pathway | ✓* | ✓ | ✓ | ||
| Tight junction | ✓ | ✓ | ✓ | ||
| Oxidative stress response | ✓ | ✓ | ✓ | ||
| Melanogenesis | ✓ | ✓ | |||
| B-cell activation | ✓ | ✓ | |||
| Telomeres, telomerase, cellular aging, and immortality | ✓ | ✓ | |||
| Cell cycle | ✓ | ✓ | ✓ | ||
| mTOR signaling pathway | ✓ | ✓ | ✓ | ||
| Ras pathway | ✓ | ✓ | ✓ | ||
| Glutathione metabolism | ✓ | ✓ | |||
| Control of gene expression by vitamin D receptor | ✓* | ✓ | |||
| Aminoacyl-tRNA biosynthesis | ✓ | ✓ | |||
| Amyotrophic lateral sclerosis | ✓ | ✓ | |||
| B-cell–receptor signaling pathway | ✓ | ✓ | |||
| Basal transcription factors | ✓ | ✓ | |||
| Endometrial cancer | ✓ | ✓ | |||
| Glyoxylate and dicarboxylate metabolism | ✓ | ✓ | |||
| Heme biosynthesis | ✓ | ✓ | |||
| Huntington disease | ✓ | ✓ | |||
| Interferon-γ signaling pathway | ✓ | ✓ | |||
| JAK/STAT signaling pathway | ✓ | ✓ | |||
| Lysosome | ✓ | ✓ | |||
| Transcription regulation by bZIP transcription factor | ✓ | ✓ | |||
| Adipocytokine signaling pathway | ✓ | ✓ | |||
| Alzheimer disease | ✓ | ✓ | |||
| Ubiquitin mediated proteolysis | ✓ | ✓ | |||
| Non–small-cell lung cancer | ✓ | ✓ | |||
| Nucleotide excision repair | ✓ | ✓ | |||
| Oxidative phosphorylation | ✓ | ✓ | |||
| p38 MAPK signaling pathway | ✓ | ✓ | |||
| p53 signaling pathway | ✓ | ✓ | |||
| Parkinson disease | ✓ | ✓ | |||
| Proteasome | ✓ | ✓ | |||
| Renal cell carcinoma | ✓ | ✓ | |||
| Ribosome | ✓ | ✓ | |||
| RNA degradation | ✓ | ✓ | |||
| Spliceosome | ✓ | ✓ | |||
| Apoptotic signaling in response to DNA damage | ✓ | ||||
| Arginine and proline metabolism | ✓ | ||||
| Base excision repair | ✓ | ||||
| Biosynthesis of unsaturated fatty acids | ✓ | ||||
| Chemokine signaling pathway | ✓ | ||||
| Chondroitin sulfate biosynthesis | ✓ | ||||
| Circadian clock system | ✓ | ||||
| Citrate cycle (TCA cycle) | ✓ | ||||
| CXCR4 signaling pathway | ✓ | ||||
| Cysteine and methionine metabolism | ✓ | ||||
| DNA replication | ✓ | ||||
| Dorsoventral axis formation | ✓ | ||||
| Endothelin signaling pathway | ✓ | ||||
| Fatty acid metabolism | ✓ | ||||
| Fc γ R–mediated phagocytosis | ✓ | ||||
| fMLP-induced chemokine gene expression in HMC-1 cells | ✓ | ||||
| Fructose and mannose metabolism | ✓ | ||||
| Gap junction | ✓ | ||||
| General transcription regulation | ✓ | ||||
| Glycerophospholipid metabolism | ✓ | ||||
| Glycosylphosphatidylinositol-anchor biosynthesis | ✓ | ||||
| Gonadotropin-releasing hormone signaling pathway | ✓ | ||||
| HIV-I Nef: negative effector of Fas and tumor necrosis factor | ✓ | ||||
| Homologous recombination | ✓ | ||||
| Hypoxia response via HIF activation | ✓ | ||||
| Influence of Ras and Rho proteins on G1 to S transition | ✓ | ||||
| Inositol phosphate metabolism | ✓ | ||||
| Keratan sulfate biosynthesis | ✓ | ||||
| Limonene and pinene degradation | ✓ | ||||
| Links between Pyk2 and MAP kinases | ✓ | ||||
| Long-term depression | ✓ | ||||
| Long-term potentiation | ✓ | ||||
| Lysine degradation | ✓ | ||||
| Mismatch repair | ✓ | ||||
| N-glycan biosynthesis | ✓ | ||||
| Notch signaling pathway | ✓ | ||||
| One carbon pool by folate | ✓ | ||||
| Oocyte meiosis | ✓ | ||||
| Other glycan degradation | ✓ | ||||
| Phosphatidylinositol signaling system | ✓ | ||||
| Phospholipids as signaling intermediaries | ✓ | ||||
| Progesterone-mediated oocyte maturation | ✓ | ||||
| Propanoate metabolism | ✓ | ||||
| Purine metabolism | ✓ | ||||
| Pyrimidine metabolism | ✓ | ||||
| Pyruvate metabolism | ✓ | ||||
| Ras-signaling pathway | ✓ | ||||
| Regulation of eIF4e and p7 S6 kinase | ✓ | ||||
| RNA polymerase | ✓ | ||||
| Role of BRCA1, BRCA2, and ATR in cancer susceptibility | ✓ | ||||
| Role of mitochondria in apoptotic signaling | ✓ | ||||
| Selenoamino acid metabolism | ✓ | ||||
| Signaling of hepatocyte growth factor receptor | ✓ | ||||
| Skeletal muscle hypertrophy is regulated via AKT/mTOR pathway | ✓ | ||||
| SNARE interactions in vesicular transport | ✓ | ||||
| Steroid biosynthesis | ✓ | ||||
| Amino sugar and nucleotide sugar metabolism | ✓ | ||||
| Valine, leucine, and isoleucine degradation | ✓ | ||||
| Thyroid cancer | ✓ |
Column A: Signaling pathways enriched for genes expressed in Flk1+ cells. Column B: Signaling pathways enriched for genes expressed in Flk1+ cells and bound by Ldb1. Column C: Signaling pathways perturbed in Ldb1−/− Flk1+ cells. Column D: Signaling pathways enriched with genes differentially expressed in Ldb1−/− Flk1+ cells and bound by Ldb1 (Set1*). Column E: Signaling pathways enriched with genes differentially expressed in Ldb1−/− Flk1+ cells and not bound by Ldb1 (Set3). Signaling pathways were selected based on significance (P ≤ .05) unless stated otherwise.
*P = .07 to .09.
Ldb1 plays a specific and major role in the BL-CFCs’ gene regulatory network
To identify the direct genomic targets of Ldb1 in Flk1+ BL-CFCs, ChIP sequencing in sorted Flk1+ cells showed 4252 significant Ldb1 binding peaks. Of all genes expressed in Flk1+ cells, more than one quarter, or 3264, genes contain Ldb1 peak(s) in their vicinity. The group of Ldb1-bound genes were found to participate in approximately half of the signaling pathways active in BL-CFCs (Table 1, Columns A vs B).
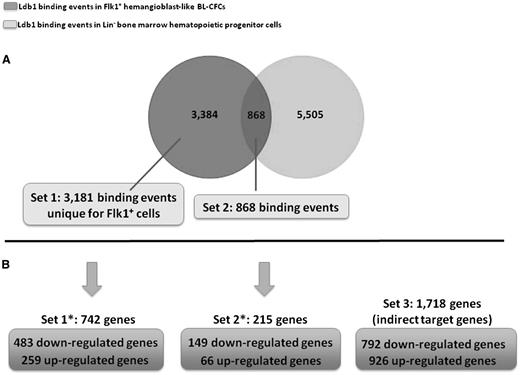
We compared the Ldb1 ChIP-sequencing dataset of primitive Flk1+ BL-CFCs to the Li et al dataset in more committed adult hematopoietic Lin– bone marrow progenitors.11 This revealed that the majority of the 4252 Ldb1-binding events in Flk1+ cells is specific to these early progenitor cells (79.6%, or 3384 binding events) (Figure 2A). Of note, among those specific binding events, a few (respectively, 35 and 168 events) have also been detected in previously published Ldb1 ChIP-sequencing datasets from fetal liver or definitive erythroleukemic cells.15 We therefore excluded those 203 binding events from the 3384 Flk1+-specific events for further investigation. The resulting 3181 Flk1+-specific binding events are referred to as “Set1” and will be compared with “Set2”, the common binding events between Flk1+ cells and Lin-bone marrow cells (20.4% or 868 Ldb1-binding events in Flk1+ cells).
Genome-wide Ldb1 binding in BL-CFCs vs adult hematopoietic progenitor cells and transcriptional function of Ldb1 on BL-CFC gene expression. (A) Venn diagram comparing Ldb1-binding events in Flk1+ BL-CFCs vs Lin– bone marrow hematopoietic progenitor cells.11 (B) Correlative crossing between Ldb1 ChIP-sequencing dataset in BL-CFCs and gene expression dataset of Ldb1−/− vs Ldb1+/+ BL-CFCs.
Genome-wide Ldb1 binding in BL-CFCs vs adult hematopoietic progenitor cells and transcriptional function of Ldb1 on BL-CFC gene expression. (A) Venn diagram comparing Ldb1-binding events in Flk1+ BL-CFCs vs Lin– bone marrow hematopoietic progenitor cells.11 (B) Correlative crossing between Ldb1 ChIP-sequencing dataset in BL-CFCs and gene expression dataset of Ldb1−/− vs Ldb1+/+ BL-CFCs.
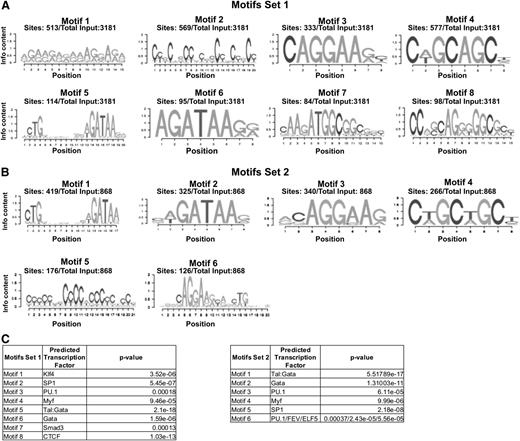
We then performed a functional study to identify genes and associated signaling pathways regulated by Ldb1 in BL-CFCs. Day 4 Ldb1−/− Flk1+ cells were used for microarray and RNA sequencing. The corresponding gene expression profiles were compared with the profile of wild-type Flk1+ cells. Of the 12 468 genes found to be expressed in Flk1+ cells, 2675 genes were identified as differentially expressed between the Ldb1+/+ and the Ldb1−/− Flk1+ cells (supplemental Table 1). Of those, 1424 genes were downregulated (53.23%) and 1251 were upregulated (46.77%). The differentially expressed genes were classified into 3 independent sets: Set1*, Set2*, and Set3, respectively, depending on whether they were included in the Ldb1-bound genes of Set1, the Ldb1-bound genes of Set2, or they were unbound in Flk1+ cells (Figure 2B). Interestingly, the majority of Ldb1 direct targets (Set1* and Set2*) were observed downregulated genes; 65% (483 genes) were downregulated and 35% (259 genes) were upregulated in Set1*, and 69.3% (149 genes) were downregulated and 30.7% (66 genes) were upregulated in Set2*. This trend is absent in indirect target genes (Set3), which present 46.1% (792 genes) and 53.9% (926 genes) of down- and upregulated genes, respectively. The fact that the majority of direct target genes is downregulated as a result of Ldb1 deletion shows that Ldb1 primarily acts as a positive transcriptional regulator in BL-CFCs. However, the one-third of upregulated genes in this group suggests an additional role for Ldb1 complexes as repressors of transcription. A search for enriched DNA-binding motifs carried out on sequences targeted by Ldb1 in Set1 revealed the known E-box(Tal1):Gata motif (motif 5) in only 3.6% of peaks (114 of 3181) (Figure 3A,C). The motifs found with the highest frequency were those associated with Klf transcription factors (motif 1: 513 peaks out of 3181 or 16%), SP1 (motif 2: 569 peaks or 18%), and PU.1 (motif 3: 333 peaks or 10%). In addition, 3% of Ldb1 peaks from Set1 are associated with a CTCF motif (motif 8: 98 of 3181 peaks) (Figure 3A,C). Conversely, in Set2 peaks (common with adult bone marrow progenitor peaks), a prominent E-box:Gata motif was found (motif 1: 419 peaks of 868 or 48%) as described,11,15 and no CTCF motif was detected (Figure 3B,C). This suggests that Ldb1 forms different complexes in primitive Flk1+ cells, targeting different regulatory sites. Alternatively, Ldb1 complex activity may be regulated by different cofactors in these cells as suggested by the differences in recognition motifs, potentially explaining the different repressing and activating transcriptional functions of Ldb1 in BL-CFCs.
Enrichment in DNA-binding motifs within BL-CFC Ldb1 binding peaks. (A) Most prevalent motifs enriched within Ldb1-binding peaks of Set1. (B) Most prevalent motifs enriched within Ldb1-binding peaks of Set2. Frequency of each motif within the total number of studied binding peaks is given. (C) Each identified motif has been associated with one or more transcription factors found in the JASPAR database, and the significance of this association is determined by the P value. Each factor represents a family of transcription factors binding the same motif.
Enrichment in DNA-binding motifs within BL-CFC Ldb1 binding peaks. (A) Most prevalent motifs enriched within Ldb1-binding peaks of Set1. (B) Most prevalent motifs enriched within Ldb1-binding peaks of Set2. Frequency of each motif within the total number of studied binding peaks is given. (C) Each identified motif has been associated with one or more transcription factors found in the JASPAR database, and the significance of this association is determined by the P value. Each factor represents a family of transcription factors binding the same motif.
Ldb1 preferentially controls developmental genes in BL-CFCs and regulates a wide range of important signaling pathways
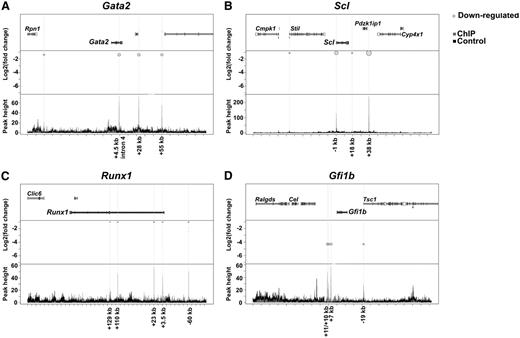
Among the misregulated genes in Ldb1−/− Flk1+ BL-CFCs directly bound by Ldb1 (Set1*), we found genes previously shown to participate in the regulation of mouse embryonic development and hematopoiesis such as Gata2,20,21 Gata1,22 Runx1,23 Fli1,24 Lyl1,25 Id2, Id4,26 Klf4,27 Gfi1b,28 Cbfa2t3/Eto2,29 Myb,30 Dkk1, Sfrp1,31,32 Meis1,33 Raldh2,34 and Fgfr2.35 The changes observed in some of those essential genes were confirmed by real-time polymerase chain reaction (supplemental Figure 3). Of interest is the downregulation of Stat5a and Sox7, Sox17, and Sox18 all having been reported as hematopoietic regulators.36-38 Figure 4 shows 4 examples of hematopoietic-essential Set1* genes. Because BL-CFCs can differentiate into both the hematopoietic and endothelial lineages, we further analyzed the hematopoietic- and endothelial-specific genes.39 Figure 5 shows that the majority of these is downregulated in the absence of Ldb1, illustrating the role of Ldb1 as an activator of transcription and to a lesser extent as a repressor in hematopoiesis and endothelial development (like the larger group of Set1* containing those genes, see Figure 2B).
Bubble plot representation of Ldb1-binding peaks around 4 selected hematopoiesis-specific genes downregulated in the Ldb1−/− BL-CFCs. Each of the figures (A-D) are divided into three panels. The upper panel shows the studied gene and the neighboring genomic genes. The middle panel shows the differential expression (presented as log2fold change on the y-axis) of the studied gene in Ldb1−/− Flk1+ cells in correlation with the Ldb1-binding peaks identified within or around the gene body. The light gray circles (allocated to binding peaks) represent downregulation of gene expression. On the bottom panel, ChIP binding peak heights are represented on the y-axis and genome location on the x-axis. The dark gray color represents Ldb1 ChIP peaks and the black color represents IgG ChIP peaks (used as control).
Bubble plot representation of Ldb1-binding peaks around 4 selected hematopoiesis-specific genes downregulated in the Ldb1−/− BL-CFCs. Each of the figures (A-D) are divided into three panels. The upper panel shows the studied gene and the neighboring genomic genes. The middle panel shows the differential expression (presented as log2fold change on the y-axis) of the studied gene in Ldb1−/− Flk1+ cells in correlation with the Ldb1-binding peaks identified within or around the gene body. The light gray circles (allocated to binding peaks) represent downregulation of gene expression. On the bottom panel, ChIP binding peak heights are represented on the y-axis and genome location on the x-axis. The dark gray color represents Ldb1 ChIP peaks and the black color represents IgG ChIP peaks (used as control).
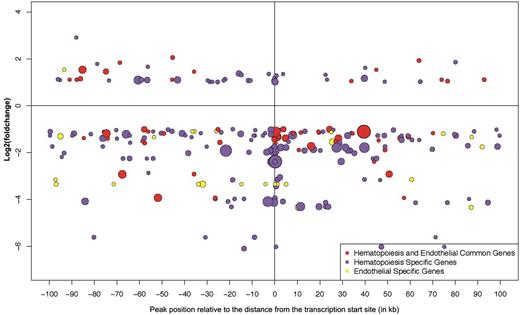
Bubble plot representation of Ldb1-binding peaks around differentially regulated developmental hematopoietic/endothelial genes in Ldb1−/− BL-CFCs. Each bubble in the plot represents one Ldb1-binding peak allocated to a hematopoietic-specific gene (purple bubble), an endothelial-specific gene (yellow bubble), or a gene involved in both hematopoietic and endothelial development (red bubble). The distance of each binding peak relative to a transcription start site of each gene is represented on the x-axis, whereas changes in gene expression in the Ldb1−/− vs Ldb1+/+ Flk1+ cells are represented on the y-axis as the log2fold change. The height of the binding signal/peak is represented by the diameter of the bubble. Hematopoietic- and endothelial-specific genes were identified through the use of IPA (Ingenuity Systems, www.ingenuity.com) and showed a 13.67% (29 of 212 genes) overlap, with the subset of endothelial genes identified through the study of Takase et al.39 The lists of hematopoietic- and endothelial-specific genes, as well as the common hematopoietic/endothelial genes, used to generate the plot are provided in supplemental Table 2.
Bubble plot representation of Ldb1-binding peaks around differentially regulated developmental hematopoietic/endothelial genes in Ldb1−/− BL-CFCs. Each bubble in the plot represents one Ldb1-binding peak allocated to a hematopoietic-specific gene (purple bubble), an endothelial-specific gene (yellow bubble), or a gene involved in both hematopoietic and endothelial development (red bubble). The distance of each binding peak relative to a transcription start site of each gene is represented on the x-axis, whereas changes in gene expression in the Ldb1−/− vs Ldb1+/+ Flk1+ cells are represented on the y-axis as the log2fold change. The height of the binding signal/peak is represented by the diameter of the bubble. Hematopoietic- and endothelial-specific genes were identified through the use of IPA (Ingenuity Systems, www.ingenuity.com) and showed a 13.67% (29 of 212 genes) overlap, with the subset of endothelial genes identified through the study of Takase et al.39 The lists of hematopoietic- and endothelial-specific genes, as well as the common hematopoietic/endothelial genes, used to generate the plot are provided in supplemental Table 2.
To decipher the essential functions of Ldb1 in the maintenance/differentiation of BL-CFCs, we performed a pathway enrichment analysis on the total number of genes misregulated in Flk1+ cells in the absence of Ldb1 (Table 1, Column C). Approximately one-third (37 of 127) of the pathways enriched for genes expressed in Flk1+ cells are misregulated in the Ldb1−/− Flk1+ cells. To distinguish between direct and indirect functions of Ldb1, we carried out in parallel pathway enrichment analyses on the Ldb1 direct-target genes (Set1*; Table 1, Column D) and the indirect Ldb1-regulated genes (Set3; Table 1, Column E). Approximately half of the misregulated pathways in the Ldb1−/− Flk1+ cells were directly targeted by Ldb1 (Table1, Column C vs Column D). Among these, acute myeloid leukemia, basal cell carcinoma, interleukin signaling pathway, melanoma, and T-cell receptor signaling pathways were found. Four examples of perturbed signaling pathways are provided, illustrating how the genes are affected by the absence of Ldb1 (supplemental Figures 4-7). Signaling pathways such as WNT (Wingless), MAPK (mitogen-activated protein kinase), hedgehog, ErbB, focal adhesion, integrin, insulin, and angiogenesis were commonly enriched for both direct and indirect genes of Set1* and Set3. Not unexpectedly, we also found myeloid and lymphoid specific pathways in that context. In contrast, the VEGF-, epidermal growth factor (EGF)-, platelet-derived growth factor (PDGF), and endocytosis signaling pathways were enriched only in the indirect Set3 genes compared with Set1*.
In conclusion, the large number of pathways that are (potentially) affected in the Ldb1−/− Flk1+ BL-CFCs easily explains the severe phenotype of the Ldb1−/− embryos. Importantly, the fact that some Flk1+ cells still emerge during Ldb1−/− ES cell differentiation suggests that most of these pathways may play some role in hemangioblast formation but become essential at subsequent developmental stages before the hematopoietic and endothelial lineages diverge.
Discussion
Primitive hematopoiesis begins in the yolk-sac after the migration of brachyury expressing mesodermal cells through the primitive streak and their differentiation toward hematopoietic or endothelial precursors that collectively form blood islands. Hematopoietic precursors give rise to primitive erythroblasts and endothelial precursors to the vasculature. Both are absent in Ldb1−/− embryo yolk-sacs, questioning the role of Ldb1 in this process.
Many of the misregulated factors identified in the gene-expression profiling of Flk1+ BL-CFCs from day 4 Ldb1+/+ and Ldb1−/− EBs act in the nuclear “response” of well-known signaling pathways. WNT signaling has a positive role in regulating primitive hematopoiesis.32,40 Addition of the inhibitor Dkk1 (downregulated in the Ldb1−/− Flk1+ cells) blocks the pathway, reducing the potential of BL-CFCs to generate colonies.31 Both WNT and Notch signaling pathways are involved in the differentiation of hemangioblast cells toward the primitive erythroid lineage. WNT signaling is active in Flk1+ BL-CFCs during the first hours of differentiation toward blast colonies, whereas Notch signaling remains inactive through Numb inhibition. Between 12 and 24 hours of differentiation, the inhibitory effect exerted on Notch signaling is reduced and its activation leads to the expression of WNT inhibitors blocking the WNT pathway.31 Both pathways were found to be active in Flk1+ cells; however, only WNT signaling was perturbed in Ldb1−/− Flk1+ cells. We propose that WNT misregulation can be attributed to changes in expression of genes bound by Ldb1. In Drosophila, the importance of interplay among Notch, fibroblast growth factor (FGF), MAPK, and EGFR signaling pathways has been documented as a requirement for blood specification,41 with the last 2 pathways affected by the deletion of Ldb1 in Flk1+ cells.
Changes in gene expression of the WNT signaling–associated transcription factors Lef1, Tcf7, and Tcf7l1 in Ldb1−/− Flk1+ cells confirm the importance of this pathway in early hematopoiesis. The downregulation of Etv2/Er71 in knockout cells leads to the same conclusion because it acts downstream of not only the WNT but also the bone morphogenic protein (BMP) and Notch pathways.42,43 Also, expression of the transforming growth factor-beta (TGF-β) signaling receptors, endoglin and Alk-1 (Acvrlk1), was reduced in Ldb1−/− Flk1+ cells. ES cell knockout and rescue experiments showed that these receptors are important for hemangioblast development and primitive hematopoiesis.44 Recently, Ldb1 has been implicated in cell migration and focal adhesion through an interaction with the Ste20-like kinase (SLK), a microtubule-associated protein necessary for migration.45 Our data show that expression of a number of focal adhesion proteins is affected, although SLK did not appear to change. Among the misregulated genes in Ldb1−/− Flk1+ BL-CFCs, we found Stat5a, Sox7, Sox17, and Sox18. Interestingly, Stat5a overexpression in differentiating ES cells leads to an increase in hematopoietic progenitors.36 Sox7, Sox18, and Sox17 participate in different stages of hematopoietic development in the mouse embryo, with Sox7 and Sox18 being involved at the earliest stages of yolk-sac hematopoiesis and Sox17 participating at later stages with the emergence of long-term repopulating hematopoietic stem cells.37,38
Ldb1 acts in a core complex with the essential hematopoietic regulators Scl, Lmo2, and Gata1/Gata2 and associates with other essential factors such as Runx1.14,15 When Scl is absent, primitive hematopoiesis is impaired and hematopoietic colonies are absent in culture, similar to Ldb1−/− embryo phenotype.20,46,47 In vitro generation of Scl−/− EBs further showed that this factor is dispensable for hemangioblast generation, but is essential for its commitment toward hematopoietic and endothelial lineages.48 Gata1-deficient embryos fail to generate erythroid cells, but Gata1-null ES cells can form other hematopoietic lineages.49,50 Gata2 deficiency shows reduced yolk-sac hematopoiesis and reduced number of ES cell–derived colonies. Finally, Lmo2 absence results in a phenotype similar to that of Scl and Gata2, although macrophage colonies were still present in colony assays.47 Our data show that all of these factors are regulated by Ldb1, placing it as a key regulator of the transcription factor network at the origin of the hematopoietic system. Accordingly, Ldb1 deletion in the mouse results in a more severe hematopoietic phenotype than that observed with the factors mentioned before. The additional defect observed in endothelial development can also be explained by the EB results. There are still some CD31+ endothelial cells in the Ldb1−/− EBs, but their number is severely reduced. We conclude that the defect in primitive hematopoiesis in the absence of Ldb1 occurs at a stage in development before the hematopoietic and endothelial lineages diverge, i.e., Ldb1 is involved in the proliferation and differentiation of Flk1+ BL-CFCs, resulting in half the number of BL-CFCs that do not develop into blast colonies when compared with normal.
It is not yet clear how the hemangioblast develops from the embryonic mesoderm and how differentiation into hematopoietic and endothelial cells is regulated. Our data show that this must be a process orchestrated by a number of signal transduction pathways and transcription factors, many of which are directly or indirectly affected by the loss of Ldb1. One of the best-studied transcription factors in hematopoietic development, Runx1, is affected by the absence of Ldb1. It is expressed at low levels after the first 24 hours of blast colony formation and increases as the first hematopoietic progenitors of the erythroid and macrophage lineages emerge.31 Although Runx1−/− blast colonies and primitive hematopoietic colonies are still able to grow,23 its absence results in the emergence of fewer colonies from EBs.31 Thus, downregulation of Runx1 in the Ldb1−/− EBs would be yet another cause of the decrease seen in the number of BL-CFCs. A large number of key hematopoietic transcription factors is misregulated in the absence of Ldb1, showing that this factor is critically required to properly orchestrate the gene expression network of hemangioblasts and their progeny (Figure 6).
Speculative model of Ldb1 function in hemangioblast cells. Schematic representation of Ldb1 functions in the hemangioblast based on whole-genome and transcriptome analyses, revealing Ldb1 involvement in the regulation of key hematopoietic gene expression and essential developmental signaling pathways.
Speculative model of Ldb1 function in hemangioblast cells. Schematic representation of Ldb1 functions in the hemangioblast based on whole-genome and transcriptome analyses, revealing Ldb1 involvement in the regulation of key hematopoietic gene expression and essential developmental signaling pathways.
A number of factors are also upregulated, and it is interesting to note that expression of the mesodermal marker brachyury in Ldb1−/− BL-CFCs and Ldb1−/− EBs is upregulated (data not shown). Brachyury expression is lost within the first 24 hours of blast colony formation31 and our data show that it is suppressed by Ldb1. The brachyury upregulation also suggests that Ldb1−/− BL-CFCs have not yet lost their mesodermal identity/properties and are therefore unable to differentiate into blast colonies.
Finally, our data also show that a number of genes involved in cardiovascular development are affected, suggesting that Ldb1 is also important in cardiovascular development, presumably in complex with the cardiac Gata factors.
In summary, Ldb1 was identified as an essential regulator of mouse hemangioblast proliferation and differentiation. The severity of the hematopoietic phenotype is the result of a decreased number of hemangioblast cells and their inability to differentiate further down the hematopoietic and endothelial lineages. The expression of essential transcription factors is directly affected by Ldb1, revealing its crucial role in the early hematopoietic/endothelial regulatory networks (Figure 6). In addition, the identification of Ldb1 target genes in Flk1+ cells reveals a number of novel pathways contributing to hematopoietic/endothelial development and provides better understanding of the early developmental steps of these lineages.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked ‘‘advertisement’’ in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the members of the Grosveld Laboratory for technical help and discussion. Cell sorting was done by Reinier van der Linden.
This work was supported by NWO (NL), the Dutch Academy of Sciences (KNAW), the EU (the integrated projects EUTRACC and SyBoss), and the European People Marie Curie actions Program (Marie Curie European Reintegration Grants ERG, Call: FP7-PEOPLE-2010-RG).
Authorship
Contribution: A. Mylona, C.A.-S., and F.G. designed the research. A. Mylona, C.A.-S., A. Martella, E.S., and R.J. carried out the research. C.K. carried out Illumina sequencing. S.T. and J.H. performed bioinformatics analyses. W.v.I. and B.L. supervised Illumina data processing and bioinformatics analyses. A. Mylona, C.A.-S., E.S., S.T., and F.G. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A. Mylona is the Department of Hematology, Erasmus MC, Rotterdam, The Netherlands.
Correspondence: Frank Grosveld, Department of Cell Biology, Erasmus MC, Dr. Molenwaterplein 50, 3065 GE, Rotterdam, The Netherlands; e-mail: f.grosveld@erasmusmc.nl; correspondence for computational biology: Boris Lenhard, Imperial College London, London SW7 2AZ, United Kingdom; e-mail: b.lenhard@imperial.ac.uk.
References
Author notes
A. Mylona, C.A.-S., and S.T. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal