In this issue of Blood, Hohmann et al describe a new antithrombotic strategy that involves delayed targeting of CD39 to activated platelets, which reduces thrombus size without increasing bleeding.1
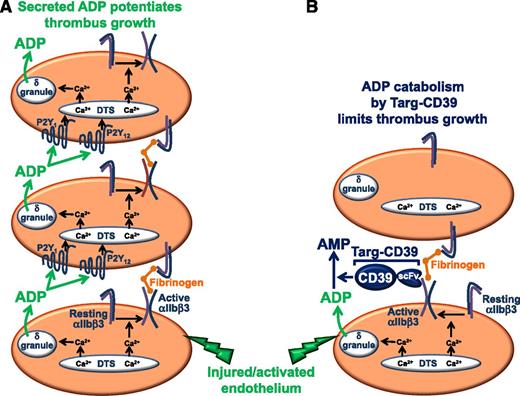
(A) Platelet thrombus formation occurs in a stepwise process. In the first phase, circulating platelets adhere to activated endothelium or exposed subendothelial matrix proteins exposed as a consequence of vascular injury. Platelet adhesion is an activating event that results in conversion of the integrin αIIbβ3 from a resting to an active conformation, in which it is competent to bind plasma fibrinogen. In the second phase, circulating platelets bind to immobilized fibrinogen and are thereby recruited into the growing thrombus. In the third phase of the process, platelets recruited to the thrombus by binding to immobilized fibrinogen are activated by soluble agonists released by activated, adherent platelets that form the initial platelet monolayer. Of particular importance is the platelet dense (δ) granule constituent, ADP, which binds to two GPCRs, including the Gαq-coupled P2Y1 receptor and the Gαi-coupled P2Y12 receptor. GPCR-mediated activation of platelets bound to immobilized fibrinogen initiates the third or perpetuation phase of the thrombus formation process because it results in activation of αIIbβ3, binding of plasma fibrinogen, and recruitment of additional platelets into the growing thrombus. (B) Targeting of soluble CD39 to activated platelets interferes with the perpetuation phase, but not the initiation or accumulation phases, of thrombus formation. Targ-CD39 represents a fusion protein composed of soluble CD39 fused to a single-chain Fv fragment (scFv) of an antibody that is specific for the active conformation of the platelet-specific integrin αIIbβ3. Targeting of CD39 to activated αIIbβ3 ensures that hydrolysis of ADP is delayed until after the first layer of platelets has already been activated and allowed to recruit the second layer of platelets. Consequently, Targ-CD39 interferes only with ADP-mediated activation of recruited platelets, which affects only the perpetuation phase of thrombus formation.
(A) Platelet thrombus formation occurs in a stepwise process. In the first phase, circulating platelets adhere to activated endothelium or exposed subendothelial matrix proteins exposed as a consequence of vascular injury. Platelet adhesion is an activating event that results in conversion of the integrin αIIbβ3 from a resting to an active conformation, in which it is competent to bind plasma fibrinogen. In the second phase, circulating platelets bind to immobilized fibrinogen and are thereby recruited into the growing thrombus. In the third phase of the process, platelets recruited to the thrombus by binding to immobilized fibrinogen are activated by soluble agonists released by activated, adherent platelets that form the initial platelet monolayer. Of particular importance is the platelet dense (δ) granule constituent, ADP, which binds to two GPCRs, including the Gαq-coupled P2Y1 receptor and the Gαi-coupled P2Y12 receptor. GPCR-mediated activation of platelets bound to immobilized fibrinogen initiates the third or perpetuation phase of the thrombus formation process because it results in activation of αIIbβ3, binding of plasma fibrinogen, and recruitment of additional platelets into the growing thrombus. (B) Targeting of soluble CD39 to activated platelets interferes with the perpetuation phase, but not the initiation or accumulation phases, of thrombus formation. Targ-CD39 represents a fusion protein composed of soluble CD39 fused to a single-chain Fv fragment (scFv) of an antibody that is specific for the active conformation of the platelet-specific integrin αIIbβ3. Targeting of CD39 to activated αIIbβ3 ensures that hydrolysis of ADP is delayed until after the first layer of platelets has already been activated and allowed to recruit the second layer of platelets. Consequently, Targ-CD39 interferes only with ADP-mediated activation of recruited platelets, which affects only the perpetuation phase of thrombus formation.
Hemostasis in the arterial circulation relies heavily on platelet activation and formation of a platelet-rich thrombus.2 When adequately controlled, this process works well to minimize blood loss from broken blood vessels. However, it becomes pathological when thrombi grow too large for the vessels in which they’ve formed. Pathological thrombi restrict or occlude blood flow to downstream tissues such as the heart, which can result in a heart attack. Antithrombotic drugs have been developed and used successfully to reduce the risk of death associated with acute cardiac events and to prevent their recurrence; however, current Food and Drug Administration–approved antiplatelet drugs are associated with an unacceptably high risk for developing major, and sometimes life-threatening, bleeding.3 Thus, efforts to identify novel antithrombotic strategies that reduce thrombus size without increasing bleeding risk are justified.
Platelet thrombus formation occurs in a stepwise process, in which activation of the integrin αIIbβ3 and secretion of the soluble agonist adenosine diphosphate (ADP) play important roles (see figure).4 The first or initiation phase of this process involves formation of a platelet monolayer at the site of vessel injury, which requires adhesion of platelets to activated endothelium or to subendothelial matrix proteins exposed as a consequence of vascular injury. Platelets respond to these activating environments in 2 important ways. First, the integrin αIIbβ3 on adherent platelets is converted from a resting to an active conformation in which it is competent to bind plasma fibrinogen. Fibrinogen immobilized in this manner captures platelets from the circulation, thereby recruiting them into the growing thrombus and initiating the second or accumulation phase of platelet thrombus formation. Second, activated adherent platelets generate and release soluble secondary agonists, such as thromboxane A2 and ADP, which activate recruited platelets on binding to G protein–coupled receptors (GPCRs) on their surfaces. GPCR-mediated activation of recruited platelets enables the third or perpetuation phase of the thrombus formation process because it results in activation of αIIbβ3, binding of plasma fibrinogen, and recruitment of additional platelets into the growing thrombus.
Optimal platelet activation by GPCRs, which contributes importantly to thrombus growth, requires involvement of both Gαq- and Gαi-coupled receptors.4 ADP is a particularly potent GPCR agonist because it serves as a ligand for both the Gαq-coupled P2Y1 receptor and the Gαi-coupled P2Y12 receptor. Existing antithrombotic therapies that interfere with this mechanism of platelet activation include the P2Y12 receptor antagonists, which block ADP binding to the P2Y12 receptor. Physiological mechanisms for regulation of ADP-mediated platelet activation also exist, among which are the activities of ecto-nucleotidases that hydrolyze ADP (and ATP) to AMP and adenosine.5-7 CD39 is one such ecto-nucleotidase. CD39 is normally expressed on the surfaces of endothelial cells in the vasculature, where it helps to keep platelets from becoming activated in the presence of an intact endothelium. Soluble forms of CD39 have been made and found to have antithrombotic activity in animal models; however, like other currently available antithrombotic agents, they are limited by their tendency to cause bleeding. To overcome this limitation, Hohmann et al1 take a novel approach of targeting soluble CD39 specifically to activated platelets within a growing thrombus. They accomplish this by creating a fusion protein, Targ-CD39, in which soluble CD39 is fused to a single chain Fv fragment (scFv) of an antibody that is specific for the active conformation of the platelet-specific integrin, αIIbβ3 (see figure). Targeting of CD39 to activated αIIbβ3 ensures that CD39 does not arrive to hydrolyze ADP until after the first layer of platelets has already been activated and allowed to recruit the second layer of platelets. Presumably, Targ-CD39 interferes only with ADP-mediated activation of recruited platelets, which affects only the perpetuation phase of thrombus formation. Targ-CD39 therefore represents a potentially promising new antithrombotic agent with minimal bleeding risks.
Conflict-of-interest disclosure: The author declares no competing financial interests.