Key Points
pDCs functionally express the IL-21 receptor and produce granzyme B in response to IL-21.
IL-21–induced granzyme B in pDC impairs pDC's capacity to induce T-cell proliferation.
Abstract
Plasmacytoid dendritic cells (pDCs) play a crucial role during innate immunity by secreting bulk amounts of type I interferons (IFNs) in response to Toll-like receptor (TLR)–mediated pathogen recognition. In addition, pDCs can also contribute to adaptive immunity by activation of antigen-specific T cells. Furthermore, it is well established that pDCs contribute to the pathogenesis of autoimmune diseases, including lupus. Interleukin-21 (IL-21) is a cytokine produced by activated CD4+ T and natural killer T (NKT) cells and has a pleiotropic role in immunity by controlling myeloid DC-, NKT-, T-, and B-cell functions. It has remained elusive whether IL-21 affects pDCs. Here we investigate the role of IL-21 in human pDC activation and function and observe that IL-21 activates signal transducer and activator of transcription 3 in line with the finding that pDCs express the IL-21 receptor. Although IL-21 did not affect TLR-induced type I IFNs, IL-6, and TNF-α nor expression of major-histocompatibility-complex class II or costimulatory molecules, IL-21 markedly increased expression of the serine protease granzyme B (GrB). We demonstrate that GrB induction was, in part, responsible for IL-21-mediated downmodulation of CD4+ T-cell proliferation induced by TLR preactivated pDCs. Collectively, our data provide evidence that pDCs are important cells to consider when investigating the role of IL-21 in immunity or pathogenesis.
Introduction
Plasmacytoid dendritic cells (pDCs) constitute a separate subset within the DC lineage and have been shown to exert both immunostimulatory and immunosuppressive functions. pDCs express Toll-like receptor 7 (TLR7) and TLR9,1 which upon sensing viral RNA or bacterial DNA, respectively, are able to produce large amounts of type I interferon-α (IFN-α) and IFN-β.2 These are pleiotropic cytokines that can activate multiple arms of the immune system, including T cells, B cells, natural killer (NK) cells, and conventional (c)-DCs,3 and have a direct antireplicative effect on the virus.4 Further, TLR triggering induces secretion of additional cytokines, such as interleukin-6 (IL-6) and tumor necrosis factor-α (TNF-α), that mediate maturation of pDCs into Ag-presenting cells (APCs) that can prime both CD4 and CD8 T-cell responses.5 Conversely, pDCs have been implicated in dampening of immune responses. TLR7/TLR9 engagement induces expression of the immunosuppressive enzyme indoleamine-2,3-dioxygenase (IDO), which degrades the essential amino acid tryptophan, thereby suppressing T-cell responses.6 In addition, pDCs constitutively express the serine protease granzyme B (GrB),7,8 which is upregulated and secreted in response to IL-3, either alone or in combination with IL-10.9 PDC-derived GrB is active, as it was shown in cytotoxicity experiments using the erythroleukemic cell line K562.7 In addition, it yields suppression of T-cell proliferation in a perforin-independent manner; the mechanism underlying this effect remains elusive.9
Following activation and polarization of T cells by APCs, T cells produce cytokines that impact on the immune response. In a classic division, T helper (Th)-1 cells produce IFN-γ, while IL-4, IL-5, and IL-13 are the signature cytokines produced by Th2 cells.10 In addition, other Th subsets have been defined, including Th17 cells, which predominantly make IL-17.11 Another cytokine that more recently attracted attention is IL-21, which is a member of the common γ-chain family of cytokines, to which IL-2, IL-4, IL-7, IL-9, and IL-15 belong as well.12,13 Production of IL-21 was originally documented to be restricted to CD4+ T cells, in particular to T-follicular helper cells found in or near the B-cell areas of secondary lymphoid tissue. In mice it is clear that IL-21 is produced also by other types of T cells, including Th17 cells and NKT cells.14 The functional receptor for IL-21 exists as a heterodimer that comprises the IL-21R and the common γ chain (γc; CD132).15 In the absence of the γc, IL-21 can bind the IL-21R; however, it does not transduce intracellular signaling. Expression of the IL-21R complex is detected in lymphoid tissues, including spleen, thymus, and peripheral blood cells, indicating that IL-21 has regulatory functions on many cell types. Although the IL-21R is shown to be expressed on resting and activated B cells, T cells, NK cells, DCs, macrophages, and keratinocytes,16,17 it has remained elusive whether pDCs express this cytokine receptor.
Here, we investigated the role of IL-21 on the phenotype and function of human pDCs. We observed that pDCs expressed a functional IL-21R as signal transducer and activator of transcription 3 (STAT-3) was rapidly phosphorylated in response to IL-21. IL-21 did not have an effect on TLR-induced production of type I IFNs, IL-6, or TNF-α by pDCs. IL-21 also did not interfere with TLR-induced maturation of pDCs, since expression of costimulatory molecules, such as CD40, CD80, and CD86, and major-histocompatibility-complex (MHC) molecules was upregulated to a similar level either in the absence or presence of IL-21. Notably, we observed that IL-21 induced the expression and secretion of GrB in pDCs. Moreover, GrB secreted from TLR/IL-21–preactivated pDCs inhibited proliferation of T cells.
Our findings demonstrate a novel role for IL-21 in controlling pDCs. We hypothesize that activated T cells in a negative feedback loop may be controlled by pDCs through production of GrB, which impairs the expansion of the T-cell pool.
Materials and methods
Human pDC and T- and B-cell isolations
Peripheral blood of healthy volunteers was used for isolation of pDCs upon donor consent in accordance with the Declaration of Helsinki (Sanquin Bloodbank, Amsterdam, The Netherlands). Postnatal thymus tissue was obtained from surgical specimens removed from children aged ≤3 years undergoing open-heart surgery (Leids Universitair Medisch Centrum, Leiden, the Netherlands). Tonsils were obtained after routine tonsillectomies (Department of Otolaryngology, Academisch Medisch Centrum, Amsterdam, the Netherlands). The AMC medical ethics committee approved use of these tissues. Thymocytes and lymphocytes from peripheral blood and from tonsils were isolated from a Ficoll-Hypaque density gradient (Lymphoprep; Nycomed Pharma, Oslo, Norway). Subsequently, BDCA4+ cells were enriched by immunomagnetic bead selection using the BDCA4 cell separation kit (MACS [magnetic-activated cell sorting]; Miltenyi Biotec, Bergisch Gladbach, Germany). CD123+CD45RA+ pDCs were sorted by flow cytometry on a FACSAria (BD Biosciences). Peripheral blood T cells and B cells were enriched using the CD3 and CD19 cell selection kits (Miltenyi Biotec), respectively, and sorted using anti-CD3 and anti-CD19 antibodies. Purity was ≥99% and confirmed by reanalysis of sorted cells.
Reagents for functional assay
To test activation and maturation of pDCs, cells were cultured in Yssel’s medium,18 supplemented with 2% human serum (Invitrogen). Oligodeoxynucleotides CpG-A (ODN2216) and CpG-B (ODN2006) and R848 (Invivogen) were used at 10 μg/mL. For IL-21 stimulation, we used mouse recombinant IL-21 (25 ng/mL, R&D Systems), which is cross-reactive between mouse and human. The Z-AAD-CMK–specific inhibitor of GrB (Enzo Life Sciences, New York) was used at 5 μg/mL. Recombinant GrB (Sigma-Aldrich, St. Louis, MO) was used at 10 ng/mL or 100 ng/mL. Recombinant IFN-α (Roferon-A; Hoffmann La Roche, Basel, Switzerland) was used at 1000 U/mL.
Flow cytometry
For flow cytometric analysis, single cell suspensions were stained with fluorescein isothiocyanate, phycoerythrin (PE), PE-Cy7, allophycocyanin (APC), APC-Cy7, or peridinin chlorophyll–coupled antihuman monoclonal antibodies (Abs) targeting the following cell surface markers: CCR7, CD40, CD45RA, CD80, CD86, CD123, HLA-DR, CD3, CD11c, CD14, CD19, CD56, or isotype controls (BD Bioscience), and BDCA2 (Miltenyi Biotech). Human IL-21R was detected on the cell surface using an APC-mouse-antihuman-IL-21R Ab (R&D systems). For detection of phosphorylated STAT (pSTAT-1, pSTAT-3, pSTAT-5) proteins, cells were fixed using cytofix/cytoperm buffer, permeabilized in ice-cold methanol, and washed with Perm/Wash buffer (BD Pharmingen) before incubation with APC-conjugated pSTAT-3 Ab, Alexa-Fluor-647–conjugated pSTAT-3 or pSTAT-5 Abs, or Alexa-Fluor-488 pSTAT-1 Ab (all BD Pharmingen). For intracellular detection of IFN-α protein and GrB protein, cells were cultured for 4 hours with GolgiPlug prior to fixation and permeabilization using a fixation/permeabilization kit (BD) according to manufacturer’s protocol. Fixed cells were stained either with a PE-conjugated IFN-α[2b] Ab (BD Pharmingen) or with a PE- or APC-conjugated GrB Ab (Sanquin, Amsterdam, The Netherlands). In some experiments, Alexa-Fluor-647–conjugated GrB Ab was used and compared with a matching isotype control Ab (Biolegend). For apoptosis staining, we used either annexin-PE or -fluorescein isothiocyanate (BD Pharmingen) and 7-AAD viability staining solution (Ebioscience). Samples were analyzed on a LSRII fluorescence-activated cell sorter (FACS) analyzer (BD Bioscience) and analyzed using FlowJo software (TreeStar).
ELISA and CBA assays
pDCs were activated overnight with CpG-A (10 µg/mL), CpG-B (10 µg/mL), or R848 (10 µg/mL) in the presence or absence of recombinant mouse IL-21 (25 ng/mL). Cell-free culture supernatants were collected and analyzed for the presence of cytokines IL-6 and TNF-α using enzyme-linked immunosorbent assay (ELISA; IL-6; U-CyTech biosciences, Utrecht, The Netherlands; TNF-α, eBiosciences, San Diego, CA) or using the cytometric bead array (CBA; Human Inflammation kit, BD Biosciences) according to the manufacturer’s protocol. GrB present in the culture medium was quantified using the PeliKine Compact human Granzyme B ELISA kit (M1936; Sanquin) according to the manufacturer’s protocol.
PCR
For polymerase chain reactions (PCRs), total RNA was extracted using TRIzol reagent (Invitrogen). RNA concentration and quality was determined using the Nanodrop spectrophotometer (Thermo Fisher Scientific). Equal amounts of total RNA were reverse transcribed into cDNA using the RNA-to-cDNA kit (Roche) according to the manufacturer’s instructions. cDNA was amplified using a PCR machine for conventional reverse transcription-PCR (RT-PCR) and separated on a 1.5% agarose gel or amplified using an iCycler and SYBR green supermix (BioRad) for quantitative PCR (qPCR) using specific primer sets (supplemental Table 1). Each sample was analyzed in triplicate, and expression levels were normalized to the 3 housekeeping genes: β-actin, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), and hypoxanthine-guanine phosphoribosyltransferase (HPRT).
Allogeneic T-cell stimulation
Lymphocytes from peripheral blood were isolated by Ficoll gradient centrifugation as described in our “Materials and methods” section. CD4+ T cells were sorted as lineage (CD11c−CD14−CD19−CD56−BDCA2−) negative cells using a FACSAria instrument (BD Biosciences). Sorted pDCs from peripheral blood were incubated with or without the TLR7 ligand R848 for 48 hours in the absence or presence of IL-21 (25 ng/mL). Subsequently, pDCs were cocultured with T cells at a 1:5 ratio for 6 days in Yssel’s medium18 supplemented with 2% human serum. Cell proliferation was assessed using the CellTrace-violet proliferation kit (Invitrogen) according to the manufacturer’s instructions. T cells activated with human T-expander CD3/CD28 beads (Dynabeads, Dynal, Invitrogen) were used as a positive control.
Statistical analyses
Data were subjected to a 2-tailed paired Student t test analysis using Graphpad Prism 5 for Windows (Graphpad Software, San Diego, CA) and considered significant when at least P < .05.
Results
pDCs express a functional IL-21R
The IL-21R was shown to be expressed by several immune cell types, including B cells and T cells.19 Evidence that pDCs express this receptor is lacking. We therefore analyzed human pDCs from 2 independent donors for expression of the IL-21R transcript by RT-PCR (Figure 1A) and compared expression to that in primary B cells (CD19 MACS-enriched) and T cells (CD3 MACS-enriched or sorted total CD4+ T cells). We observed that pDCs, B cells, and T cells expressed the IL-21RA chain. Furthermore, protein expression of the IL-21Rα chain was detected by flow cytometry at the cell surface of freshly isolated pDCs from peripheral blood, thymus, and tonsil (Figure 1B and data not shown). The expression level of the IL-21R on peripheral blood pDCs was comparable to the levels detected on T cells, B cells, and other (non-pDC/T/B) cells from the same donor (Figure 1B). To determine whether the IL-21R expressed on pDCs was functional, we stimulated human pDCs from blood, thymus, and tonsils with IL-21 and evaluated the phosphorylation of STAT-3, which is a key component of the IL-21R downstream signaling pathway.19 As shown by flow cytometric analysis, IL-21 upregulated pSTAT-3 levels as compared with unstimulated cells independent of their source of origin (Figure 1C). pSTAT-3 levels in unstimulated tonsil pDCs appeared to have increased compared with thymus and blood pDCs, which may suggest that STAT-3 is constitutively activated in tonsil pDCs. While this may reflect the inflamed condition of tonsil, it may well be independent of IL-21. IL-21 did not phosphorylate STAT-1 in pDCs (supplemental Figure 1A) in contrast to IFN-α (supplemental Figure 1B). Increased levels of pSTAT-5 in response to IL-21 could only be observed in pDCs isolated from blood, but not thymus or tonsil, although the results may suggest that STAT-5 may be constitutively activated in thymus and tonsil pDCs (supplemental Figure 1). Taken together, these data show that human pDCs not only express the IL-21R chain but that the receptor is functional when engaged by its physiological ligand.
IL-21R is expressed by human pDCs and is functional. (A) Expression of IL-21 receptor was measured by RT-PCR on RNA extracted from human-sorted pDCs from thymus (2 donors) and compared with peripheral blood human T cells (sorted or MACS-enriched for CD3) and human B cells (sorted or MACS-enriched for CD19). Actin amplification was done as a loading control. (B) Total human peripheral blood mononuclear cells were analyzed for expression of IL-21R by flow cytometry. IL-21R surface expression levels on pDCs, T cells (CD4+ MACS), B cells (CD19+ MACS), and other cells (other) are shown (black lines). Isotype control stainings are shown as gray-filled histograms. IL-21R expression on total B cells is shown as positive control. (C) Flow cytometric analysis of phosphorylated (p) STAT-3 protein in primary pDCs from peripheral blood, thymus, and tonsils stimulated in the presence (black lines) or absence (gray-filled histograms) of IL-21 (25 ng/mL) for 20 minutes. One representative experiment out of 3 is depicted. RCN, relative cell number.
IL-21R is expressed by human pDCs and is functional. (A) Expression of IL-21 receptor was measured by RT-PCR on RNA extracted from human-sorted pDCs from thymus (2 donors) and compared with peripheral blood human T cells (sorted or MACS-enriched for CD3) and human B cells (sorted or MACS-enriched for CD19). Actin amplification was done as a loading control. (B) Total human peripheral blood mononuclear cells were analyzed for expression of IL-21R by flow cytometry. IL-21R surface expression levels on pDCs, T cells (CD4+ MACS), B cells (CD19+ MACS), and other cells (other) are shown (black lines). Isotype control stainings are shown as gray-filled histograms. IL-21R expression on total B cells is shown as positive control. (C) Flow cytometric analysis of phosphorylated (p) STAT-3 protein in primary pDCs from peripheral blood, thymus, and tonsils stimulated in the presence (black lines) or absence (gray-filled histograms) of IL-21 (25 ng/mL) for 20 minutes. One representative experiment out of 3 is depicted. RCN, relative cell number.
IL-21 does not affect pDC survival and cytokine production
To unravel the putative role of the IL-21R on pDCs, we first investigated the effect of IL-21 on pDC survival, both in the presence and absence of TLR stimulation. We observed no significant differences of IL-21 on cell survival after 4 days, neither in TLR unstimulated cells nor after stimulation with the TLR7 ligand R848, as shown by apoptosis staining using annexinV and 7-AAD (Figure 2A). To determine the role of IL-21 in combination with TLR9 engagement, pDCs were activated using the TLR9 agonist CpG-A in the presence or absence of IL-21 (Figure 2B). Equal percentages of IFN-α–expressing cells were found in response to CpG-A either with or without IL-21, as shown by intracellular cytokine staining. Furthermore, the presence of IL-21 during TLR triggering did not influence the ability of pDC to secrete the proinflammatory cytokines IL-6 and TNF-α in response to stimulation with CpG-B or R848, as detected by cytokine bead array analysis (Figure 2C-D) or ELISA (data not shown).
IL-21 stimulation does not affect survival and does not alter cytokine production and secretion in pDCs upon TLR activation. (A) Freshly isolated human thymic pDCs were cultured with and without TLR7 agonist R848 (10 μg/mL) in the presence or absence of IL-21 (25 ng/mL) for 4 days and subsequently stained with an annexinV-conjugated APC antibody and 7-AAD. The percentages of annexinV+7-AAD−/+ early and late apoptotic cells were analyzed by flow cytometry. Numbers represent percentages of cells in the indicated gates. (B) Freshly isolated pDCs were cultured overnight with and without CpG–A (10 μg/mL) in the presence or absence of IL-21, as indicated. Cells were incubated with GolgiPlug for the last 4 hours and analyzed by flow cytometry after intracellular staining using a PE-conjugated antibody directed against IFN-α[2b] protein. CD45RA expression was analyzed to confirm the presence of pDCs. Numbers represent percentages of cells in the indicated gates, which were set on the basis of an IgG-PE isotype control antibody. (C,D) Freshly isolated pDCs were cultured in the presence of CpG-B (10 μg/mL) (C) or R848 (10 μg/mL) (D) with IL-21 (black bars) or without IL-21 (white bars) for 20 hours. Culture supernatants were analyzed for the presence of IL-6 and TNF-α by CBA using flow cytometry. One representative experiment out of 2 is depicted.
IL-21 stimulation does not affect survival and does not alter cytokine production and secretion in pDCs upon TLR activation. (A) Freshly isolated human thymic pDCs were cultured with and without TLR7 agonist R848 (10 μg/mL) in the presence or absence of IL-21 (25 ng/mL) for 4 days and subsequently stained with an annexinV-conjugated APC antibody and 7-AAD. The percentages of annexinV+7-AAD−/+ early and late apoptotic cells were analyzed by flow cytometry. Numbers represent percentages of cells in the indicated gates. (B) Freshly isolated pDCs were cultured overnight with and without CpG–A (10 μg/mL) in the presence or absence of IL-21, as indicated. Cells were incubated with GolgiPlug for the last 4 hours and analyzed by flow cytometry after intracellular staining using a PE-conjugated antibody directed against IFN-α[2b] protein. CD45RA expression was analyzed to confirm the presence of pDCs. Numbers represent percentages of cells in the indicated gates, which were set on the basis of an IgG-PE isotype control antibody. (C,D) Freshly isolated pDCs were cultured in the presence of CpG-B (10 μg/mL) (C) or R848 (10 μg/mL) (D) with IL-21 (black bars) or without IL-21 (white bars) for 20 hours. Culture supernatants were analyzed for the presence of IL-6 and TNF-α by CBA using flow cytometry. One representative experiment out of 2 is depicted.
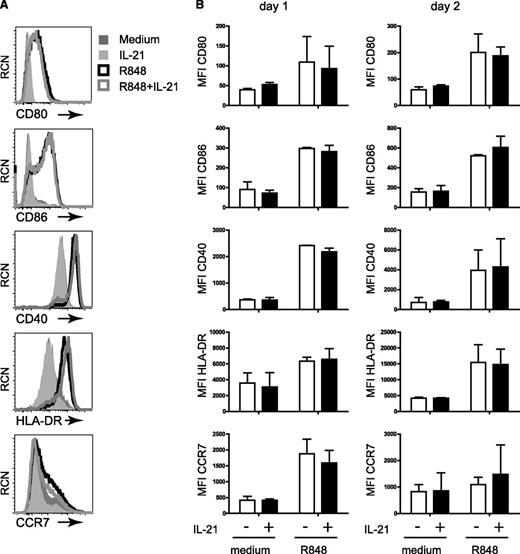
IL-21 does not interfere with TLR-induced maturation of pDCs
Engagement of TLR induces differentiation of pDCs into mature DCs that have upregulated expression levels of MHC-II, chemokine receptor CCR7, and costimulatory molecules, including CD40, CD80, and CD86.3 In our determination of whether IL-21 affects this process, we observed that the maturation of pDCs from blood, tonsil, or thymus upon CpG-B or R848 stimulation was not changed by IL-21, as equal levels of the costimulatory molecules CD40, CD80, and CD86 were detected by flow cytometry after 1 day and after 2 days of stimulation (Figure 3; supplemental Figures 2 and 3; data not shown). Similarly, no effect of IL-21 on expression of HLA-DR and CCR7 was observed. Taken together, these results indicate that IL-21 does not interfere with the TLR-induced functionality of pDCs in terms of activation and maturation.
IL-21 stimulation does not affect TLR-induced pDC maturation. Surface expression of the costimulatory molecules CD80, CD86, and CD40 and expression of HLA-DR and CCR7 were measured by flow cytometry on blood pDCs that were cultured in medium, IL-21 (25 ng/mL), R848 (10 μg/mL), or R848 plus IL-21 for 1 or 2 days. (A) Histograms representing the level of protein expression as indicated after 2 days of culture. One representative experiment out of 2 is depicted. Medium, dark gray–shaded histogram; IL-21, light gray–shaded histogram; R848, black line open histogram; R848 plus IL-21, gray line open histogram. (B) Mean fluorescence intensities (MFIs) were calculated based on the flow cytometry data as described in A after 1 and 2 days. Shown are the mean MFIs of 2 experiments. Error bars indicate standard deviation values.
IL-21 stimulation does not affect TLR-induced pDC maturation. Surface expression of the costimulatory molecules CD80, CD86, and CD40 and expression of HLA-DR and CCR7 were measured by flow cytometry on blood pDCs that were cultured in medium, IL-21 (25 ng/mL), R848 (10 μg/mL), or R848 plus IL-21 for 1 or 2 days. (A) Histograms representing the level of protein expression as indicated after 2 days of culture. One representative experiment out of 2 is depicted. Medium, dark gray–shaded histogram; IL-21, light gray–shaded histogram; R848, black line open histogram; R848 plus IL-21, gray line open histogram. (B) Mean fluorescence intensities (MFIs) were calculated based on the flow cytometry data as described in A after 1 and 2 days. Shown are the mean MFIs of 2 experiments. Error bars indicate standard deviation values.
IL-21 induces GrB expression in pDCs and is modulated by TLR stimulation
PDCs, unlike conventional DCs, constitutively express GrB, although conflicting data have been reported in the literature.7-9 To further elucidate this and to address whether IL-21 affects GrB expression, we cultured pDCs in the presence or absence of IL-21. We confirmed earlier observations7,8 that freshly isolated pDCs constitutively express GrB independent of their source of origin (tonsil, thymus, peripheral blood; Figure 4A). Notably, IL-21 had a major impact on GrB levels in pDCs as IL-21 induced GrB expression both at the transcriptional level (Figure 4B) and at the protein (Figure 4C) level. Four hours after stimulation of pDCs, IL-21 induced the level of GrB mRNA 3.2-fold, as measured by qPCR analysis; after 6 hours, this was even higher at 7.5-fold as compared with medium-cultured pDCs (Figure 4B). Secretion of active GrB from pDCs (tonsil, n = 5; blood, n = 2) was measured in the supernatant after culture in the presence or absence of IL-21 in serum-free medium for 16 hours by ELISA (Figure 4C). In all donors, IL-21 induced higher levels of GrB in pDCs as compared with medium-cultured cells. Tonsil pDCs secreted 2.5-fold more GrB in response to IL-21 (515+/−195 pg/mL; P < .05) compared with medium (209+/−134 pg/mL). Blood pDCs secreted 2.3-fold higher GrB levels in response to IL-21 (566+/−261 pg/mL) compared with medium (245+/−205 pg/mL). Notably, low levels of GrB could be detected when cells were cultured in the absence of IL-21; this is in line with our findings that pDCs constitutively express GrB (Figure 4C).
GrB is endogenously expressed in pDCs and is strongly induced upon IL-21 stimulation. (A) Human pDCs purified from sources as indicated were stained for intracellular GrB expression (black lines) and analyzed by flow cytometry using a GrB-PE–conjugated (thymus and tonsil) or a GrB-APC–conjugated (peripheral blood) antibody. Gray-filled histograms represent appropriate isotype control stainings. (B) GrB RNA levels were measured by qPCR in pDCs after incubation in the presence (black bars) or absence (white bars) of IL-21 for 4 hours and 6 hours as indicated. Expressions of the housekeeping genes (β-actin, GAPDH, and HPRT) were used to control for the amount of RNA used. Values were normalized to cells incubated without IL-21, which was set to 1. (C) Freshly isolated pDCs from tonsil (n = 5) and blood (n = 2) were cultured overnight in the presence or absence of IL-21 in serum-free medium. Culture supernatants were analyzed for the presence of GrB by ELISA. Shown are the mean GrB levels of the pDC donors tested +/− standard deviation. *P < .05. (D,E) Freshly isolated pDCs from 2 tonsil donors (D, upper and lower panels) and from blood (E) were cultured for 1 day (D) or for 2 days (E) in medium (dark gray–shaded histograms) or stimulated (black lines) with either IL-21 alone or with CpG-B (10 μg/mL) or R848 (10 μg/mL) in the presence or absence of IL-21 as indicated. Intracellular GrB expression was analyzed by flow cytometry. Isotype control stainings are shown as light gray–filled histograms. The numbers in E indicate the differences in mean fluorescence intensity (ΔMFI), which were calculated by subtracting the MFI of medium-cultured pDCs from the MFI of stimulated (IL-21, R848, or both) pDCs.
GrB is endogenously expressed in pDCs and is strongly induced upon IL-21 stimulation. (A) Human pDCs purified from sources as indicated were stained for intracellular GrB expression (black lines) and analyzed by flow cytometry using a GrB-PE–conjugated (thymus and tonsil) or a GrB-APC–conjugated (peripheral blood) antibody. Gray-filled histograms represent appropriate isotype control stainings. (B) GrB RNA levels were measured by qPCR in pDCs after incubation in the presence (black bars) or absence (white bars) of IL-21 for 4 hours and 6 hours as indicated. Expressions of the housekeeping genes (β-actin, GAPDH, and HPRT) were used to control for the amount of RNA used. Values were normalized to cells incubated without IL-21, which was set to 1. (C) Freshly isolated pDCs from tonsil (n = 5) and blood (n = 2) were cultured overnight in the presence or absence of IL-21 in serum-free medium. Culture supernatants were analyzed for the presence of GrB by ELISA. Shown are the mean GrB levels of the pDC donors tested +/− standard deviation. *P < .05. (D,E) Freshly isolated pDCs from 2 tonsil donors (D, upper and lower panels) and from blood (E) were cultured for 1 day (D) or for 2 days (E) in medium (dark gray–shaded histograms) or stimulated (black lines) with either IL-21 alone or with CpG-B (10 μg/mL) or R848 (10 μg/mL) in the presence or absence of IL-21 as indicated. Intracellular GrB expression was analyzed by flow cytometry. Isotype control stainings are shown as light gray–filled histograms. The numbers in E indicate the differences in mean fluorescence intensity (ΔMFI), which were calculated by subtracting the MFI of medium-cultured pDCs from the MFI of stimulated (IL-21, R848, or both) pDCs.
To investigate the effect of IL-21 on GrB production upon pDC activation, ex vivo pDCs from tonsil or blood were stimulated with the TLR9 agonist CpG-B or with the TLR7 agonist R848 in the presence or absence of IL-21. Overnight or after 2 days of stimulation, GrB expression was assessed by flow cytometry after intracellular staining (Figure 4D-E). We observed that stimulation of pDCs with either CpG-B or R848 reduced GrB levels as compared with medium-cultured pDCs. This is in contrast to pDCs incubated with IL-21 alone in the absence of TLR ligands, where GrB expression increased as compared with the medium control–cultured pDCs. Notably, TLR-induced GrB inhibition in the concomitant presence of IL-21 was only partial. Under this condition, GrB levels were comparable to the levels that were expressed in medium-cultured cells (Figure 4D-E). The effect of IL-21 on the GrB levels in R848-stimulated blood pDCs for 2 days was comparable to overnight stimulation, although less pronounced (Figure 4E). Analysis of the secreted levels of GrB in the supernatant of overnight-cultured pDCs (supplemental Figure 4) reflected our findings when we analyzed the intracellular GrB levels (Figure 4C). Collectively these results show that pDCs constitutively express and secrete GrB and that IL-21 potently induces GrB expression, which can be partially antagonized by TLR coligation.
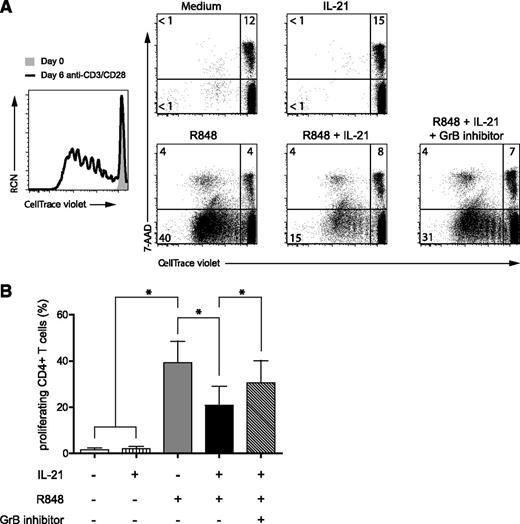
IL-21–induced GrB production by pDCs inhibits T-cell proliferation
During a viral or bacterial infection, CD4+ T cells and NKT cells are the main producers of IL-21.12 To investigate the role of IL-21 in the interactions between pDCs and T cells during an immune response, we performed in vitro allogeneic T-cell stimulation assays using preactivated pDCs. First, pDCs were activated with the TLR7 ligand R848 or with the TLR9 ligand CpG-B in the presence or absence of IL-21 for 48 hours (Figure 5 and data not shown). After extensive washing, preactivated pDCs were cocultured with resting allogeneic CD4+ T cells for 6 days. T-cell proliferation was assessed by measuring the loss of the dye CellTrace-violet upon cell division using flow cytometry. In addition, we included 7-AAD in our analyses to measure cell death. Both TLR7- and TLR9-activated pDCs were able to induce T-cell proliferation (Figure 5A and data not shown). Allogeneic T-cell proliferation induced by activated pDCs was lower than that induced by polyclonal stimulation using anti-CD3/CD28 beads. IL-21–preactivated pDCs did not induce the proliferation of T cells as compared with medium-cultured pDCs. Notably, when pDCs were preactivated by the TLR agonists in the presence of IL-21, they were less capable of inducing T-cell proliferation as compared with TLR-activated pDCs in the absence of IL-21 (R848, 40% vs R848 + IL-21, 15%). This decrease in the percentage of expanded T cells was not due to increased cell death, since the percentages of 7-AAD+ T cells were similar under both conditions (Figure 5A). To demonstrate that GrB could be involved in blocking T-cell proliferation, we added a GrB inhibitor during the pDC–T-cell coculture. Indeed, T-cell proliferation was restored, at least in part, by impairing the activity of GrB. Under all conditions, statistically significant differences were observed when multiple donors (n = 4) were analyzed (Figure 5B). Culture of CD4+ T cells in the presence of recombinant GrB did not affect proliferation (supplemental Figure 5). Hence, our data support the notion that GrB, which is increased in pDCs upon stimulation with IL-21, affects the level of T-cell proliferation induced by TLR-activated pDCs in a cell contact–dependent manner.
IL-21-induced GrB production in pDCs inhibits allogeneic CD4+ T cell proliferation. Freshly isolated pDCs from blood were preactivated for 2 days in medium with or without the TLR7 agonist R848 (10 μg/mL) and in the presence or absence of IL-21 (25 ng/mL) as indicated. After extensive washing, pDCs were cocultured with freshly isolated allogeneic CD4+ T cells (ratio pDC:T cell = 1:5) after labeling with the CellTrace violet dye. After 6 days, T cells were analyzed by flow cytometry for expression of CD3, the fluorescent CellTrace violet dye, and 7-AAD. Dot plots shown are gated on CD3+ T cells. Numbers represent percentages of cells in the indicated quadrants. CellTrace-violetlo7-AAD−CD3+ cells (lower left panel) represent living T cells that have proliferated. CD4+ T cells activated with anti-CD3/CD28 beads are shown as a positive control for proliferation (black line histogram) in comparison with CD4+ T cells cultured with medium only (gray-filled histogram). The GrB inhibitor Z-AAD-CMK (5 μg/mL) was added during the pDC/T cell coculture. (B) CD4+ T cell expansion was measured as described in A. Shown are the mean percentages of CellTrace-violetlo7-AAD−CD3+ cells from 4 donors (*P < .05). Error bars indicate standard deviation values.
IL-21-induced GrB production in pDCs inhibits allogeneic CD4+ T cell proliferation. Freshly isolated pDCs from blood were preactivated for 2 days in medium with or without the TLR7 agonist R848 (10 μg/mL) and in the presence or absence of IL-21 (25 ng/mL) as indicated. After extensive washing, pDCs were cocultured with freshly isolated allogeneic CD4+ T cells (ratio pDC:T cell = 1:5) after labeling with the CellTrace violet dye. After 6 days, T cells were analyzed by flow cytometry for expression of CD3, the fluorescent CellTrace violet dye, and 7-AAD. Dot plots shown are gated on CD3+ T cells. Numbers represent percentages of cells in the indicated quadrants. CellTrace-violetlo7-AAD−CD3+ cells (lower left panel) represent living T cells that have proliferated. CD4+ T cells activated with anti-CD3/CD28 beads are shown as a positive control for proliferation (black line histogram) in comparison with CD4+ T cells cultured with medium only (gray-filled histogram). The GrB inhibitor Z-AAD-CMK (5 μg/mL) was added during the pDC/T cell coculture. (B) CD4+ T cell expansion was measured as described in A. Shown are the mean percentages of CellTrace-violetlo7-AAD−CD3+ cells from 4 donors (*P < .05). Error bars indicate standard deviation values.
Discussion
To our knowledge, this study is the first to show constitutive expression of the IL-21R on human pDCs. We show that the IL-21R is functionally expressed, since IL-21 stimulation of pDCs resulted in activation of the downstream signaling molecule STAT-3. IL-21 by itself did not affect pDC activation, maturation, or cytokine production in response to TLR ligation. However, IL-21 induced GrB expression in pDCs. Moreover, we demonstrate that IL-21–induced GrB was secreted by pDCs, which contributes to impairing CD4+ T-cell proliferation in an allogeneic setting.
Despite the pleiotropic effect of IL-21 on a wide range of immune cells, its effect on pDCs has previously not been investigated. In mice, bone marrow–derived conventional DCs (BMDCs) generated in the presence of IL-21 (IL-21–DCs) showed an impaired activation and maturation capacity.20 Consistent with this, IL-21–pretreated BMDCs, when adoptively transferred, failed to induce T-cell activation in vivo.21 Human monocyte-derived DCs pretreated with IL-21 also failed to upregulate CD86 and MHCII expression in response to lipopolysaccharide, although the consequence of this with respect to T-cell stimulatory capacity was not analyzed.22 In our studies using human pDCs, we did not observe significant differences in expression of maturation markers, including CD40, CD80, CD86, MHCII, or CCR7, in response to TLR agonists either in the presence or absence of IL-21. Therefore, this cannot explain the impaired T-cell proliferation we observed in the presence of TLR/IL-21–stimulated pDCs as compared with pDCs stimulated only by TLR. Moreover, the impaired ability of TLR/IL21 pDCs to activate T cells cannot be due to increased apoptosis induction of the pDCs themselves, since IL-21 did not affect pDC survival. Because increased apoptosis induction of T cells was not observed, it is more likely that GrB secreted by IL-21–stimulated pDCs hampered T-cell proliferation. This is underscored by our finding that the addition of a GrB inhibitor partially restored T-cell expansion.
It is generally accepted that T-cell differentiation, expansion, and survival are enforced in response to cues delivered by DCs. While antigen presentation is key to DCs functioning, additional signals will polarize T cells to induce a tailored immune response against microbial infections. In return, T-cell responses should be downmodulated in order to avoid overactivation of the immune system. Under physiological conditions, activated T cells and pDCs may meet in the lymph node where follicular helper CD4+ T (Tfh) cells produce its primary cytokine IL-21, which provides B cells with signals that are important for the generation of high-affinity antibodies and immunological memory.23 It is reasonable to assume that, reversely, IL-21 may serve to dampen Tfh responses through induction of GrB in pDCs and thereby help the immune system to return to homeostasis at the stage where antibodies have been formed and there is resolution of infection.
Previously, GrB was shown to enter T cells and, via an as yet unknown mechanism, impair proliferation.9 A molecule known to steer T-cell responses is Notch. The Notch pathway is evolutionarily conserved in multicellular eukaryotes, playing a broad and important role during embryonic development and in adult tissue homeostasis.24 Notch proteins coordinate cell–cell communication through receptor–ligand (ie, Δ, Jagged) interactions. Activation cleaves the Notch intracellular domain, which translocates into the nucleus where it binds the transcription factor CSL (CBF1/suppressor of Hairless/Lag-1) in human or RBP-Jκ (recombination signal-binding protein 1 for Jκ) in mice, thereby activating Notch target genes.25 Notch activity is tightly controlled by proteases that activate (ie, γ–secretase) Notch receptor signaling. Within the CD4+ T-cell lineage, Notch can skew T-cell responses toward a Th2 phenotype by controlling expression of the lineage-specific factor GATA-3.26-28 More recently, it was demonstrated that Notch can also control T-cell proliferation29 and longevity.30 Interestingly, NOTCH1 has been described as a substrate of GrB,31 resulting in loss of its transcriptional activity.32 Hence, impaired T-cell proliferation induced by IL-21–pretreated pDCs may be the result of GrB-mediated NOTCH1 degradation. Taken together, a model is emerging that CD4+ T cells, after activation, will inhibit their own expansion via production of IL-21 as well as induction of GrB by pDCs, forming a negative feedback loop in the regulation of an adaptive immune response.
In primary human B cells, IL-21 induced activation of STAT-3.33,34 Here, we show that IL-21 also activates STAT-3 in human primary pDCs, leading to increased levels of GrB. Other cytokines, including IL-3 and IL-10, were previously reported to induce expression of GrB in pDCs.9 As these cytokines act through activation of the JAK/STAT-3 as well, it is likely that this is a common pathway for induction of GrB. Interestingly, in mice, targeting Stat3 in CD11c+ myeloid cells by CpG-siRNA improved key effector functions and proliferation of adoptively transferred T cells.35 While direct evidence is lacking, it is reasonable to consider that this may partially be due to reduced levels of GrB expressed in Stat3−/− DCs, thereby allowing more extensive T-cell proliferation.
PDCs form the first line of defense against viruses and bacteria by rapid production of IFN-α.36 Conversely, uncontrolled or unwanted pDC-derived production of IFN-α plays a key role in human autoimmune pathogenesis, such as systemic lupus erythematosus (SLE),37 Sjögren’s syndrome,38 and psoriasis.39 In addition to IFN-α, elevated serum levels of IL-21 were detected in patients suffering from Sjögren's syndrome, SLE, and RA compared with healthy controls.40,41 Furthermore, SLE is associated with 2 single nucleotide polymorphisms of the IL-21 gene.42 In agreement with these data, elevated IL-21 levels were found in SLE mouse models.43 It is known that IL-21 enhances anti-CD3–induced proliferation of T cells.44 IL-21 also controls the functional activity of effector Th cells, controls the differentiation of Th17 cells, and counteracts the suppressive effects of regulatory T cells.45 Moreover, IL-21, either alone or in synergy with B-cell activating factor, is capable of promoting B-cell expansion and plasma B-cell differentiation.44,46 Adding to this list, we show that IL-21 induced GrB production in pDCs. Interestingly, GrB plays a role in autoimmunity, as GrB-mediated cleavages of intracellular autoantigens may enhance their immunogenicity via the generation of new antigenic epitopes (reviewed in Darrah and Rosen47 ). Also, CD5+ SLE B cells constitutively express GrB,48 and IL-21 was previously shown to induce GrB in B cells.49 This, together with our findings that IL-21 induced the production of GrB in pDCs, enforces the crucial role of IL-21 in the pathogenesis of autoimmune diseases.
Our study highlights the dichotomic role of IL-21 in the activation of pDCs. On the one hand, IL-21–induced GrB may drive or accelerate autoimmunity by cleavage of autoantigenic epitopes,50 and on the other hand, IL-21–induced GrB may be causal for the termination of immune responses by inhibiting T-cell expansion. These findings open new perspectives in the development of therapies specifically targeting the IL-21/IL-21R pathway in autoimmunity and inflammation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Berend Hooibrink and Toni van Capel for maintenance of the AMC FACS facility and help during cell sorting, Dr Mark Hazekamp and staff from the LUMC for providing human thymus tissue, and Dr Wytske Fokkens (Department of Otolaryngology, AMC) for providing tonsil tissue. Rosa de Groot (Department of Cell Biology & Histology, AMC) is acknowledged for her technical help performing ELISA.
J.J.K. is financially supported through a personal VIDI grant to B.B. (Dutch Science Foundation, grant 917.66.310). L.C.M.J. and C.H.U. are supported by a grant from the National Institutes of Health (AI 080564; University of California Los Angeles, Center for AIDS Research).
Authorship
Contribution: J.J.K. designed research, performed experiments, analyzed data, and wrote the manuscript. L.C.M.J., M.N., A.K., and M.B. performed experiments and analyzed data. M.C.W., C.H.U., and S.M.v.H. analyzed data. B.B. designed research, analyzed data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bianca Blom, Academic Medical Center, University of Amsterdam, Department of Cell Biology & Histology, Meibergdreef 15, 1105 AZ, Amsterdam, The Netherlands; e-mail: b.blom@amc.uva.nl.


![Figure 2. IL-21 stimulation does not affect survival and does not alter cytokine production and secretion in pDCs upon TLR activation. (A) Freshly isolated human thymic pDCs were cultured with and without TLR7 agonist R848 (10 μg/mL) in the presence or absence of IL-21 (25 ng/mL) for 4 days and subsequently stained with an annexinV-conjugated APC antibody and 7-AAD. The percentages of annexinV+7-AAD−/+ early and late apoptotic cells were analyzed by flow cytometry. Numbers represent percentages of cells in the indicated gates. (B) Freshly isolated pDCs were cultured overnight with and without CpG–A (10 μg/mL) in the presence or absence of IL-21, as indicated. Cells were incubated with GolgiPlug for the last 4 hours and analyzed by flow cytometry after intracellular staining using a PE-conjugated antibody directed against IFN-α[2b] protein. CD45RA expression was analyzed to confirm the presence of pDCs. Numbers represent percentages of cells in the indicated gates, which were set on the basis of an IgG-PE isotype control antibody. (C,D) Freshly isolated pDCs were cultured in the presence of CpG-B (10 μg/mL) (C) or R848 (10 μg/mL) (D) with IL-21 (black bars) or without IL-21 (white bars) for 20 hours. Culture supernatants were analyzed for the presence of IL-6 and TNF-α by CBA using flow cytometry. One representative experiment out of 2 is depicted.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/121/16/10.1182_blood-2012-08-452995/4/m_3103f2.jpeg?Expires=1763630702&Signature=hbSrNu9l5XJNGShfkqVTaCZptr2Prm0GOrVlacaAp1ptJlFMBByvrFJ3KCj8T5z96CI28DmWZiClfRF1AENtqxPXT7gjm0PUJ08Q2XuVA5gFYT93K64iwtpdqKZjijspOUihI0CKf-dKc0-zxj2i-ectes8ebIeMZOgePD~sDunocqzLML6uOdPNGQ6DGvxlqRFfD~OIOPK5QQHqkpwByQVXkdQXHUNCHw7AbumwiQWb~i0OL0u2SX0eEuakxdJyj1I--8anNyQAUKJIC1CFBO-CUi5g0CyZBI3iEF467m-MzevKG~ZZn2KfJfl1fuvjBSmYlgLGaoGjVQgis6dk8A__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal