Key Points
Two distinct proximal signaling complexes involving SLP-76 and LAT1/LAT2 or ADAP are formed by immunoreceptor-activated NK cells.
Both signaling pathways formed by LAT1/LAT2 and ADAP with SLP-76 are required for the optimal functioning of immunoreceptor-activated NK cells.
Abstract
Signaling pathways leading to natural killer (NK)–cell effector function are complex and incompletely understood. Here, we investigated the proximal signaling pathways downstream of the immunotyrosine-based activation motif (ITAM) bearing activating receptors. We found that the adaptor molecule SH2 domain-containing leukocyte protein of 76 kD (SLP-76) is recruited to microclusters at the plasma membrane in activated NK cells and that this is required for initiation of downstream signaling and multiple NK-cell effector functions in vitro and in vivo. Surprisingly, we found that 2 types of proximal signaling complexes involving SLP-76 were formed. In addition to the canonical membrane complex formed between SLP-76 and linker for activation of T cells (LAT) family members, a novel LAT family–independent SLP-76–dependent signaling pathway was identified. The LAT family–independent pathway involved the SH2 domain of SLP-76 and adhesion and degranulation-promoting adaptor protein (ADAP). Both the LAT family–dependent and ADAP-dependent pathway contributed to interferon-gamma production and cytotoxicity; however, they were not essential for other SLP-76–dependent events, including phosphorylation of AKT and extracellular signal–related kinase and cellular proliferation. These results demonstrate that NK cells possess an unexpected bifurcation of proximal ITAM-mediated signaling, each involving SLP-76 and contributing to optimal NK-cell function.
Introduction
Natural killer (NK) cells provide protection from intracellular pathogens and tumors via production of cytokines, including interferon-gamma (IFN-γ), and by direct cytotoxicity against target cells.1 NK cells do not have a single defining receptor for activation but instead integrate signals from multiple activating and inhibitory receptors.2 One example of NK-cell receptors is the Ly49 family, which contains both activating (D, H) and inhibitory (G2, A, C, I) members that are differentially expressed on murine NK cells.3
Many NK-cell–activating receptors associate with the immunotyrosine-based activation motif (ITAM)-containing adaptor proteins DNAX-activating protein of molecular mass 12 kD (DAP12) or FcRγ.4 Although not fully characterized in NK cells, studies of a variety of hematopoietic cell types, such as T cells and mast cells, have demonstrated that the triggering of ITAM-bearing receptors leads to phosphorylation of ITAMs, which become docking sites for Syk family protein tyrosine kinases (PTKs). Localization to the ITAM-bearing receptor allows Syk family PTKs to become activated and to phosphorylate the membrane-bound adaptor protein LAT1 (linker for activation of T cells). This enables LAT1 to associate with growth factor receptor–bound protein 2 (Grb2)-related adaptor protein 2 (Gads) and phospholipase C-gamma (PLC-γ), which are constitutively bound to the cytosolic adaptor protein SLP-76, allowing for SLP-76 recruitment to the cellular surface and subsequent phosphorylation by Syk family PTKs.5 SLP-76 has 4 main protein-binding domains: a sterile-α motif domain, an amino-terminal acidic region with 3 conserved tyrosine residues, a central proline-rich region, and a C-terminal SH2 domain.6 SLP-76 recruitment to the cellular membrane after ligation of ITAM-bearing receptors is mediated via LAT1 and/or the LAT1 homolog LAT2 through the Gads binding domain in the central proline-rich region.7 Tyrosine-phosphorylated SLP-76 can then associate with other proteins, including Vav, the noncatalytic region of tyrosine kinase adaptor protein 1 (Nck), and interleukin-2 (IL-2)-inducible T-cell kinase (Itk).5 The formation of this multimolecular signaling complex at the cellular membrane is vital for cell signaling and effector function downstream of ITAM-bearing receptors.
Proximal signaling complex formation in NK cells has not been fully elucidated and was initially thought to be similar to that of T cells. However, the investigation of LAT1 and SLP-76 involvement in NK-cell signaling has yielded mixed results. Early studies demonstrated that SLP-76 and LAT1 were dispensable for NK-cell–mediated natural cytotoxicity.8,9 Upon discovery of LAT2 and the creation of LAT1/LAT2 double-knockout (DKO) mice, it was shown that NK cells from LAT1/LAT2 DKO but not single-knockout (KO) mice displayed impaired IFN-γ production downstream of ITAM-bearing activating receptor stimulation, raising the possibility that SLP-76 may also play a role in this pathway.10 Indeed, SLP-76–deficient NK cells were later found to exhibit defective antibody-mediated cytotoxicity.11 Yet, the precise interactions required for the formation of proximal membrane-signaling complexes in NK cells still remain unknown.
To gain a better understanding of how signals are transduced through ITAM-bearing NK-cell–activating receptors, we investigated the role of proximal signaling complexes in NK-cell function. Our data suggest that immunoreceptor-mediated NK-cell function, including cytokine production, degranulation, and proliferation, is highly dependent on SLP-76. NK cells use at least 2 distinct signaling pathways that involve SLP-76. While the canonical pathway utilizes LAT1 and LAT2 for SLP-76 recruitment to the cellular surface, the alternate pathway relies upon the SH2 domain of SLP-76 and adhesion and degranulation-promoting adaptor protein (ADAP). Both pathways contribute to cytokine production and degranulation but are dispensable for NK-cell proliferation. Together, these results demonstrate that NK cells possess an unexpected bifurcation of proximal ITAM-mediated signaling, each contributing to full functional activation of NK cells.
Materials and methods
Mice
C57BL/6 (B6), RAG KO, and NOD/SKID/IL2Rγ KO (NSG) mice were purchased from the Jackson Laboratory (Bar Harbor, ME). LAT1 KO, LAT2 KO, LAT1/2 DKO, ADAP KO, SLP-76 KO, and conditional KO (cKO) mice have been previously described.12,-,16 To acutely deplete SLP-76 in NK cells, SLP-76 cKO (expressing the Tamoxifen-inducible Cre recombinase and a Cre-inducible ROSA26 promoter-driven yellow fluorescent protein [YFP] reporter gene), mice were treated orally with 200 μg/g body weight of tamoxifen (Sigma-Aldrich, St. Louis, MO) in corn oil for 5 days and sacrificed on day 11. Littermate controls were used for SLP-76 KO mice since the SLP-76 KO mice are on a B6/129 mixed background. All mice were at least age 8 weeks at the time of sacrifice. Mice were maintained under specific pathogen-free conditions. Animal care and work were in accordance with national and institutional guidelines and approved by the Institutional Animal Care and Use Committee at the University of Pennsylvania.
Reagents and antibodies
Cytokines were purchased from Peprotech (Rocky Hill, NJ). Antibodies (Abs) for cell stimulation were purchased from BD Pharmingen (San Diego, CA): rat IgG2a isotype control, NK1.1, anti-CD16/32; BioLegend (San Diego, CA): Ly49D; and eBioscience (San Diego, CA): Ly49H. Abs for flow cytometry were purchased from BD Pharmingen, Ly49D, Ly49G2, CD11b, DX5, NK1.1, Ly49C/I, CD107a, CD45.2, CD122; eBioscience, Ly49H, CD27, H-2Kb, NKp46, NKG2AB6; BioLegend, Ly49A, CD122, CD8α, CD3, CD4; Molecular Probes, Invitrogen (Carlsbad, CA): LIVE/DEAD Fixable Aqua Dead Cell Stain Kit.
Flow cytometry, cell sorting, and data analysis
Cells were stained with Dead Cell stain and the indicated Abs. Intracellular staining was performed using the Cytofix/Cytoperm Fixation/Permeabilization Kit (BD Pharmingen) per manufacturer’s instructions. Flow cytometry and fluorescence-activated cell sorter (FACS) were performed with LSR II, FACSCalibur, and FACSAria (BD Biosciences, San Jose, CA). Data were analyzed with FlowJo software (TreeStar, Ashland, OR). Dead cells were excluded from analysis with LIVE/DEAD Aqua Dead Cell staining. Statistical analysis was performed with Excel (Microsoft, Seattle, WA) or Prism (GraphPad, San Diego, CA).
Primary NK-cell cultures and stimulation
DX5+ or Ly49D+ NK cells were isolated by positive selection using anti–DX5-biotin or anti–Ly49D-FITC followed by magnetic-activated cell sorting beads per manufacturer’s instructions (Miltenyi Biotec, Auburn, CA) and expanded in NK-cell media (minimum essential medium-α [Invitrogen] with 10% fetal bovine serum [FBS], 1% penicillin/streptomycin, 10 mM N-2-hydroxyethylpiperazine-N'-2-ethanesulfonic acid [HEPES], and 1 × 10−5 M 2-mercaptoethanol) with human IL-2 (1000 U/mL) for 6 days at 37°C. The NK cells were then stimulated with plate-immobilized isotype control Ab, anti-Ly49D Ab, anti-NK1.1 Ab, anti-Ly49H Ab, or with soluble phorbol myristate acetate (PMA) (100 ng/mL) and ionomycin (1 μg/mL) in the presence of monensin (eBiosciences) and anti-CD107a-PE for 4 hours at 37°C. Following incubation, IFN-γ production and degranulation were analyzed by flow cytometry.
NK-cell proliferation assays
Freshly isolated splenocytes were depleted of T cells with Thy1.2 beads (Miltenyi Biotec) and labeled with carboxyfluorescein succinimidyl ester (CFSE) (Invitrogen) by resuspending cells in phosphate-buffered saline (PBS) containing CFSE (5 μM) and shaken for 9 minutes at 37°C. The reaction was quenched with 100% FBS. CFSE-labeled splenocytes were cultured for 3 days at 5 × 105 cells/well in 200 μL NK-cell media with IL-2 (40 to 100 U/mL) in 96-well flat-bottom plates previously coated with 20 μg/mL isotype-control Ab or anti-Ly49D Ab. The percentage of maximal proliferation was calculated as follows: [(% of CFSE-diluted NK cells stimulated with anti-Ly49D Ab) − (% of CFSE-diluted NK cells stimulated with isotype control Ab)]/[100% − (% of CFSE-diluted NK cells stimulated with isotype control Ab)].
Western blots
IL-2–expanded NK cells were rested for 2 to 4 hours and stimulated with 30 μg/mL of soluble isotype control Ab or anti-Ly49D Ab for the indicated times. The cells were then lysed in 1% Ipegal in Tris-buffered saline with protease/phosphatase inhibitors (protease inhibitor cocktail solution [Roche, Sigma], 1 mM sodium orthovanadate, 50 mM sodium fluoride, 50 mM sodium pyrophosphate, 0.2 mM dichloroisocoumarin, and 1 mM benzamidine), and the proteins were resolved by sodium dodecyl sulfate polyacrylamide gel electrophoresis (Bio-Rad Laboratories, Hercules, CA). The phosphorylation of ERK1/2 (Thr202/Tyr204), SLP-76 (Tyr128), and AKT (Ser473) was analyzed by western blotting. Total PLC-γ2 was used as a loading control. All antibodies were from Cell Signaling (Danvers, MA), except for the anti-phospho-SLP-76 antibody (eBioscience).
Generation of retrovirally transduced BM chimeras
Bone marrow (BM) cells B6, LAT1/LAT2 DKO, or ADAP KO mice were cultured in media with IL-3 (10 ng/mL), IL-6 (10 ng/mL), and stem cell factor (50 ng/mL) at 37°C overnight. The BM cells were then centrifuged with Polybrene (Sigma; 4 μg/mL) and murine stem cell viral supernatant (MigR1) encoding green fluorescent protein (GFP)-tagged forms of SLP-76, SLP-76 Gads-binding mutant (SLP-76.G2), SLP-76 SH2 domain mutant (SLP-76.RK), SLP-76 Gads-binding and SH2 domain double mutant (SLP-76.G2.RK), and the SLP-76 Gads-binding and SH2 domain double mutant with 3 N-terminal tyrosines (Y112, 128, and 145) mutated to phenylalanines (SLP-76.Y3F.G2.RK) for 90 minutes at 1250xg and was repeated the next day. The MigR1 and SLP-76 mutant constructs have been described previously.17,18 Twenty-four hours later, the cells were injected intravenously into irradiated (950 cGy) B6, LAT1/LAT2 DKO, or ADAP KO mice. The BM chimeras were kept on sulfamethoxazole/trimethoprim drinking water (200 mg/40 mg per 250 mL water; HiTech Pharmacal, Amityville, NY) for 21 days. Splenic NK cells were isolated for analysis 8 to 10 weeks post transfer.
Imaging by TIRF microscopy
Δ T dishes (Bioptechs, Butler, PA) were coated with anti-Ly49D Ab (20 μg/mL). IL-2–expanded NK cells from BM chimeras were washed, rested for 30 minutes in Tyrode’s buffer (130 mM NaCl, 10 mM HEPES, 1 mM MgCl2, 5 mM KCl, 1.4 mM CaCl2, 5.6 mM glucose, 1 mg/mL bovine serum albumin, pH 7.4), and deposited onto δ T dishes maintained at 37°C in Tyrode’s buffer. Samples were excited using a 488-nm laser line and visualized by a 60× 1.45-numerical-aperture total internal reflection fluorescence (TIRF) objective fitted to an inverted microscope system (Olympus IX71 or IX81; Olympus, Center Valley, PA) equipped with a charge-coupled device (CCD) camera (Hamamatsu, Bridgewater, NJ). Some images were acquired using Volocity software (PerkinElmer, Waltham, MA) controlling a C9100 EM-CCD camera (Hamamatsu) on an inverted Nikon Ti microscope (Nikon Inc, Melville, NY) with apochromat 100x 1.49 NA oil-immersion objective. Images were recorded using HCImage software (Hamamatsu), while background fluorescence was equalized using a Blackman filter. Images were acquired at 1 image per second for 60 seconds. Images were acquired and analyzed using Volocity software (PerkinElmer, Waltham, MA) controlling a C9100 EM-CCD camera (Hamamatsu). Images were cropped and underwent photobleaching correction. Clusters were located using the find-spots feature, with an offset minimum spot intensity range of (–5 to 80) and a radius of 10 μm. The same settings were used for analysis of all cells within an experiment. Spots were counted for each time point, ranging from 30 to 126 time points/cell (same within each experiment), and averaged across all time points for each cell. The number of spots from each cell was normalized to the average number of spots detected in wild type (WT) SLP-76–transduced NK cells.
In vivo bioluminescent tumor immunosurveillance assay
NSG mice were injected intravenously with 2 × 105 luciferase-expressing Chinese hamster ovary (CHO) target cells (Clontech, Mountain View CA). Two hours later, the mice were injected intravenously with sterile PBS or 4 × 105 Ly49D+ IL-2–expanded B6, RAG KO, or SLP-76 KO NK cells. To evaluate bioluminescence, mice were given an intraperitoneal injection of 10 mg/kg body weight D-luciferin (Caliper Life Sciences, Hopkinton, MA), subsequently anesthetized via inhalational isofluorane, and imaged using a Xenogen Spectrum system and Living Image v3.2 software at the same relative time point after D-luciferin injection. Data were collected until the mid-range of the linear scale or the maximal exposure setting was achieved (f stop 1, large binning and 120 s). Data were then normalized for each image and expressed as photons/second/cm2/steradian. For display purposes, a pseudo-color bioluminescence intensity map was superimposed over the gray-scale body-surface image. Animals were imaged on days 0, 3, 7, and 10 post injection.
Results
SLP-76 is phosphorylated and recruited to plasma membrane clusters downstream of the Ly49D receptor in NK cells
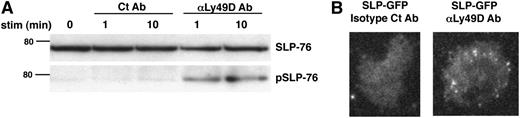
We began our investigation of proximal signaling events in NK-cell activation by examining the phosphorylation and plasma membrane recruitment of SLP-76 using the Ly49D receptor as a model-activating immunoreceptor. Like many other NK-cell–activating receptors such as Ly49H and NKG2C, Ly49D transmits its signal by associating with the ITAM-containing adaptor molecule DAP12.4 Upon stimulation with anti-Ly49D Ab but not with isotype control Ab, SLP-76 was phosphorylated in WT NK cells (Figure 1A). Moreover, visualization of GFP-tagged SLP-76 in NK cells by TIRF microscopy revealed that SLP-76 aggregated into microclusters at the plasma membrane upon stimulation with anti-Ly49D but not with isotype control Ab (Figure 1B). Thus, SLP-76 is recruited to the plasma membrane and phosphorylated downstream of Ly49D stimulation, suggesting that SLP-76 might be involved in signal transduction downstream of NK-cell–activating receptors.
SLP-76 is phosphorylated and recruited to plasma membrane clusters following Ly49D receptor stimulation. (A) Ly49D+ splenic NK cells were enriched and expanded in IL-2 for 6 days. The expanded NK cells were then left unstimulated or stimulated with soluble isotype Ct Ab or anti-Ly49D Ab for 1 or 10 minutes and analyzed for total and phosphorylated SLP-76 by western blot. One representative of 3 independent experiments is shown. (B) Splenic NK cells from GFP-tagged SLP-76–transduced BM chimeric mice were enriched for Ly49D expression, expanded in IL-2 for 6 days, and stimulated on an isotype control Ab or anti-Ly49D Ab-coated surface. Clustering of SLP-76 at the plasma membrane was imaged by TIRF microscopy. Scale bar, 45 μm. One representative of 16 independent experiments is shown.
SLP-76 is phosphorylated and recruited to plasma membrane clusters following Ly49D receptor stimulation. (A) Ly49D+ splenic NK cells were enriched and expanded in IL-2 for 6 days. The expanded NK cells were then left unstimulated or stimulated with soluble isotype Ct Ab or anti-Ly49D Ab for 1 or 10 minutes and analyzed for total and phosphorylated SLP-76 by western blot. One representative of 3 independent experiments is shown. (B) Splenic NK cells from GFP-tagged SLP-76–transduced BM chimeric mice were enriched for Ly49D expression, expanded in IL-2 for 6 days, and stimulated on an isotype control Ab or anti-Ly49D Ab-coated surface. Clustering of SLP-76 at the plasma membrane was imaged by TIRF microscopy. Scale bar, 45 μm. One representative of 16 independent experiments is shown.
SLP-76 KO mice display normal NK-cell maturation but possess decreased proportions of Ly49-expressing NK cells
To directly test the role of SLP-76 in NK-cell immunoreceptor signaling, we used NK cells from SLP-76 KO mice. Prior to functional experiments, we first compared the maturation of WT and SLP-76 KO NK cells to see if they were developmentally similar. There were no significant differences in the expression of early and late developmental markers19 between WT and SLP-76 KO splenic NK cells (supplemental Figure 1A). However, all Ly49 receptor family members examined were expressed on a significantly fewer percentage of SLP-76 KO NK cells when compared with WT NK cells (supplemental Figure 1B). This defect did not represent a global loss of receptor expression, as NK-cell lectin-like receptor 2A (NKG2A), another NK-cell inhibitory receptor, was not similarly affected in SLP-76 KO NK cells (supplemental Figure 1B). Therefore, although SLP-76 KO NK cells reach developmental maturity, they are unable to express a normal Ly49 receptor repertoire.
SLP-76 is necessary for optimal NK-cell function and proliferation downstream of activating receptors
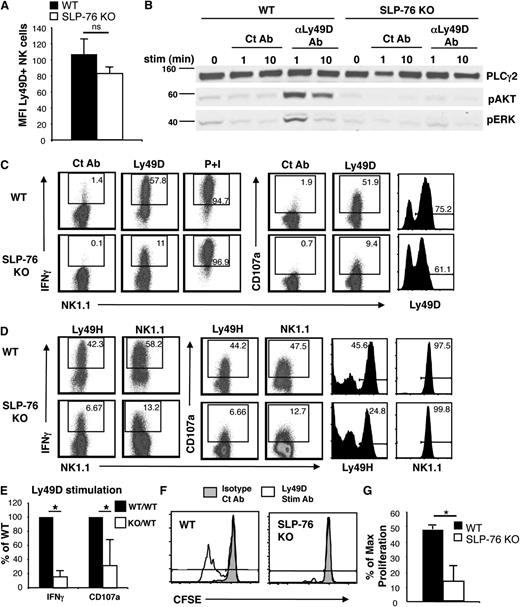
To test the importance of SLP-76 in downstream signaling of NK-cell immunoreceptors, we compared SLP-76 KO NK cells to WT NK cells after Ly49D stimulation. Because Ly49D was expressed on a lower percentage of SLP-76 KO NK cells, we first enriched for Ly49D-expressing NK cells before in vitro expansion for functional analysis. This resulted in high NK-cell purity and similar fractions of Ly49D-expressing NK cells of WT and SLP-76 KO origin. Ly49D+ NK cells showed slightly reduced levels of Ly49D expression by mean fluorescence intensity (MFI), although the Ly49D expression level between WT and SLP-76 KO NK cells was not significantly different (Figure 2A). WT and SLP-76 KO NK cells were then stimulated through Ly49D and assessed for phosphorylation of AKT and ERK, as these signaling molecules and their upstream activators, including phosphoinositide 3-kinase (PI3K), have been shown to be important for several NK-cell effector functions, including IFN-γ production and cytotoxicity.20-22 Upon Ly49D stimulation, AKT and ERK were phosphorylated in WT NK cells, but not in SLP-76 KO NK cells (Figure 2B), demonstrating that SLP-76 is necessary for activation of these important signaling molecules.
SLP-76 is necessary for optimal NK-cell function and proliferation downstream of Ly49D stimulation. Ly49D+ splenic NK cells from WT or SLP-76 KO mice were enriched and expanded in IL-2 for 6 days. (A) Expanded NK cells (CD3–NK1.1+ lymphocytes) were analyzed for the expression of Ly49D and represented as mean MFI ± standard error of the mean of 5 independent experiments. (B) Expanded NK cells were left unstimulated or stimulated with soluble isotype Ct Ab or anti-Ly49D Ab for 1 or 10 minutes and analyzed for total PLC-γ2 (loading control), pAKT, and pERK by western blot. One representative of 3 independent experiments is shown. (C) Expanded NK cells from WT or SLP-76 KO mice were stimulated with plate-immobilized Ct Ab, anti-Ly49D Ab, or soluble PMA/ionomycin for 4 hours in the presence of Monensin followed by detection of IFN-γ and CD107a expression by flow cytometry. Ly49D expression of NK cells at the time of stimulation is shown. All plots are gated on CD4–CD8–NK1.1+ lymphocytes. (D) Expanded NK cells from WT or SLP-76 KO mice were stimulated with plate-immobilized anti-Ly49H Ab or anti-NK1.1 Ab for 4 hours in the presence of Monensin followed by detection of IFN-γ and CD107a expression by flow cytometry. Ly49H and NK1.1 expression of NK cells at the time of stimulation is shown. All plots are gated on CD4–CD8–NK1.1+ lymphocytes. Dot plots are representative of 4 (Ly49H) or 2 (NK1.1) independent experiments. (E) Data compiled from 5 (IFN-γ) or 4 (CD107a) Ly49D stimulation independent experiments were normalized to the WT NK-cell response and expressed as mean ± standard error of the mean (SEM). (F) CFSE-labeled T-cell–depleted WT or SLP-76 KO splenocytes were stimulated with plate-immobilized control Ab or anti-Ly49D Ab in the presence of IL-2 (40 to 100 U/mL) for 3 days followed by flow cytometric analysis. Plots are gated on CD4–CD8–NK1.1+Ly49D+ NK cells. Cells to the left of gate have diluted CFSE and represent cells that have proliferated. One representative of 4 independent experiments is shown. (G) The percent of maximal proliferation induced by Ly49D stimulation is expressed as mean ± SEM of 4 independent experiments. * indicates statistical significance of P < .05 by paired t test; n.s., not significant.
SLP-76 is necessary for optimal NK-cell function and proliferation downstream of Ly49D stimulation. Ly49D+ splenic NK cells from WT or SLP-76 KO mice were enriched and expanded in IL-2 for 6 days. (A) Expanded NK cells (CD3–NK1.1+ lymphocytes) were analyzed for the expression of Ly49D and represented as mean MFI ± standard error of the mean of 5 independent experiments. (B) Expanded NK cells were left unstimulated or stimulated with soluble isotype Ct Ab or anti-Ly49D Ab for 1 or 10 minutes and analyzed for total PLC-γ2 (loading control), pAKT, and pERK by western blot. One representative of 3 independent experiments is shown. (C) Expanded NK cells from WT or SLP-76 KO mice were stimulated with plate-immobilized Ct Ab, anti-Ly49D Ab, or soluble PMA/ionomycin for 4 hours in the presence of Monensin followed by detection of IFN-γ and CD107a expression by flow cytometry. Ly49D expression of NK cells at the time of stimulation is shown. All plots are gated on CD4–CD8–NK1.1+ lymphocytes. (D) Expanded NK cells from WT or SLP-76 KO mice were stimulated with plate-immobilized anti-Ly49H Ab or anti-NK1.1 Ab for 4 hours in the presence of Monensin followed by detection of IFN-γ and CD107a expression by flow cytometry. Ly49H and NK1.1 expression of NK cells at the time of stimulation is shown. All plots are gated on CD4–CD8–NK1.1+ lymphocytes. Dot plots are representative of 4 (Ly49H) or 2 (NK1.1) independent experiments. (E) Data compiled from 5 (IFN-γ) or 4 (CD107a) Ly49D stimulation independent experiments were normalized to the WT NK-cell response and expressed as mean ± standard error of the mean (SEM). (F) CFSE-labeled T-cell–depleted WT or SLP-76 KO splenocytes were stimulated with plate-immobilized control Ab or anti-Ly49D Ab in the presence of IL-2 (40 to 100 U/mL) for 3 days followed by flow cytometric analysis. Plots are gated on CD4–CD8–NK1.1+Ly49D+ NK cells. Cells to the left of gate have diluted CFSE and represent cells that have proliferated. One representative of 4 independent experiments is shown. (G) The percent of maximal proliferation induced by Ly49D stimulation is expressed as mean ± SEM of 4 independent experiments. * indicates statistical significance of P < .05 by paired t test; n.s., not significant.
To examine whether these defects in signaling correlate with deficits in effector function, we tested the ability of Ly49D-activated NK cells to produce IFN-γ and upregulate CD107a on the cell surface, a marker of granule exocytosis and cytotoxic function. The proportion of NK cells expressing IFN-γ and surface-associated CD107a upon Ly49D stimulation was significantly reduced in SLP-76 KO NK cells compared with WT NK cells (Figure 2C). Similarly, SLP-76 KO NK cells displayed impaired IFN-γ production and CD107a expression when compared with WT NK cells following stimulation by other activating receptors, including Ly49H and NK1.1 (Figure 2D-E). When proximal signaling pathways were bypassed with PMA/ionomycin stimulation, both WT and SLP-76 KO NK cells produced similar levels of IFN-γ, suggesting there was no inherent defect in the ability of SLP-76 KO NK cells to produce IFN-γ (Figure 2C). Together, these results suggest that SLP-76 is necessary for maximal IFN-γ production and degranulation downstream of multiple ITAM-bearing activating receptors in NK cells.
NK cells expand during viral infections in response to activating receptor stimulation such as Ly49H.23 Because AKT and ERK are known to be important for cellular proliferation24 and the phosphorylation of these kinases were defective in SLP-76 KO NK cells, we determined whether activating receptor stimulation could enhance the proliferation of SLP-76 KO NK cells. In the presence of IL-2, Ly49D stimulation augmented the proliferation of WT NK cells. This enhancement in proliferation was significantly diminished in SLP-76 KO NK cells (Figure 2F,G). Thus, SLP-76 is necessary for maximal proliferation downstream of activating receptor stimulation in NK cells.
Functional deficiencies in SLP-76 KO NK cells are not due to developmental abnormalities
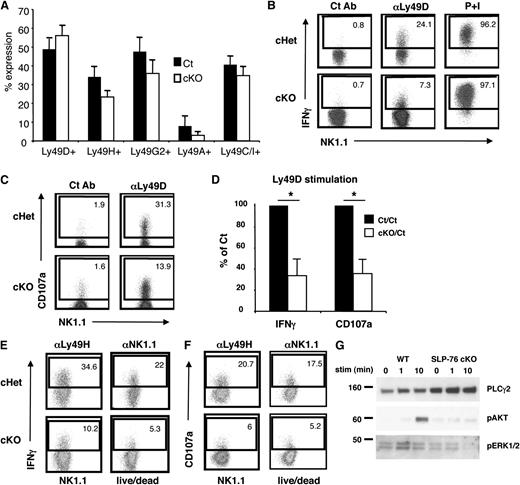
Because a defective Ly49 receptor repertoire may affect the ability of NK cells to respond appropriately to stimuli,25-27 we used SLP-76 cKO mice13 to inducibly excise a loxp-flanked allele of SLP-76 upon tamoxifen treatment in NK cells after normal development. Cells that no longer express SLP-76 were tracked using a Cre-inducible YFP reporter. After tamoxifen treatment, western blot analysis revealed that SLP-76 protein was not detected in FACS-sorted YFP-positive NK cells from tamoxifen-treated SLP-76 cKO mice, indicating successful deletion of SLP-76 in these NK cells (data not shown). Importantly, NK cells from tamoxifen-treated control (WT or cHet mice) and SLP-76 cKO mice displayed a similar Ly49 receptor repertoire (Figure 3A) and Ly49 receptor MFI (data not shown). Similar to germline SLP-76 KO mice, a significantly lower fraction of Ly49D-activated NK cells from SLP-76 cKO mice produced IFN-γ and expressed CD107a on the surface when compared with NK cells from control mice (Figure 3B-D). SLP-76 cKO NK cells also displayed impaired IFN-γ production and CD107a expression when compared to NK cells from control mice following stimulation by other activating receptors including Ly49H and NK1.1 (Figure 3E-F). In addition, we found that the phosphorylation of ERK and AKT were nearly abolished in Ly49D-activated NK cells from SLP-76 cKO mice compared with control mice (Figure 3G). These data suggest that the functional and biochemical deficiencies seen in SLP-76 KO NK cells appear independent of NK-cell developmental defects.
NK cells acutely depleted of SLP-76 display a normal Ly49 receptor repertoire but still exhibit functional defects. (A) Splenic NK cells (CD3–NK1.1+ lymphocytes) from control and SLP-76 cKO mice were analyzed for the expression of Ly49A, Ly49C/I, Ly49G2, Ly49D, and Ly49H and represented as mean percent positive ± SEM of 3 to 4 independent experiments. (B) Ly49D+ splenic NK cells from WT or SLP-76 cKO mice were enriched and expanded in IL-2 for 6 days. Expanded NK cells were stimulated by plate-bound isotype Ct Ab, anti-Ly49D Ab, or soluble PMA/ionomycin for 4 hours in the presence of Monensin followed by detection of IFN-γ and (C) CD107a expression by flow cytometry. Plots are gated on YFP+CD4–CD8–NK1.1+ lymphocytes. (D) Data compiled from 5 (IFN-γ) or 3 (CD107a) independent experiments were normalized to the control NK-cell response and expressed as mean ± SEM. * indicates statistical significance of P < .05 by paired t test. (E) The same NK cells wrtr stimulated with plate-bound anti-Ly49H or anti-NK1.1 antibody. IFN-γ and (F) CD107a surface expression was detected by flow cytometry. (G) The expanded NK cells were stimulated with soluble anti-Ly49D antibody for the indicated time and probed for pAKT and pERK1/2 by western blot analysis. PLCg2 served as the loading control. One representative of 2 independent experiments is shown.
NK cells acutely depleted of SLP-76 display a normal Ly49 receptor repertoire but still exhibit functional defects. (A) Splenic NK cells (CD3–NK1.1+ lymphocytes) from control and SLP-76 cKO mice were analyzed for the expression of Ly49A, Ly49C/I, Ly49G2, Ly49D, and Ly49H and represented as mean percent positive ± SEM of 3 to 4 independent experiments. (B) Ly49D+ splenic NK cells from WT or SLP-76 cKO mice were enriched and expanded in IL-2 for 6 days. Expanded NK cells were stimulated by plate-bound isotype Ct Ab, anti-Ly49D Ab, or soluble PMA/ionomycin for 4 hours in the presence of Monensin followed by detection of IFN-γ and (C) CD107a expression by flow cytometry. Plots are gated on YFP+CD4–CD8–NK1.1+ lymphocytes. (D) Data compiled from 5 (IFN-γ) or 3 (CD107a) independent experiments were normalized to the control NK-cell response and expressed as mean ± SEM. * indicates statistical significance of P < .05 by paired t test. (E) The same NK cells wrtr stimulated with plate-bound anti-Ly49H or anti-NK1.1 antibody. IFN-γ and (F) CD107a surface expression was detected by flow cytometry. (G) The expanded NK cells were stimulated with soluble anti-Ly49D antibody for the indicated time and probed for pAKT and pERK1/2 by western blot analysis. PLCg2 served as the loading control. One representative of 2 independent experiments is shown.
SLP-76 KO NK cells cannot prevent tumor engraftment
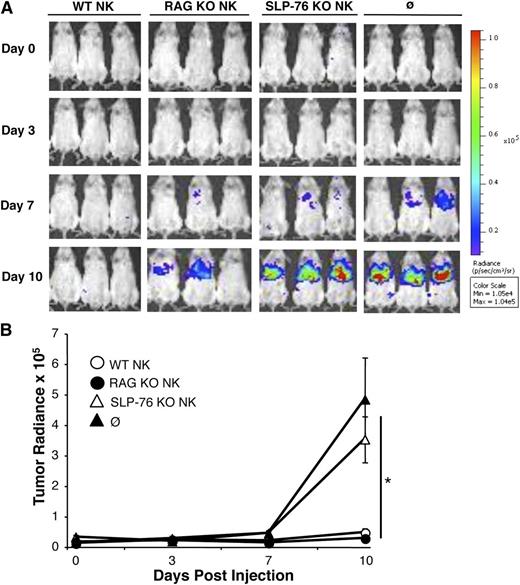
Next we tested the ability of SLP-76 KO NK cells to function in vivo using a CHO tumor cell model, as murine NK cells kill CHO cells via a Ly49D-dependent mechanism.28 NSG mice, which have no B, T, or functional NK cells,29 were injected with luciferase-expressing CHO cells followed by injection of saline or Ly49D-enriched IL-2–expanded NK cells derived from WT, RAG KO, or SLP-76 KO mice. As SLP-76 KO mice do not have T cells, RAG KO NK cells were used to control for potential T-cell contamination that may be present in the WT NK-cell preparation. Ten days after tumor challenge, SLP-76 KO NK-cell–injected mice had similar tumor radiance compared with the saline-injected group, which was significantly higher than WT or RAG KO NK-cell–injected mice (Figure 4A,B). Fewer Ly49D+ NK cells were recovered from the spleen and liver of mice injected with SLP-76 KO NK cells when compared with WT or RAG KO NK-cell–injected mice, suggesting that SLP-76 KO NK cells display decreased expansion in response to CHO cells in vivo (data not shown). These data suggest that SLP-76 KO NK cells display defective clearance of tumor cells in vivo due to diminished ITAM-mediated proliferation and/or effector function.
SLP-76 KO NK cells cannot prevent tumor engraftment in vivo. Ly49D+ splenic NK cells from WT, SLP-76 KO, or RAG KO mice were enriched and expanded in IL-2 for 6 days. Luciferase-expressing CHO cells were injected into NSG mice followed by injection of PBS (∅) or the indicated NK-cell type. (A) Images were taken on days 0, 3, 7, and 10. One representative of 3 independent experiments is shown. (B) Average tumor radiance data compiled from all experiments (n = 6 for WT, n = 7 for RAG KO, n = 10 for SLP-76 KO, and n = 9 for PBS) are expressed as mean ± standard error of the mean. * indicates significance of P < .05 for WT vs SLP-76 KO, WT vs PBS, RAG KO vs SLP-76 KO, and RAG KO vs PBS.
SLP-76 KO NK cells cannot prevent tumor engraftment in vivo. Ly49D+ splenic NK cells from WT, SLP-76 KO, or RAG KO mice were enriched and expanded in IL-2 for 6 days. Luciferase-expressing CHO cells were injected into NSG mice followed by injection of PBS (∅) or the indicated NK-cell type. (A) Images were taken on days 0, 3, 7, and 10. One representative of 3 independent experiments is shown. (B) Average tumor radiance data compiled from all experiments (n = 6 for WT, n = 7 for RAG KO, n = 10 for SLP-76 KO, and n = 9 for PBS) are expressed as mean ± standard error of the mean. * indicates significance of P < .05 for WT vs SLP-76 KO, WT vs PBS, RAG KO vs SLP-76 KO, and RAG KO vs PBS.
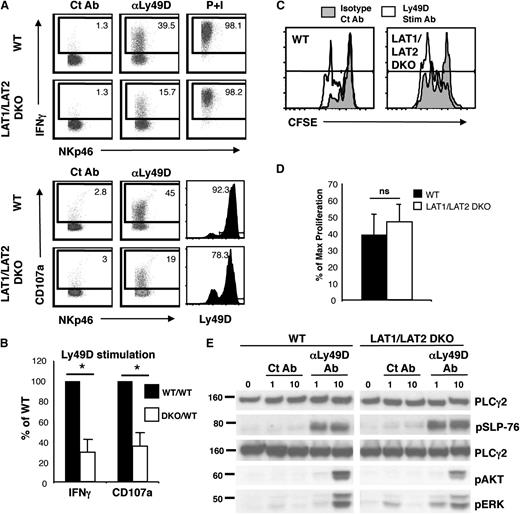
LAT1/LAT2 are required for IFN-γ production and cytotoxicity but are dispensable for NK-cell proliferation and for phosphorylation of AKT and ERK
In T cells and mast cells, LAT family members are responsible for forming a multimolecular complex with SLP-76 downstream of ITAM-bearing receptors.5 Thus, ITAM-mediated activation of SLP-76 KO and LAT1/LAT2 DKO mast cells are similarly defective.7 To determine whether this is also true in NK cells, the effector function of LAT1/LAT2 DKO NK cells was examined. As expected, LAT1/LAT2 DKO NK cells exhibited defects in the expression of IFN-γ and cell surface CD107a after stimulation with Ly49D, similar to that seen in SLP-76 KO NK cells (Figure 5A,B). Surprisingly, however, no significant differences were seen in Ly49D-enhanced proliferation of WT and LAT1/LAT2 DKO NK cells (Figure 5C,D). Because AKT and ERK are responsible for cellular proliferation and survival, we determined whether the activation of these molecules was also retained in LAT1/LAT2 DKO NK cells. Upon stimulation with Ly49D, the phosphorylation of AKT and ERK was partially retained in the absence of LAT1/LAT2 in NK cells (Figure 5E). In contrast, phosphorylation of AKT and ERK was completely absent in SLP-76 KO NK cells (Figure 2B). These data suggest that NK cells possess a novel SLP-76–dependent but LAT1/LAT2–independent signaling pathway that leads to phosphorylation of AKT and ERK and to NK-cell proliferation.
LAT1/LAT2 are required for IFN-γ production and cytotoxicity but are dispensable for phosphorylation of SLP-76, AKT, and ERK and for NK-cell proliferation. Ly49D+ splenic NK cells from WT and LAT1/LAT2 DKO mice were enriched and expanded in IL-2 for 6 days. (A) Expanded NK cells were stimulated with plate-bound isotype Ct Ab, anti-Ly49D Ab, or soluble PMA/ionomycin for 4 hours in the presence of Monensin followed by detection of IFN-γ and LAMP-1 expression by flow cytometry. Ly49D expression of cells at time of stimulation is shown. Plots are gated on CD4–CD8–NK1.1+ lymphocytes. (B) Data compiled from 3 independent experiments were normalized to the control NK-cell response and expressed as mean ± standard error of the mean (SEM). (C) CFSE-labeled T-cell–depleted WT or LAT1/LAT2 DKO splenocytes were stimulated with plate-bound control Ab or anti-Ly49D Ab in the presence of IL-2 (40 to 100 U/ml) for 3 days followed by flow cytometric analysis. Plots are gated on CD4–CD8–NK1.1+Ly49D+ NK cells. (D) The percent of maximal proliferation induced by Ly49D stimulation was calculated from 4 independent experiments and expressed as mean ± SEM. (E) Ly49D-enriched IL-2–expanded NK cells were left unstimulated or stimulated with Ct Ab or anti-Ly49D Ab for 1 or 10 minutes and analyzed for PLC-γ2 (loading control), pSLP-76, pAKT, and pERK. One representative of 3 to 6 independent experiments is shown. * indicates statistical significance of P < .05 by paired t test; n.s., not significant.
LAT1/LAT2 are required for IFN-γ production and cytotoxicity but are dispensable for phosphorylation of SLP-76, AKT, and ERK and for NK-cell proliferation. Ly49D+ splenic NK cells from WT and LAT1/LAT2 DKO mice were enriched and expanded in IL-2 for 6 days. (A) Expanded NK cells were stimulated with plate-bound isotype Ct Ab, anti-Ly49D Ab, or soluble PMA/ionomycin for 4 hours in the presence of Monensin followed by detection of IFN-γ and LAMP-1 expression by flow cytometry. Ly49D expression of cells at time of stimulation is shown. Plots are gated on CD4–CD8–NK1.1+ lymphocytes. (B) Data compiled from 3 independent experiments were normalized to the control NK-cell response and expressed as mean ± standard error of the mean (SEM). (C) CFSE-labeled T-cell–depleted WT or LAT1/LAT2 DKO splenocytes were stimulated with plate-bound control Ab or anti-Ly49D Ab in the presence of IL-2 (40 to 100 U/ml) for 3 days followed by flow cytometric analysis. Plots are gated on CD4–CD8–NK1.1+Ly49D+ NK cells. (D) The percent of maximal proliferation induced by Ly49D stimulation was calculated from 4 independent experiments and expressed as mean ± SEM. (E) Ly49D-enriched IL-2–expanded NK cells were left unstimulated or stimulated with Ct Ab or anti-Ly49D Ab for 1 or 10 minutes and analyzed for PLC-γ2 (loading control), pSLP-76, pAKT, and pERK. One representative of 3 to 6 independent experiments is shown. * indicates statistical significance of P < .05 by paired t test; n.s., not significant.
Membrane recruitment and phosphorylation of SLP-76 after Ly49D stimulation can occur independently of LAT1/LAT2 in NK cells
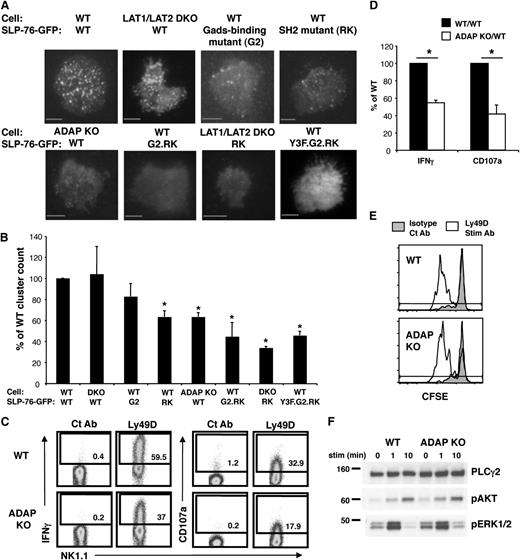
The presence of a SLP-76–dependent but LAT1/LAT2–independent signaling pathway in Ly49D-activated NK cells suggests that SLP-76 membrane recruitment and subsequent phosphorylation might occur in the absence of LAT1/LAT2. Thus, we examined Ly49D-induced phosphorylation and membrane localization of SLP-76 in WT vs LAT1/LAT2 DKO NK cells. Upon stimulation through Ly49D, we found that SLP-76 was phosphorylated normally (Figure 5E) and recruited to the surface independently of LAT1/LAT2 (Figure 6A,B). As a complementary approach, we used a Gads-binding mutant of SLP-76 (SLP-76.G2), which prevents the association of SLP-76 with LAT family members.17 Similar to results obtained with LAT1/LAT2 DKO NK cells, GFP-tagged SLP-76.G2 was recruited normally to the NK surface, forming microclusters following Ly49D stimulation (Figure 6A,B). Together, these data suggest that SLP-76 localization to the NK surface can occur independently of LAT family members.
The membrane recruitment of SLP-76 can occur independently of LAT1/LAT2 in NK cells. IL-2–expanded Ly49D-enriched WT or LAT/LAT2 DKO NK cells expressing GFP-tagged SLP-76, SLP-76.G2, SLP-76.RK, or SLP-76 G2.RK mutant were stimulated on an anti-Ly49D Ab-coated surface. (A) Clustering of SLP-76 at the plasma membrane was imaged by TIRF microscopy. (B) Summary of TIRF data represented as mean ± SEM of WT clustering in 3-8 independent experiments. Scale bar, 5 μm. (C) Ly49D+ splenic NK cells from WT or ADAP KO mice were enriched and expanded in IL-2 for 6 days. Expanded NK cells from WT or ADAP KO mice were stimulated with plate-immobilized Ct Ab or anti-Ly49D Ab for 4 hours in the presence of Monensin followed by detection of IFN and CD107a expression by flow cytometry. Ly49D expression of NK cells at the time of stimulation is shown. All plots are gated on CD4–CD8–NK1.1+ lymphocytes. (D) Data compiled from 3 Ly49D stimulation experiments were normalized to the WT NK cell response and expressed as mean ± SEM. (E) CFSE-labeled T cell-depleted WT or ADAP KO splenocytes were stimulated with plate-immobilized control Ab or anti-Ly49D Ab in the presence of 25 U/mL IL-2 for 3 days followed by flow cytometric analysis. Plots are gated on CD4–CD8–NK1.1+Ly49D+ NK cells. Cells to the left of gate have diluted CFSE and represent cells that have proliferated. One representative of 2 independent experiments is shown. (F) Expanded splenic NK cells were left unstimulated or stimulated with soluble anti-Ly49D Ab for 1 or 10 minutes and analyzed for total PLCγ2 (loading control), pAKT, and pERK1/2 by western blot. One representative of 2 independent experiments is shown. *P < .03 by t-test.
The membrane recruitment of SLP-76 can occur independently of LAT1/LAT2 in NK cells. IL-2–expanded Ly49D-enriched WT or LAT/LAT2 DKO NK cells expressing GFP-tagged SLP-76, SLP-76.G2, SLP-76.RK, or SLP-76 G2.RK mutant were stimulated on an anti-Ly49D Ab-coated surface. (A) Clustering of SLP-76 at the plasma membrane was imaged by TIRF microscopy. (B) Summary of TIRF data represented as mean ± SEM of WT clustering in 3-8 independent experiments. Scale bar, 5 μm. (C) Ly49D+ splenic NK cells from WT or ADAP KO mice were enriched and expanded in IL-2 for 6 days. Expanded NK cells from WT or ADAP KO mice were stimulated with plate-immobilized Ct Ab or anti-Ly49D Ab for 4 hours in the presence of Monensin followed by detection of IFN and CD107a expression by flow cytometry. Ly49D expression of NK cells at the time of stimulation is shown. All plots are gated on CD4–CD8–NK1.1+ lymphocytes. (D) Data compiled from 3 Ly49D stimulation experiments were normalized to the WT NK cell response and expressed as mean ± SEM. (E) CFSE-labeled T cell-depleted WT or ADAP KO splenocytes were stimulated with plate-immobilized control Ab or anti-Ly49D Ab in the presence of 25 U/mL IL-2 for 3 days followed by flow cytometric analysis. Plots are gated on CD4–CD8–NK1.1+Ly49D+ NK cells. Cells to the left of gate have diluted CFSE and represent cells that have proliferated. One representative of 2 independent experiments is shown. (F) Expanded splenic NK cells were left unstimulated or stimulated with soluble anti-Ly49D Ab for 1 or 10 minutes and analyzed for total PLCγ2 (loading control), pAKT, and pERK1/2 by western blot. One representative of 2 independent experiments is shown. *P < .03 by t-test.
The SH2 and Gads-binding domains of SLP-76 contribute to SLP-76 localization to the plasma membrane following Ly49D stimulation
To investigate how SLP-76 is recruited to the plasma membrane to form microclusters in NK cells independently of LAT1/LAT2, we examined the localization of several mutant forms of SLP-76. First we tested an SH2 domain mutant of SLP-76 (SLP-76.RK), which prevents the association of SLP-76 with ADAP.18 Post Ly49D stimulation, SLP-76.RK was recruited at mildly but significantly reduced levels compared with WT SLP-76 at the NK surface (Figure 6A,B), suggesting that the SH2 domain is partially responsible for SLP-76 membrane recruitment in NK cells. Consistent with this observation, we found that SLP-76 recruitment to the plasma membrane was similarly diminished in ADAP KO NK cells (Figure 6A,B).
Because the residual SLP-76 microcluster formation in ADAP KO NK cells might be due to localization by LAT1/LAT2 through the Gads-binding domain of SLP-76, we constructed a SLP-76.G2.RK double mutant, where both the Gads-binding domain and the SH2 domain were made nonfunctional. This double mutant formed significantly fewer clusters at the NK-cell membrane compared with the single mutants (Figure 6A,B), suggesting that either the Gads-binding domain or the SH2 domain could be used for SLP-76 recruitment to the NK-cell membrane. As a complementary approach, we expressed GFP-tagged SLP-76.RK in NK cells lacking LAT1/LAT2. We found that the clustering of SLP-76.RK in LAT1/LAT2 DKO NK cells was reduced to levels seen with the SLP-76.G2.RK double mutant, suggesting that LAT1/LAT2 were the responsible upstream molecules in SLP-76 localization to the plasma membrane via the Gads-binding domain of SLP-76 (Figure 6A,B). Since the SLP-76.G2/RK mutant still displayed some residual clustering at the cell surface, we also tested the role of the N-terminal tyrosines, which could associate with DAP10 via Vav, in SLP-76 recruitment. SLP-76 with 3 mutated tyrosines (SLP-76.Y3F) alone or in conjunction with G2/RK mutation did not further diminish SLP-76 clustering, suggesting that the N-terminal tyrosines are not required for SLP-76 recruitment in immunoreceptor-activated NK cells (Figure 6A-B). (See supplemental representative movies of cluster formation for all conditions.)
Given that ADAP was involved in SLP-76 membrane localization, we tested the function of ADAP KO NK cells. Compared with WT NK cells, we found that ADAP KO NK cells displayed diminished IFN-γ and degranulation responses (Figure 6C-D). However, similar to LAT1/LAT2 DKO NK cells, the proliferation of NK cells (Figure 6E) and the phosphorylation of AKT and ERK were intact in ADAP KO NK cells (Figure 6F). These data suggest that although both pathways are required for IFN-γ production and degranulation, either LAT1/LAT2 or ADAP-mediated localization of SLP-76 is sufficient to maintain immunoreceptor-induced NK-cell proliferation and AKT and ERK phosphorylation.
Discussion
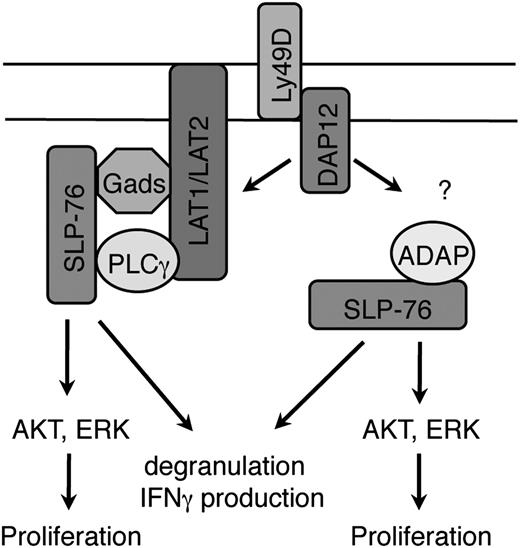
We have demonstrated that the adaptor molecule SLP-76 is critical for signaling downstream of ITAM-bearing activating receptors in NK cells. The lack of SLP-76–mediated signaling in NK cells hampers multiple effector functions downstream of these receptors, including IFN-γ production, cytotoxicity (degranulation), and proliferation in vitro and prevention of tumor engraftment in vivo. The reduced function of SLP-76 KO NK cells was not due to altered development, as NK cells inducibly deleted of SLP-76 after normal development were also similarly defective. Surprisingly, our experiments unraveled 2 distinct signaling pathways involving SLP-76 downstream of NK-cell activation, 1 using LAT family members and the other involving ADAP. Both pathways were required for optimal IFN-γ production and cytotoxicity. However, each pathway alone was sufficient to maintain other SLP-76–dependent events, including AKT and ERK phosphorylation, and for enhancing NK-cell proliferation. Together, these results demonstrate that NK cells possess an unexpected bifurcation of proximal ITAM-mediated signaling involving SLP-76, each contributing to optimal NK-cell function (Figure 7).
SLP-76–dependent signaling pathways in NK cells stimulated through ITAM-bearing receptors. SLP-76 is recruited to the plasma membrane by LAT1/LAT2 indirectly through Gads in the canonical signaling pathway. In addition, the SH2 domain of SLP-76 is necessary for recruitment via ADAP to the plasma membrane through an unknown mechanism. The strength of downstream signals generated by the LAT family-dependent or ADAP-dependent SLP-76 pathway alone is sufficient for phosphorylation of AKT and ERK and for NK cell proliferation. However, both pathways are necessary for optimal IFN-γ production and cytotoxicity. Although depicted in the figure, note only LAT1, not LAT2, cooperatively binds PLC with SLP-76.
SLP-76–dependent signaling pathways in NK cells stimulated through ITAM-bearing receptors. SLP-76 is recruited to the plasma membrane by LAT1/LAT2 indirectly through Gads in the canonical signaling pathway. In addition, the SH2 domain of SLP-76 is necessary for recruitment via ADAP to the plasma membrane through an unknown mechanism. The strength of downstream signals generated by the LAT family-dependent or ADAP-dependent SLP-76 pathway alone is sufficient for phosphorylation of AKT and ERK and for NK cell proliferation. However, both pathways are necessary for optimal IFN-γ production and cytotoxicity. Although depicted in the figure, note only LAT1, not LAT2, cooperatively binds PLC with SLP-76.
In other hematopoietic cell types such as T cells and mast cells, SLP-76 is recruited to the cell surface via LAT family members, where a multimolecular signaling complex is formed, allowing for downstream signaling events. Although the same mechanism for SLP-76 recruitment and phosphorylation was expected in NK cells, we unexpectedly found that LAT1/LAT2 DKO NK cells displayed intact SLP-76 microcluster formation and phosphorylation. As SLP-76 is recruited indirectly to LAT family members through Gads, these findings were corroborated by the observation that SLP-76 with a mutation in the Gads-binding domain also localized normally upon Ly49D stimulation. Subsequently, we found that membrane localization of SLP-76 was mildly reduced when harboring an SH2 domain mutation or when placed in ADAP KO NK cells. SLP-76 clustering was markedly reduced when containing mutations in both the SH2 and Gads-binding domain. These data suggest that SLP-76 could be recruited to the membrane by either LAT family members or via ADAP though its SH2 domain.
The 2 distinct SLP-76–dependent proximal signaling pathways both contribute to NK-cell activation. The degranulation and IFN-γ production by NK cells from SLP-76 KO, LAT1/LAT2 DKO, and ADAP KO mice were defective, suggesting that the interaction of either LAT1/LAT2 or ADAP with SLP-76 is necessary for these NK-cell effector functions. In contrast, LAT1/LAT2 DKO and ADAP KO but not SLP-76 KO NK cells displayed normal Ly49D-enhanced proliferation, suggesting that either the LAT family-dependent or ADAP-dependent SLP-76 signaling pathway is sufficient to enhance NK-cell proliferation through ITAM-bearing activating receptors. The intact proliferative response of LAT1/LAT2 DKO and ADAP KO NK cells might be related to preserved AKT and ERK phosphorylation in these cells, as AKT and ERK phosphorylation was completely abrogated in SLP-76 KO NK cells but not in LAT1/LAT2 DKO or ADAP KO NK cells. These results suggest that the strength of downstream signals generated by the LAT family-dependent or ADAP-dependent SLP-76 pathway alone might be sufficient to enhance NK-cell proliferation, while both pathways are necessary for optimal IFN-γ production and cytotoxicity. A previous report has suggested that ADAP is not necessary for NK cell function.30 However, our results clearly show a role for ADAP in achieving maximal NK cell immunoreceptor signaling.
To our knowledge, we are the first to demonstrate that 2 distinct domains of SLP-76 can mediate SLP-76 recruitment to proximal signaling complexes downstream of a single ITAM-bearing receptor. However, it is possible that this mechanism is not unique to NK cells. TCR stimulation of T cells lacking LAT1 results in the phosphorylation of SLP-76,31 suggesting that T cells may also possess a LAT1-independent mechanism of SLP-76 recruitment and phosphorylation. Like mast cells, this may be explained by LAT2 expression in a subset of activated T cells.32 However, quantitative polymerase chain reaction results have shown that LAT2 is expressed at very low levels in T cells.33 Thus, it would be interesting to test whether SLP-76 is recruited in TCR-stimulated T cells lacking both LAT1 and LAT2.
In addition to their attenuated effector function, SLP-76 KO NK cells displayed a striking defect in their ability to express the Ly49 family of receptors. These data suggest that a signal generated through an ITAM-bearing receptor is necessary for acquisition of inhibitory Ly49 receptor family members on NK cells during development. This notion is concordant with data from mice lacking various other signaling molecules, although the Ly49 receptor repertoire defects in these mouse models are not as severe.10,34-39 Thus, SLP-76 may play a central role in orchestrating the positive signals necessary for Ly49 receptor induction. Interestingly, the Ly49 receptor repertoire of LAT1/LAT2 DKO and ADAP KO NK cells is only slightly defective (R.M.M., unpublished observations), suggesting that both pathways contribute to SLP-76–mediated Ly49 receptor acquisition.
NK-cell–activating receptors do not contain intrinsic signaling ability and transmit their signals by associating with other signaling adaptors. Ly49D associates with the ITAM-containing adaptor protein DAP12. In addition to DAP12, Ly49D can also associate with DNAX-activation protein 10 (DAP10), which contains a YINM motif rather than an ITAM and signals though PI3K or Grb2.40 Given that SLP-76 can be phosphorylated post stimulation through DAP10,41 it is potentially possible that SLP-76 is recruited in a LAT family–independent manner after Ly49D stimulation because of the presence of both Ly49D-associated DAP12 and DAP10. Thus, the residual SLP-76 surface clustering seen with the G2.RK SLP-76 double mutant could be due to its association with DAP10 through Vav.41 However, we believe that this scenario is unlikely as DAP10-mediated signaling appears to play a minimal role in Ly49D-mediated activation.40 Moreover, our data show that the association of N-terminal tyrosines with Vav is not required for SLP-76 clustering. Furthermore, SLP-76 recruitment to the plasma membrane was found to be intact in NK cells expressing a Gads-binding mutant of SLP-76 when stimulated through another ITAM-bearing receptor, NK1.1 (data not shown). NK1.1 only associates with the ITAM-bearing adaptor FcRγ,42 which suggests that SLP-76 can be localized in a LAT1/LAT2–independent manner downstream of an ITAM alone. Thus, we favor the notion that SLP-76 is recruited differentially through its Gads-binding and SH2 domain by ITAM-bearing receptors in NK cells.
In conclusion, we have found that ITAM-based signals lead to the recruitment of SLP-76 to microclusters at the cellular membrane via at least 2 distinct mechanisms in NK cells. The canonical pathway utilizes LAT family members for SLP-76 recruitment, while the alternate pathway relies upon ADAP and the SH2 domain of SLP-76. We have shown that both pathways contribute to the optimal function of NK cells. Further investigation is required to decipher the exact downstream signaling pathways that result from these alternate mechanisms of recruitment. A complete understanding of these signaling pathways will be necessary for therapeutic manipulation of NK cells to fight viral infections and cancer.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Gary Koretzky, Sheila Rao, Martha Jordan, and Jiyeon Kim for careful reading of the manuscript; and the Koretzky, Nichols, Behrens, and Kambayashi Labs for support and helpful discussions.
This work was supported by grants from the National Blood Foundation, American Society of Hematology, the University of Pennsylvania internal funds, Innovation Award from the University of Pennsylvania Nano/Bio Interface, and the National Institutes of Health (R01HL107589, R01HL111501, K08HL086503, T32AR007442, R01AI067946, R01HL089745, R21AI073409).
Authorship
Contribution: R.M.M., M.O., C.-J.H., H.B., E.Y., G.R., E.M.M., N.H.P., and T.K. performed experiments; W.Z., T.B., J.S.O., and K.E.N. provided reagents and equipment; and R.M.M. and T.K. designed research, analyzed data, and prepared the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Taku Kambayashi, 288 John Morgan Building, 3620 Hamilton Walk, Philadelphia, PA, 19104; e-mail: taku.kambayashi@uphs.upenn.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal