Key Points
The expression of CD16 by immature slanDCs equips these cells with a unique capacity to handle immune complexes.
CD16 expression on slanDCs is rapidly downregulated during maturation by activation of ADAM10 and ADAM17.
Abstract
Binding and uptake of immune complexes (ICs) via low-affinity Fcγ receptors (FcγRs) on dendritic cells (DCs) is well known as a booster of immune responses. It can be helpful when stimulating immunity against pathogenic microbes but may be harmful when antibodies form complexes with autologous antigens. To date, no human DC subtype specialized in handling ICs has been identified. By incubating human blood mononuclear cells with ICs and studying their cellular binding, we identified 6-sulfo LacNAc-expressing DCs (slanDCs) as having an outstanding capacity to bind ICs compared with other myeloid DCs, plasmacytoid DCs, or monocytes. Using selective blocking of different (FcγRs), we identified CD16 (FcγRIII) as the major IC-binding structure on slanDCs. In addition, CD16 proved critical for phagocytosis of IgG-coated erythrocytes, and CD16-targeted antigen led to a more efficient proliferation of CD4+ T cells than CD32 (FcγRII)-targeted antigen. Interestingly, these CD16-mediated functions are short-lived and restricted to the immature stage of slanDCs in blood. We show that CD16 is rapidly shed from the surface of maturing slanDCs, resulting from the combined action of the metalloproteinases ADAM10 and ADAM17. In conclusion, these data provide strong evidence that slanDCs play an important role in IC-driven immune responses.
Introduction
Human dendritic cells (DCs) comprise a group of phenotypically and functionally distinct MHC class II–positive cells that are differentially distributed throughout the body and also circulate in the blood.1,2 DCs display a powerful ability to receive and integrate signals of the innate and the adaptive arm of the immune system and are therefore regarded as central regulators of immunity. In general, DCs can be divided into myeloid and plasmacytoid DCs, both of which are further split into phenotypically and functionally diverging cell populations.3,4 Among the phenotypic differences of DC subsets, the differential equipment with Fcγ receptors (FcγRs) provides DC subtypes with specific qualitative and quantitative sensors for the detection of either monomeric or complexed immunoglobulin.5,6
In general, three main subclasses of human FcγRs are distinguished whose members are broadly and differentially expressed on hematopoietic cells. CD16 (FcγRIII) and CD32 (FcγRII) display low to medium affinity and mediate signaling after cross-linking of the receptors on the cell surface, whereas CD64 is a receptor for monomeric IgG with high affinity.7 The human FcγRs include both activating and inhibitory receptors. Downstream signaling via immunoreceptor tyrosine–based activation motifs and immunoreceptor tyrosine–based inhibition motifs integrates signals from other receptor systems such as complement or toll-like receptors (TLRs), which together play a fundamental role in immune regulation.8,9
Antigen-antibody or immune complex (IC) formation is a natural process that not only occurs during disease but also occurs constantly in health. Binding of ICs to Fc receptors on DCs can lead to IC internalization and activation of the DCs. Therefore, ICs may well account for the perpetuation of inflammatory responses.10 For example, it has been shown that autoantibodies in human lupus erythematosus and in mouse models of the disease are directed against nuclear antigens, which leads to the formation of autoimmune complexes containing ssRNA and DNA.11 Such autoimmune complexes stimulate DCs, which consequently drive pathogenic T-cell responses.12
Human CD1c+ DCs express CD32a/b and CD64,13,14 and in various studies the implications of both receptors for antigen uptake and presentation have been demonstrated.6 The rare subset of human CD141+ myeloid DCs also expresses CD32a, and its functionality for enhancing cross-presentation has recently been shown.15 The FcγR expression by plasmacytoid DCs is restricted and limited to a low expression of CD32a,16 and there is no concomitant expression of the inhibitory CD32b.14 Although expressed at only low levels, CD32a on pDCs is functional and can contribute to antigen presentation.16,17 Human 6-sulfo LacNAc-expressing DCs (slanDCs) are a highly proinflammatory subpopulation of human DCs characterized by their expression of the carbohydrate modification 6-sulfo LacNAc on PSGL-1.18,19 Like CD1c+ DCs and pDCs, slanDCs express CD32. However, in contrast to both CD1c+ DCs and pDCs, slanDCs additionally and strongly express the low- to medium-affinity FcγR CD16. Little is known about the functional relevance of this protein on slanDCs. One previous study has described the induction of a potent antibody-dependent cell-mediated cytotoxicity by slanDCs; however, this was mediated through CD16 and CD32.20
In this paper, we show that a population of human DCs exists that is specialized in handling IgG-ICs. We recognized that coexpression of CD16 and CD32 only on one subset of human blood DCs mediated an outstanding IC-binding capacity. We further show that expression of CD16 on this DC subset broadly affects its immune-related functions.
Materials and methods
Human ethics
This study was approved by the Institutional Review Board of the University of Heidelberg, Germany. Healthy donors and patients gave informed consent in accordance with the ethics principles stated in the Declaration of Helsinki.
Flow cytometry and cell separation
Peripheral blood mononuclear cells (PBMCs) were isolated from buffy coats of healthy donors and blood samples of a patient with paroxysmal nocturnal hemoglobinuria (PNH) by density-gradient centrifugation over Ficoll (Biochrom, Berlin, Germany). Flowcytometric identification of cell types in PBMCs was as follows: slanDCs by positive staining for slan (clone M-DC8), monocytes by positive staining for CD14 (ImmunoTools, Friesoythe, Germany), CD1c+ DCs by negative staining for CD19 (BD, Heidelberg, Germany) and positive staining for CD1c (Miltenyi, Bergisch Gladbach, Germany), and pDCs by positive staining for CD123 and BDCA2 (Miltenyi). For flow cytometric measurement of IC binding, mDCs and pDCs were identified by negative staining for slan, CD3, CD14, CD16, CD19, CD34, CD56; positive staining for HLA-DR; and positive (mDCs) or negative (pDCs) staining for CD11c (all BD). Additional antibodies were specific for ADAM10 (Abcam, Cambridge, UK), ADAM17 (R&D, Minneapolis, MN), fluorescein isothiocyanate (FITC) (Acris Antibodies; Herford, Germany), CD16b (Beckman Coulter, Krefeld, Germany), CD32, CD64 (BioLegend, San Diego, CA), CD32b (clone 2B6, kind gift of J.V. Ravetch, New York, USA), CD55, CD83, and CD86 (BD Biosciences).
Magnetic cell sorting of slanDCs was carried out as described previously.21 Briefly, PBMCs were incubated in 1:20 M-DC8 hybridoma supernatant (1 × 108 PBMCs/400 µL, stock concentration 10 μg/mL) for 15 minutes at 4°C, then washed and incubated with 0.5 × 107 PBMCs/µL anti-mouse-IgM microbeads (Miltenyi) for 15 minutes at 4°C. slanDCs were isolated by positive selection (purity ≥94%) using an autoMACS Pro Separator (Miltenyi). Isolation of monocytes was performed using CD14 microbeads (Miltenyi) according to the manufacturer’s recommendations (purity ≥97%). Flow-cytometric analysis of granulocytes was performed after staining of whole blood and subsequent hypotonic lysis of erythrocytes. Flow cytometric analysis was performed using a FACScan or FACSCanto (BD Biosciences).
Cell culture
Cells were cultured in complete RPMI (cRPMI, RPMI 1640 supplemented with 2 mM l-glutamine, 1% nonessential amino acids, 100 U/mL penicillin, and 100 µg/mL streptomycin [Biochrom]) with 10% heat-inactivated pooled human serum (PHS) (CCPro, Neustadt, Germany). Cell culture of slanDCs in Figure 5D was done in cRPMI with 0.125% PHS. During inhibition experiments, slanDCs were cultured in the presence of Ro327315, Ro327066, Ro4002855, Ro282653 (Dr. H.-W. Krell, Roche, Grenzach-Wyhlen, Germany), GW280264x, GI254023x, and dimethyl sulfoxide (DMSO) (Sigma-Aldrich, St. Louis, MO).
IC binding and phagocytosis assays
ICs were prepared by incubating FITC-labeled human serum albumin (HSA; Abcam)—conjugated with 2.6 moles FITC per mol HSA—with polyclonal anti-FITC (rabbit IgG22 ; Acris Antibodies) at a ratio of 1: 6. For comparative IC binding of DC subtypes and monocytes, PBMCs were incubated with ICs for 15 minutes at 4°C and then washed in phosphate-buffered saline (PBS)/2 mM Ethylenediaminetetraacetic acid (EDTA). Receptor-specific IC binding was achieved in the presence of 25 µg/mL blocking mAbs for CD16 (clone 3G8), CD32 (clone AT10), or a CD8 mAb (clone RPA-T8) as control. In Figure 5D, purified slanDCs were first cultured for 8 hours, then washed twice in PBS/2 mM EDTA and then incubated with diluted ICs for 15 minutes at 4°C, followed by washing in PBS/2 mM EDTA. IC binding was quantified by flow cytometry after cell type–specific staining.
Internalization of fluorescent latex beads (Life Technologies, Carlsbad, CA) was studied by incubating 10 to 90 × 107 IgG-coated or untreated latex beads/mL with 50 × 106 PBMCs/mL in cRPMI with 10% PHS for 30 min at 37°C. Thereafter, all samples were washed with lactic acid buffer (pH = 4.5) to release cell surface–bound latex beads. Uptake of latex beads was quantified by flow cytometry.
The phagocytic capacity of slanDCs and monocytes was studied as described in Salmon et al.23 Briefly, 5 × 105 MACS-purified slanDCs or monocytes were co-cultured for 30 minutes with 1.25 × 107 IgG-opsonized sheep red blood cells (SRBCs) (coupled with the IgG anti-SRBC fraction of serum of immunized rabbits) in cRPMI with 10% fetal calf serum. Receptor-specific uptake was achieved in the presence of 100 µg/mL blocking mAbs against CD16 (clone 3G8), CD32 (clone AT10), or CD8 (clone RPA-T8) as control. A high concentration of the blocking mAbs was chosen to account for the antibody uptake or receptor turnover during the culture period at 37°C. After incubation, nonphagocytosed SRBCs were removed by hypotonic lysis. Finally, Pappenheim-stained cytospins were prepared with 1 × 105 cells per microscope slide. Phagocytosis of SRBCs was quantified by light microscopic analysis of 400 random cells per sample.
Internalization assay
5 × 105 MACS-purified slanDCs were incubated for 15 minutes at 4°C with FITC-anti–human-CD16 (clone 3G8) to cross-link CD16 and then washed in PBS to deplete unbound antibodies. The cells were then resuspended in 500 µL cRPMI with 10% PHS and incubated for 1 hour at 37°C. Uncultured controls were stored on ice. After incubation, all samples were washed in PBS/0.1% NaN3, fixed for 15 minutes with ice-cold 4% paraformaldehyde/PBS, and then washed in 1:100 saponin solution (in PBS/0.1% NaN3). Intracytoplasmatic staining was carried out with FITC-anti–mouse-IgG1 and PE-anti–human-HLA-DR for 15 minutes at 4°C. A fraction of the cells was resuspended in 40°C gelatin and immediately transferred to microscope slides. Internalization was analyzed by confocal laser scanning microscopy.
T-cell proliferation
The ability of slanDCs to restimulate antigen-specific T cells after antigen uptake via CD16, CD32, or the fluid phase was tested using the mouse IgG1-specific T-cell clone B13 (kindly provided by M. Cella, St. Louis, MO).24 B13 T cells recognize mouse IgG1-specific peptides on HLA-DR1. 1 × 105 B13 T cells/mL were seeded 10:1 with HLA-DR–matched slanDCs in 96-well plates. The monoclonal mouse IgG1 mAbs against CD16 (clone 3G8), CD32 (clone AT10), or a CD8-mAb (clone RPA-T8) as control were added, followed by cell culture at 37°C. All mAbs were ultracentrifuged to prevent nonspecific binding of aggregated immunoglobulin to slanDCs. After 20 hours, the cells were pulsed with 2 µCi of 3H-thymidine and incubated for additional 15 hours. 3H-thymidine incorporation was then measured on a scintillation beta counter.
Western blotting
1 × 106 MACS-purified slanDCs, monocytes, or T cells were incubated in 100 µL PBS for 1 hour at 37°C. Supernatants were then separated on a 12% SDS-PAGE gel, transferred to a polyvinylidene difluoride membrane, and blocked in 1% milk powder solution before incubation with anti-CD16 (clone DJ130c, Santa Cruz Biotechnology, Santa Cruz, CA). The primary antibody was detected with horseradish peroxidase–conjugated goat anti-mouse IgG (Santa Cruz Biotechnology) and an Amersham ECL Advance Western Blot Detection Kit (GE Healthcare, Little Chalfont, UK)
Immunofluorescence
Paraffin-embedded tissue sections (1 µm) of skin biopsies from psoriatic plaques of patients with psoriasis were deparaffinized in xylol and ethanol. Antigen retrieval was performed with citrate buffer at a pH of 7.0. The slides were then incubated with dilutions of primary and secondary antibodies (listed in supplemental Table 2). Nuclei were counterstained with DAPI (4,6 diamidino-2-phenylindole, Sigma Aldrich). All samples were analyzed using a Leica DM 5500 B microscope (Leica Microsystems, Wetzlar, Germany) and MetaVue software (Molecular Devices, Sunnyvale, CA).
Statistical analysis
Statistical differences were calculated using analysis of variance (ANOVA) with Tukey’s post hoc test, with P < .05 (*), P < .01 (**), and P < .001 (***) representing different levels of significance.
Results
FcγRs on human blood DCs and monocytes
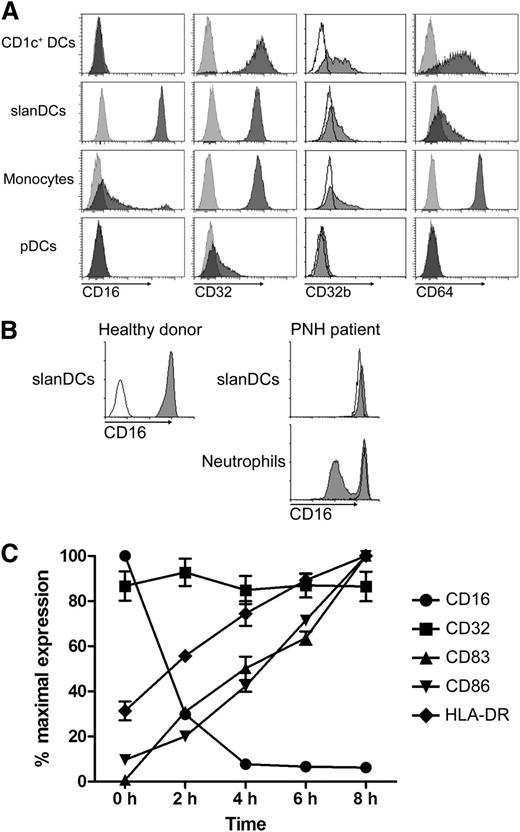
Human PBMCs were stained with mAbs for CD16 (FcγRIII), CD32 (FcγRII), CD32b (FcγRIIb), and CD64 (FcγRI). After electronic gating on CD1c+ DCs, slanDCs, monocytes, and pDCs (for gating strategy; see supplemental Figure 1A), expression levels of these receptors were determined for each cell type (Figure 1A). slanDCs stand out among blood DCs for their high-level expression of the medium-affinity FcγR CD16 that is not expressed by CD1c+ DCs or pDCs.1 slanDCs, CD1c+ DCs, and monocytes are positive for CD32, which is only weakly expressed by pDCs. Using the CD32b-specific mAb 2B6, positive staining was clearly found on CD1c+ DCs, and to a lesser extent on monocytes and a subset of slanDCs. Expression of the high-affinity FcγR CD64 was pronounced on monocytes and CD1c+ DCs but was weak on slanDCs.
Fcγ receptor expression of slanDCs. (A) FcγR expression pattern of CD1c+ DCs, slanDCs, monocytes, and pDCs obtained through staining of PBMCs from buffy coats and subsequent flow cytometric analysis. Light gray and open histograms show control staining. Dark gray histograms refer to the mAb as indicated. Data are representative for 5 experiments. (B) Flow cytometric analysis of CD16 expression on slanDCs and neutrophils in PNH. Staining was performed with a mAb binding to both CD16a and CD16b (clone 3G8). The left panel shows CD16 control staining of slanDCs of a healthy donor. Open histogram: isotype control. Dark gray histogram: CD16-specific staining. The right panel shows CD16 expression on slanDCs and neutrophils of a healthy donor (open histograms) compared with a patient with PNH (gray histograms). (C) Freshly isolated and cultured PBMCs were stained for slanDCs and CD16, CD32, CD83, CD86, and HLA-DR to visualize the kinetics of FcγRs and maturation markers during maturation. Expression of any marker at the different time points was normalized to the isotype control and the sample with the highest expression. Data represent mean ± SEM (N = 5).
Fcγ receptor expression of slanDCs. (A) FcγR expression pattern of CD1c+ DCs, slanDCs, monocytes, and pDCs obtained through staining of PBMCs from buffy coats and subsequent flow cytometric analysis. Light gray and open histograms show control staining. Dark gray histograms refer to the mAb as indicated. Data are representative for 5 experiments. (B) Flow cytometric analysis of CD16 expression on slanDCs and neutrophils in PNH. Staining was performed with a mAb binding to both CD16a and CD16b (clone 3G8). The left panel shows CD16 control staining of slanDCs of a healthy donor. Open histogram: isotype control. Dark gray histogram: CD16-specific staining. The right panel shows CD16 expression on slanDCs and neutrophils of a healthy donor (open histograms) compared with a patient with PNH (gray histograms). (C) Freshly isolated and cultured PBMCs were stained for slanDCs and CD16, CD32, CD83, CD86, and HLA-DR to visualize the kinetics of FcγRs and maturation markers during maturation. Expression of any marker at the different time points was normalized to the isotype control and the sample with the highest expression. Data represent mean ± SEM (N = 5).
CD16 comprises 2 isoforms: the transmembrane molecule CD16a and the GPI-anchored CD16b, which are products of 2 different but nearly identical genes with cell-specific promoter activity.25,26 To identify the isoform expressed by slanDCs, we used leukocytes obtained from a patient with PNH. Due to a mutation in the phosphatidylinositol glycan-class A (PIGA) gene, hematopoietic cells descending from a mutated stem cell show reduced synthesis of the GPI-anchor,27 resulting in diminished or missing expression of GPI-anchored molecules such as CD16b, CD14 and CD55. The bimodal expression of CD55 on slanDCs and CD14 on monocytes of a patient with PNH demonstrates that a major population of these cell types was derived from mutated hematopoietic stem cells (supplemental Figure 2). slanDCs from this PNH patient revealed a homogeneous expression of CD16, indicating the exclusive expression of the transmembrane form CD16a (Figure 1B). In comparison, a clearly bimodal expression of CD16b was evident on granulocytes. Applying the CD16-specific mAb 1D3, which has a strong bias toward CD16b,28 supports these results. Staining of neutrophils yielded a strong signal for CD16b, whereas slanDCs were only weakly stained (supplemental Figure 3).
High-level expression of CD16 is restricted to immature slanDCs
The immature phenotype of slanDCs within blood is actively maintained by erythrocytes because of their expression of CD47 and the molecule’s binding to the inhibitory molecule SIRP-α on slanDCs.19 Ex vivo–cultured isolated slanDCs lack this interaction and rapidly undergo spontaneous phenotypic and functional maturation without further stimulation. This is well documented and demonstrated by upregulation of markers indicative for mature DCs (CD83, CD86, CD40, and HLA-DR) and the acquisition of a capacity to produce high amounts of IL-12.19 Early and very rapidly during maturation of slanDCs, CD16 is lost from the cell surface. In contrast, the expression level of CD32 remains unaltered (Figure 1C). This rapid loss is a clear difference from CD16-expressing neutrophils or natural killer (NK) cells on which CD16 expression remains stable during culture (data not shown).
Coexpression of CD16 and CD32 equips slanDCs with an outstanding capacity to bind ICs
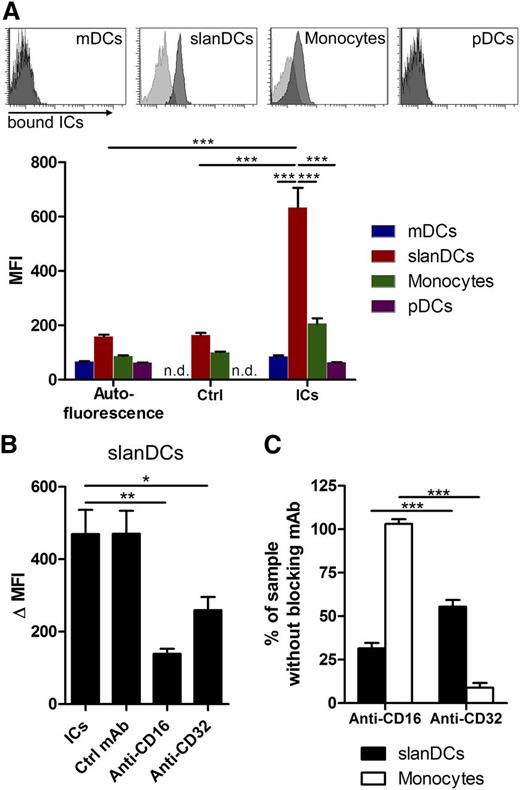
We next investigated the functional relevance of CD16 expressed by immature slanDCs. CD16 is a medium-affinity FcγR with a low capacity to bind uncomplexed IgG but high affinity to ICs.7 To evaluate the influence of CD16 on the ability of slanDCs to bind and internalize complexed antigen, we performed a series of experiments starting with small soluble ICs. These IgG-ICs were prepared by incubating FITC-labeled HSA with polyclonal IgG–anti-FITC. Incubation of PBMCs with these ICs was followed by specific staining for slanDCs, mDCs, pDCs, and monocytes (for gating strategy, see supplemental Figure 1B). Using this assay, we observed a capacity of slanDCs to bind ICs that was 4 times higher when compared with monocytes (Figure 2A). IC binding of mDCs and pDCs was low, which may be a result of their lack of CD16 (mDCs) or lack of both CD16 and CD32 (pDCs). Applying a blocking mAb for CD16 before incubation with ICs greatly reduced the capacity of slanDCs to bind ICs (Figure 2B-C). Interestingly, pre-incubation with a blocking CD32 mAb had a less profound effect on slanDCs but nearly completely abrogated IC binding by monocytes. Taken together, these data suggest that slanDCs have a superior capacity to bind small IgG-ICs, which is to a great extent achieved by binding of ICs to CD16a.
Superior IC binding of slanDCs compared with other human blood DCs and monocytes. (A) Measurement of FITC-labeled IC binding (ICs consisted of HSA-FITC and polyclonal IgG anti-FITC) within freshly isolated PBMCs. Binding of ICs to specific cell types was quantified by staining for slanDCs, monocytes, mDCs, and pDCs, followed by flow cytometric analysis. The histograms are a representative example of the IC-binding assay. Light gray histograms show control staining with HSA-FITC. Dark gray histograms show IC binding. The diagram summarizes 5 independent experiments (mean ± SEM). n.d. = not determined. (B) CD16- and CD32-specific IC binding of slanDCs. PBMCs were incubated with 25 µg/mL blocking mAbs for either CD16 (clone 3G8) or CD32 (clone AT10). IC binding of slanDCs in unblocked, and blocked samples were subsequently quantified by flow cytometric analysis. ΔMFI is calculated by subtraction of the MFI of the control staining from the MFI of the samples. (C) Normalized CD16- and CD32-specific IC binding of slanDCs and monocytes. Data are normalized to the HSA-FITC control (0%) and the sample with the control mAb (100%). Data represent mean ± SEM (N = 5).
Superior IC binding of slanDCs compared with other human blood DCs and monocytes. (A) Measurement of FITC-labeled IC binding (ICs consisted of HSA-FITC and polyclonal IgG anti-FITC) within freshly isolated PBMCs. Binding of ICs to specific cell types was quantified by staining for slanDCs, monocytes, mDCs, and pDCs, followed by flow cytometric analysis. The histograms are a representative example of the IC-binding assay. Light gray histograms show control staining with HSA-FITC. Dark gray histograms show IC binding. The diagram summarizes 5 independent experiments (mean ± SEM). n.d. = not determined. (B) CD16- and CD32-specific IC binding of slanDCs. PBMCs were incubated with 25 µg/mL blocking mAbs for either CD16 (clone 3G8) or CD32 (clone AT10). IC binding of slanDCs in unblocked, and blocked samples were subsequently quantified by flow cytometric analysis. ΔMFI is calculated by subtraction of the MFI of the control staining from the MFI of the samples. (C) Normalized CD16- and CD32-specific IC binding of slanDCs and monocytes. Data are normalized to the HSA-FITC control (0%) and the sample with the control mAb (100%). Data represent mean ± SEM (N = 5).
CD16 but not CD32 is the main phagocytic receptor of slanDCs for opsonized particulate antigen
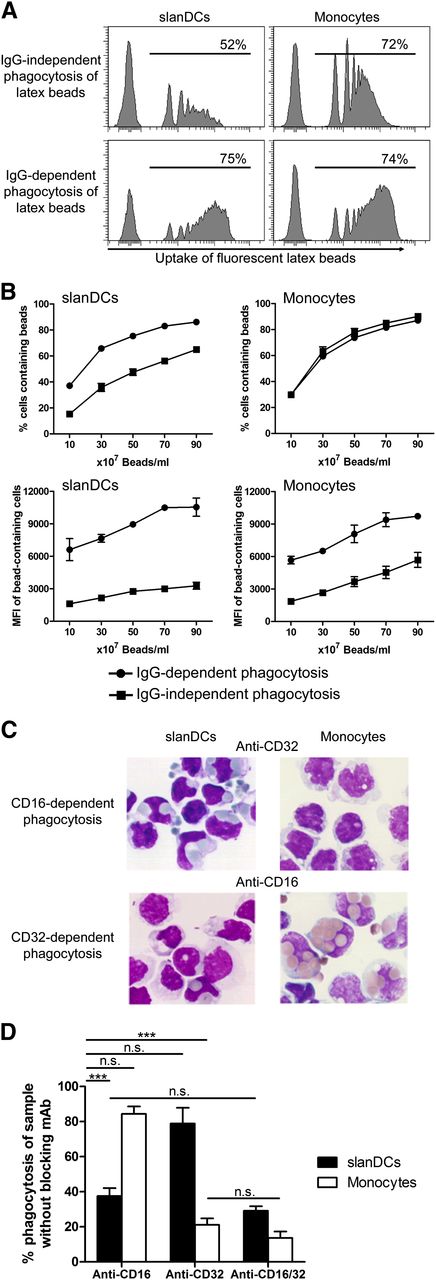
Because FcγRs can bind complexed antigen not only in the form of ICs but also in the form of particulate antigen, we investigated the phagocytosis of nonopsonized and opsonized small spherical latex beads with a diameter of 1 µm. When we directly incubated untreated fluorescent latex beads with freshly isolated PBMCs for 30 minutes, we observed that a comparatively low amount of slanDCs had phagocytosed a single bead, whereas the percentage of monocytes that captured these untreated latex beads was high (Figure 3A-B). In contrast, when we used human IgG-coated latex beads, a high number of slanDCs were able to take up one or more latex beads, whereas the percentage of monocytes that have taken up one or more beads was not increased. Coating of latex beads with IgG before phagocytosis strongly increased the amount of beads per cell in both slanDCs and monocytes, as indicated by the higher median fluorescence intensity (MFI) of the bead-containing cells (Figure 3A-B).
CD16 but not CD32 enables a high phagocytic activity of slanDCs. (A) FcγR-dependent uptake of particulate antigens by slanDCs. PBMCs were incubated for 30 minutes at 37°C with untreated or IgG-coated fluorescent latex particles. Internalization of latex particles was quantified by specific staining of slanDCs and monocytes and subsequent flow cytometric analysis. Data in the histograms are 1 representative experiment, where 50 × 107 latex beads/mL were incubated with PBMCs. The numbers give the percentage of cells that contain at least 1 latex bead. (B) Quantification of the experiments over a range of bead concentrations. The upper graphs quantify the percentage of cells that have internalized at least 1 bead. The lower graphs show the MFI of the bead-containing cells as a measure of the amount of internalized beads. Data of the graphs represent mean ± SEM (N = 3). (C) CD16- and CD32-specific uptake of opsonized SRBCs by slanDCs and monocytes. slanDCs and monocytes were incubated for 30 minutes at 37°C with IgG-opsonized SRBCs. To analyze receptor-specific phagocytosis, incubation was performed in the presence of blocking mAbs for either CD16 (clone 3G8) or CD32 (clone AT10). Pappenheim-stained cytospins of cells containing SRBCs were prepared with 1 × 105 cells per microscope slide. The microscopic pictures are of 1 representative experiment. (D) The quantification is presented relative to the sample with the nonblocking isotype-matched control mAb (100%). Data represent mean ± SEM (N = 3).
CD16 but not CD32 enables a high phagocytic activity of slanDCs. (A) FcγR-dependent uptake of particulate antigens by slanDCs. PBMCs were incubated for 30 minutes at 37°C with untreated or IgG-coated fluorescent latex particles. Internalization of latex particles was quantified by specific staining of slanDCs and monocytes and subsequent flow cytometric analysis. Data in the histograms are 1 representative experiment, where 50 × 107 latex beads/mL were incubated with PBMCs. The numbers give the percentage of cells that contain at least 1 latex bead. (B) Quantification of the experiments over a range of bead concentrations. The upper graphs quantify the percentage of cells that have internalized at least 1 bead. The lower graphs show the MFI of the bead-containing cells as a measure of the amount of internalized beads. Data of the graphs represent mean ± SEM (N = 3). (C) CD16- and CD32-specific uptake of opsonized SRBCs by slanDCs and monocytes. slanDCs and monocytes were incubated for 30 minutes at 37°C with IgG-opsonized SRBCs. To analyze receptor-specific phagocytosis, incubation was performed in the presence of blocking mAbs for either CD16 (clone 3G8) or CD32 (clone AT10). Pappenheim-stained cytospins of cells containing SRBCs were prepared with 1 × 105 cells per microscope slide. The microscopic pictures are of 1 representative experiment. (D) The quantification is presented relative to the sample with the nonblocking isotype-matched control mAb (100%). Data represent mean ± SEM (N = 3).
In further experiments, we used IgG-opsonized SRBCs with an average diameter of 4.5 µm to compare the phagocytosis of large particles by slanDCs and monocytes. The samples were prepared by incubating slanDCs and monocytes with SRBCs under both nonblocking and blocking conditions for either CD16 or CD32, or both, and subsequent preparation of cytospins. The amount of internalized SRBCs was determined from 400 random cells per sample. IgG-independent phagocytosis of nonopsonized SRBCs by both slanDCs and monocytes was below 1% compared with the uptake of opsonized SRBCs. The overall amount of slanDCs that had internalized IgG-opsonized SRBCs under nonblocking conditions was about 50% of that of monocytes. The average number of phagocytosed opsonized SRBCs by slanDCs and monocytes was 1.3 and 2.5, respectively (data not shown). Under blocking conditions for CD16, the phagocytic capacity of slanDCs was reduced by >60%, whereas there was only a slight effect on phagocytosis by monocytes. Conversely, blocking of CD32 had only a slight effect on the phagocytic capacity of slanDCs but led to 80% reduction of phagocytosis by monocytes (Figure 3C-D). Taken together, these data indicate that slanDCs have a comparatively high threshold for nonspecific phagocytosis but exhibit a low threshold for FcγR-mediated phagocytosis, and this FcγR-mediated phagocytosis was dependent on CD16.
Targeting antigen to CD16 mediates high-level restimulation of antigen-specific T cells
When slanDCs were cultured in the presence of cross-linking antibodies for CD16 or CD32 and were additionally stimulated with TLR ligands, we observed only a mild modulation of cytokine production in the case of IL-6, tumor necrosis factor-α (TNF-α) and IL-1β (not shown). Cross-linking of CD16 or CD32 gave similar results in these experiments. A moderate inhibition was exerted on the IL-12 production after TLR7/8 stimulation (supplemental Figure 4A). A differential effect of cross-linking either CD16 or CD32 was observed when studying the induction of an oxidative burst. Only cross-linking with a CD16-specific mAb induced a signal that could be detected with the reactive oxygen species indicator dihydrorhodamine 123 (supplemental Figure 4B). When measuring the induction of calcium release as a general measure for downstream signaling after cross-linking of CD16 or CD32, we again found comparable results for both receptors (supplemental Figure 4C).
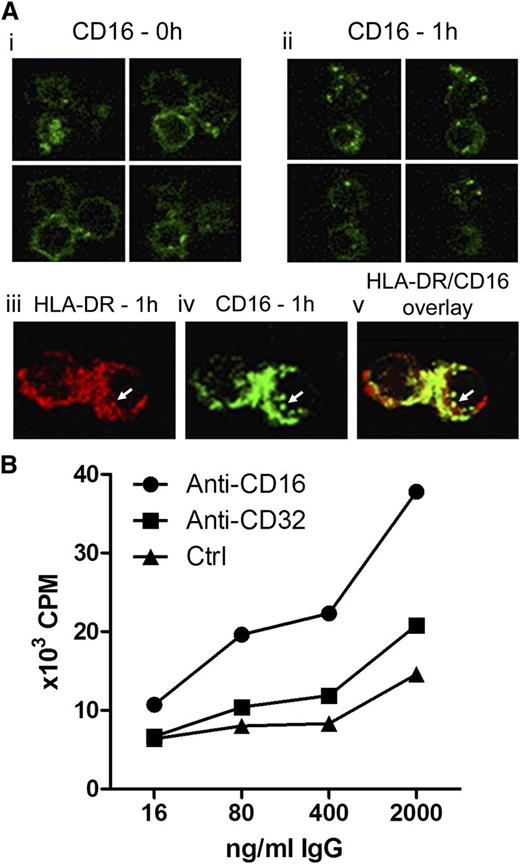
To analyze the intracellular routing of cross-linked CD16, we applied confocal laser scanning microscopy. Freshly isolated slanDCs showed a strong homogeneous CD16-specific labeling of the cell surface (Figure 4Ai). After cross-linking of CD16 and cell culture for 1 hour at 37°C, the ring-shaped staining could no longer be observed, whereas intracytoplasmatic signals pointed to internalization of cross-linked CD16 (Figure 4Aii). Double-immunofluorescence staining for HLA-DR and internalized CD16 revealed an intracellular colocalization of internalized CD16 and MHC class II compartments (Figure 4Aiii-v), indicating the involvement of CD16 in pathways leading to antigen presentation. To further address this question, we cultivated freshly isolated slanDCs and monocytes with autologous CD4+ T cells in the presence of tetanus toxoid and human anti-tetanus toxin immunoglobulin. When IC formation was allowed, a much stronger T-cell proliferative response was observed for slanDCs but not for monocytes, a finding in line with the outstanding capacity of slanDCs to bind ICs (supplemental Figure 5). In another set of experiments, we used the HLA-DR1–restricted CD4+ T-cell clone B13, which is specific for murine IgG1.24 Because the blocking mAbs for CD16 and CD32 were IgG1 isotypes, we set up HLA-DR–matched settings to check the proliferative response of the B13 T cells. Figure 4B demonstrates that the proliferation of B13 T cells was about twofold higher (259% ± 15% at 2000 ng/mL) when antigen was targeted to CD16 instead of CD32 (143% ± 11% at 2000 ng/mL). Nevertheless, a B13 T-cell proliferation was also observed in the settings with the control mAb (anti-CD8, clone RPA-T8, mIgG1) that does not directly bind to slanDCs. This may be a result of nonspecific antigen uptake via FcγRs or macropinocytosis. Taken together, these data show that when targeted to CD16, antigen is potently presented by slanDCs via MHC class II to efficiently stimulate antigen-specific proliferation of CD4+ T cells.
Targeting antigen to CD16 on slanDCs enables efficient internalization and restimulation of antigen-specific CD4+ T cells. (A) Internalization of CD16-targeted antigen into MHC class II–containing compartments. slanDCs were preincubated with FITC–anti-CD16 at 4°C. Internalization of cross-linked CD16 was induced by incubation for 1 hour at 37°C. Control cells were stored at 4°C. Localization of cross-linked CD16 in incubated and control cells was then analyzed by confocal laser scanning microscopy. (i) Distribution of CD16 on the surface of control cells. Four sections of the same cells are shown. (ii) Distribution of cross-linked CD16 after incubation for 1 hour. Four sections of the same cells are shown. (iii-v) Costaining of incubated slanDCs for HLA-DR (red, iii) and internalized CD16 (green, iv) shows partial colocalization of both signals in the overlay (v). (B) Efficient restimulation of antigen-specific T cells after targeting CD16 on slanDCs. Targeted antigen presentation was measured with specific mAbs to either CD16 (clone 3G8, mouse IgG1) or CD32 (clone AT10, mouse IgG1) followed by coculture with mouse IgG1–specific B13 T cells. Antigen presentation after nonspecific antigen uptake was measured using an anti-CD8 mAb (clone RPA-T8, mouse IgG1) with no affinity to slanDCs. Proliferation rates of IgG1-specific B13 T cells were measured by determining the 3H-thymidine incorporation into the DNA. Results are representative of 3 experiments.
Targeting antigen to CD16 on slanDCs enables efficient internalization and restimulation of antigen-specific CD4+ T cells. (A) Internalization of CD16-targeted antigen into MHC class II–containing compartments. slanDCs were preincubated with FITC–anti-CD16 at 4°C. Internalization of cross-linked CD16 was induced by incubation for 1 hour at 37°C. Control cells were stored at 4°C. Localization of cross-linked CD16 in incubated and control cells was then analyzed by confocal laser scanning microscopy. (i) Distribution of CD16 on the surface of control cells. Four sections of the same cells are shown. (ii) Distribution of cross-linked CD16 after incubation for 1 hour. Four sections of the same cells are shown. (iii-v) Costaining of incubated slanDCs for HLA-DR (red, iii) and internalized CD16 (green, iv) shows partial colocalization of both signals in the overlay (v). (B) Efficient restimulation of antigen-specific T cells after targeting CD16 on slanDCs. Targeted antigen presentation was measured with specific mAbs to either CD16 (clone 3G8, mouse IgG1) or CD32 (clone AT10, mouse IgG1) followed by coculture with mouse IgG1–specific B13 T cells. Antigen presentation after nonspecific antigen uptake was measured using an anti-CD8 mAb (clone RPA-T8, mouse IgG1) with no affinity to slanDCs. Proliferation rates of IgG1-specific B13 T cells were measured by determining the 3H-thymidine incorporation into the DNA. Results are representative of 3 experiments.
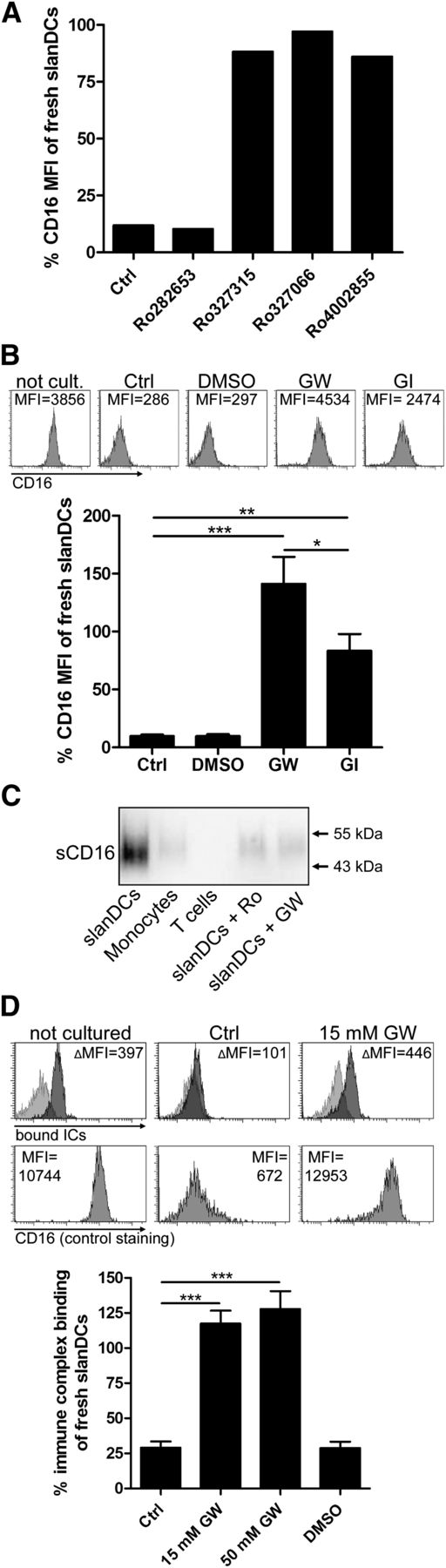
ADAM10 and ADAM17 promote downregulation of CD16 by proteolytic cleavage. (A) Broad-spectrum metalloproteinase inhibitors prevent downregulation of CD16 on slanDCs. Freshly isolated slanDCs were cultured for 6 hours in the presence of broad-spectrum metalloproteinase inhibitors with distinct specificities for certain MMPs and ADAMs (supplemental Table 1). Cultured controls did not contain an inhibitor. Uncultured controls were stored on ice. After cell culture, CD16 expression was measured by flow cytometry. Expression of CD16 is depicted as percent expression of uncultured slanDCs. (B) Inhibiting ADAM10 and ADAM17 activity with specific inhibitors prevents downregulation of CD16 on slanDCs. slanDCs were cultured for 8 hours in the presence of GW280264x, blocking both ADAM10 and ADAM17, and GI254023x, preferentially blocking ADAM10. Controls did not contain an inhibitor or did contain the inhibitor’s solvent (DMSO). Uncultured control cells were left on ice. CD16 expression was measured by flow cytometry and is depicted as percent expression of uncultured slanDCs. The histograms above the diagram show the CD16 staining of 1 representative experiment. (C) Soluble CD16 is produced in cell cultures of freshly isolated slanDCs but is hardly produced in the presence of metalloproteinase inhibitors. 1 × 106 slanDCs, monocytes, and CD4+ T cells, as well as slanDCs in the presence of 50 µM Ro327315 and 50 µM GW280264x, were incubated for 1 hour at 37°C. Supernatants of the cell cultures were then analyzed by Western blot for the presence of soluble CD16. (D) Preventing loss of CD16 prevents loss of IC binding. Freshly isolated slanDCs were cultured without the addition of metalloproteinase inhibitors, in the presence of GW280264x and the inhibitor’s solvent (DMSO). Control cells were stored on ice. After 8 hours of cell culture, binding of fluorescent ICs was quantified by flow cytometry and is depicted as percent IC binding of uncultured slanDCs. The histograms above the diagram are results of 1 representative experiment. Upper row: Light gray histograms show the control staining; dark gray histograms represent IC binding. ΔMFI is calculated by subtraction of the MFI of the control staining from the MFI of the samples with bound ICs. Lower row: CD16 expression of the respective slanDCs. Data represent mean ± SEM (N = 5).
ADAM10 and ADAM17 promote downregulation of CD16 by proteolytic cleavage. (A) Broad-spectrum metalloproteinase inhibitors prevent downregulation of CD16 on slanDCs. Freshly isolated slanDCs were cultured for 6 hours in the presence of broad-spectrum metalloproteinase inhibitors with distinct specificities for certain MMPs and ADAMs (supplemental Table 1). Cultured controls did not contain an inhibitor. Uncultured controls were stored on ice. After cell culture, CD16 expression was measured by flow cytometry. Expression of CD16 is depicted as percent expression of uncultured slanDCs. (B) Inhibiting ADAM10 and ADAM17 activity with specific inhibitors prevents downregulation of CD16 on slanDCs. slanDCs were cultured for 8 hours in the presence of GW280264x, blocking both ADAM10 and ADAM17, and GI254023x, preferentially blocking ADAM10. Controls did not contain an inhibitor or did contain the inhibitor’s solvent (DMSO). Uncultured control cells were left on ice. CD16 expression was measured by flow cytometry and is depicted as percent expression of uncultured slanDCs. The histograms above the diagram show the CD16 staining of 1 representative experiment. (C) Soluble CD16 is produced in cell cultures of freshly isolated slanDCs but is hardly produced in the presence of metalloproteinase inhibitors. 1 × 106 slanDCs, monocytes, and CD4+ T cells, as well as slanDCs in the presence of 50 µM Ro327315 and 50 µM GW280264x, were incubated for 1 hour at 37°C. Supernatants of the cell cultures were then analyzed by Western blot for the presence of soluble CD16. (D) Preventing loss of CD16 prevents loss of IC binding. Freshly isolated slanDCs were cultured without the addition of metalloproteinase inhibitors, in the presence of GW280264x and the inhibitor’s solvent (DMSO). Control cells were stored on ice. After 8 hours of cell culture, binding of fluorescent ICs was quantified by flow cytometry and is depicted as percent IC binding of uncultured slanDCs. The histograms above the diagram are results of 1 representative experiment. Upper row: Light gray histograms show the control staining; dark gray histograms represent IC binding. ΔMFI is calculated by subtraction of the MFI of the control staining from the MFI of the samples with bound ICs. Lower row: CD16 expression of the respective slanDCs. Data represent mean ± SEM (N = 5).
Direct proteolytic cleavage by ADAM10 and ADAM17 is involved in the rapid downregulation of CD16 on maturing slanDCs
Having shown the profound functional relevance of an intact CD16 expression on slanDCs, we then investigated the factors leading to downregulation of the molecule from the cell surface. A soluble form of CD16 (sCD16) was reported to be released from PMA- or fMLP-stimulated neutrophils or NK cells.29,30 Several previous studies noted that proteolytic processes may be responsible for the release of CD16.31,32 Culturing slanDCs in the presence of the general metalloproteinase inhibitors EDTA, 1,10-phenanthroline and GM6001 led to retention of CD16 on the surface of slanDCs (data not shown). To restrict these results to the action of specific metalloproteinases, we applied the matrix metalloproteinase (MMP) and a disintegrin and metalloproteinase (ADAM) inhibitors Ro327315, Ro327066, Ro4002855, and Ro282653. These inhibitors vary in their specificities for certain members of the MMP and ADAM families (supplemental Table 1). The pattern of inhibition pointed to an involvement of members of the ADAM family of metalloproteinases, especially ADAM17 (Figure 5A). Members of the ADAM family, notably ADAM10 and ADAM17, are constitutively expressed on various leukocytes as well as on slanDCs (supplemental Figure 6) and were implicated in the cell-surface shedding of numerous proteins.33 To gain insight into the involvement of ADAM10 and ADAM17, we cultured slanDCs in the presence of the hydroxamate inhibitors GW280264x, an inhibitor blocking both ADAM10 and ADAM17 with equally high potency, and GI254023x, an inhibitor with a one-hundred fold higher potency for ADAM10 than for ADAM17 (Figure 5B).34 Both inhibitors clearly inhibited the downregulation of CD16 on slanDCs. Inhibition of ADAM10 was less efficient than combined inhibition of ADAM10 and ADAM17, suggesting proteolytic targeting of CD16 on maturing slanDCs by both ADAM10 and ADAM17. To test whether activity of these proteases leads to the release of sCD16, we analyzed supernatants of slanDC cell cultures by Western blotting. We were able to detect sCD16 in slanDC supernatants devoid of metalloproteinase inhibitors (Figure 5C). In contrast, only a faint signal of sCD16 could be obtained when the cell culture supernatants were generated in the presence of Ro327315 or GW280264x. Finally, we compared the IC binding of slanDCs cultured for 8 hours without metalloproteinase inhibitor and in the presence of GW280264x (Figure 5D). IC binding of cultured slanDCs that have downregulated CD16 was about one-fourth of immature slanDCs. However, in the presence of the combined ADAM10 and ADAM17 inhibitor GW280264x, slanDCs completely retained their highly effective capacity to bind ICs.
slanDCs within inflamed tissue display low or no expression of CD16
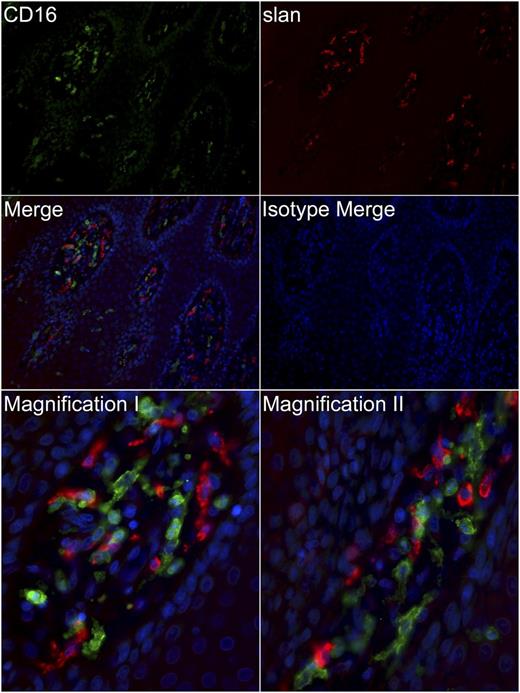
Having shown the different aspects of CD16 regulation and function on immature slanDCs and in vitro matured slanDCs, we finally searched for an in vivo correlate of activated slanDCs and investigated their expression of CD16. Activation of slanDCs in psoriatic lesions has already been shown by their expression of maturation markers and TNF-α.18,35 To this end, we phenotyped slanDCs within human psoriatic skin lesions by immunofluorescence. We observed that slanDCs in psoriatic lesions display a mostly missing expression of CD16 (Figure 6). Therefore, slanDCs become activated in inflamed tissue (with a phenotype corresponding to in-vitro matured slanDCs), have largely downregulated their CD16 expression and thus may have a reduced capacity to bind ICs.
slanDCs in inflamed tissues mostly lack expression of CD16. Paraffin-embedded tissue sections (1 µm) of psoriatic plaques were immunostained for slan (red) and CD16 (green) or with appropriate isotype controls. Nuclei were counterstained with DAPI (blue). Upper four panels: original magnification ×200. Lower two panels: original magnification ×400. The slides were analyzed using a Leica DM 5500 B microscope and the MetaVue software. Data are representative for >10 experiments.
slanDCs in inflamed tissues mostly lack expression of CD16. Paraffin-embedded tissue sections (1 µm) of psoriatic plaques were immunostained for slan (red) and CD16 (green) or with appropriate isotype controls. Nuclei were counterstained with DAPI (blue). Upper four panels: original magnification ×200. Lower two panels: original magnification ×400. The slides were analyzed using a Leica DM 5500 B microscope and the MetaVue software. Data are representative for >10 experiments.
Discussion
In this study, we identified immature CD16-expressing slanDCs as the population of human blood DCs exhibiting by far the highest capacity of IC binding. mDCs, pDCs, and CD14+ monocytes express significant levels of CD32, and they all have previously been shown to bind ICs via CD32. However, their IC-binding capacity proves to be much less pronounced than that mediated by coexpression of CD16 on slanDCs. Beyond IC binding, we demonstrated the chief role of CD16 on these immature blood DCs as a phagocytic receptor for IgG-opsonized particles and showed its relevance in antigen uptake and presentation to CD4+ T cells. These findings provide evidence for a potential pathogenic function of slanDCs in autoimmune diseases in which IC-containing autologous antigens play a crucial role in stimulating disease activity. Indeed, we recently identified increased frequencies of slanDCs in skin lesions of cutaneous and systemic lupus erythematosus (SLE).36
slanDCs have previously been described as a distinct population of human blood DCs expressing the marker 6-sulfo LacNAc (slan).18 The stable expression of slan on the cell surface allows direct isolation from blood, facilitates their identification as dermal DCs in the skin, and allows them to be studied among cultured PBMCs. Recently, we identified slanDCs as a population of DCs in the autoimmune disease psoriasis.35 In psoriatic skin lesions, slanDCs expressed IL-23p19, TNF-α, and inducible nitric oxide synthase but lacked the monocyte and macrophage markers CD14 and CD163. Freshly isolated slanDCs from the blood of healthy donors displayed a marked capacity to produce IL-23 and IL-1β in response to lipopolysaccharide. Consequently, compared with CD1c+ DCs, slanDCs programmed stronger Th17/Th1 responses. Together these data provide strong evidence for a pro-inflammatory function of slanDCs in psoriasis.
The pivotal role of FcγRs has been known since the 1990s when studies of Fc receptor gamma chain-deficient mice clearly showed the importance of FcγRs in mediating IgG-dependent effector functions.37,38 Their importance also became evident when mouse models of autoimmune diseases such as SLE and arthritis were investigated.39 In addition, allelic forms of CD16 have been associated with SLE40 or were suggested to be risk factors for rheumatoid arthritis.41 Cross-linking of FcγRs by IC ligation induces activating as well as inhibitory regulatory signals via immunoreceptor tyrosine–based activation motifs or immunoreceptor tyrosine–based inhibition motifs, respectively. One of the firsthand effects of IC binding to FcγRs is accumulation of antigen on the cell surface, which consequently leads to much more efficient internalization.42 With regard to slanDCs, we have not only observed pronounced accumulation of antigen on the cell surface but also showed efficient internalization of antigen within 1 hour when this antigen was directly delivered to CD16. In other studies, it has been shown that FcγR-mediated signal transduction promotes induction of maturation of immature DCs and FcγR-mediated antigen uptake potently enhances antigen presentation.43-46 Our results reflect these data insofar as a markedly lower amount of antigen was necessary to induce the proliferation of antigen-specific T cells when antigen was targeted to CD16 instead of CD32. Although, under some stimulatory conditions we observed a reduced expression of IL-12 after cross-linking of FcγRs, this did not seem to be relevant in the antigen-specific T-cell proliferation assays performed in this study. We did not investigate the role of CD16 in cross-presentation; however, Mende et al already showed that cross-presentation of slanDCs seemed to be CD16-independent, because no efficient cross-presentation or activation of CD8+ T cells occurred when CD16 was targeted.47 A variety of responses to cross-linking of FcγRs exist, and these responses are not only restricted to IC binding and uptake and enhanced antigen presentation by DCs, but they also include induction of an oxidative burst; release of inflammatory mediators, vasoactive substances and chemoattractants; as well as cytotoxic activity.45 In the case of slanDCs, it has already been shown that these cells potently mediate antibody-dependent cell-mediated cytotoxicity against tumor cells, which depended on both CD16 and CD32.20 The increased frequency of slanDCs in skin lesions of cutaneous and systemic lupus erythematosus, together with their concomitant expression of TLR7 and TLR8, suggest that these cells are potential mediators of autoimmune complex–mediated effector functions in this disease.36
To our knowledge, there are no reports describing major differences in IC binding by human CD16 versus CD32.48 At first hand, the high-density expression of CD16 in addition to CD32 (Figure 1A) on slanDCs could be responsible for their strong binding of ICs. Differences in the intracellular signaling of CD16a and CD32a were recently described. It has been shown that signal transmission via CD16a is not only provided by phosphorylation of its associated γ-chains but also by phosphorylation of a protein kinase C consensus motif in the CD16a α-chain. Calcium flux, Syk phosphorylation, cytokine production, and degranulation were shown to be affected by the phosphorylation state of this motif.49 Moreover, differential outcomes might also be caused by cross-talk with other receptor systems. It has for example been shown that such a cross-talk occurs between murine TLR4 and CD16.9
Our data document the functional switch from immature DCs that are specialized in antigen uptake to mature DCs that efficiently present the respective antigen. We demonstrated that immature slanDCs have an outstanding capacity to bind and internalize IgG-complexed antigens via CD16 and to a lesser extent CD32. However, within 2 to 3 hours of culture in the absence of erythrocytes, slanDCs spontaneously lose their expression of CD16 and thereby a large proportion of their capacity to take up antigen. In parallel to the loss of CD16, both the upregulation of T-cell stimulatory molecules and the translocation of HLA-DR to the cell surface allow antigen, which was initially taken up via ICs, to be efficiently presented to CD4+ T cells.
When investigating the mechanism of CD16 loss, we studied whether CD16 may be shed by proteolytic cleavage, which should result in enhanced levels of soluble CD16 in cell-culture supernatants. By using Western blot it was indeed possible to detect soluble CD16. Accordingly, when we used protease inhibitors, soluble CD16 could hardly be detected. From the inhibition profile of a set of inhibitors with different potency for selected metalloproteinases, we concluded that ADAM10 and ADAM17 critically contribute to the shedding of CD16 on slanDCs. Other CD16-expressing cell types such as neutrophils and NK cells also express ADAM17 and ADAM10. However, they do not show a comparable spontaneous shedding of CD16 when cultured in vitro. The signals leading to activation of ADAM10 and ADAM17 on slanDCs remain to be studied. The lack of CD16 on slanDCs in inflamed skin can result from proteolytic cleavage as shown here; however, the internalization of ICs via CD16 may also contribute to this phenomenon.
In summary, among the different populations of DCs, the coexpression of CD16 and CD32 by immature slanDCs equips these cells with a unique capacity to handle ICs. However, some of these functions are short lived, because CD16 is rapidly downregulated during maturation by activation of ADAM10 and ADAM17.
The online version of the article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank M. Cella (Washington University St. Louis, MO) for providing the mouse IgG1–specific T-cell clone B13, H.-W. Krell (Roche, Germany) for delivering broad-spectrum metalloproteinase inhibitors, and Medarex for providing their CD32a-specifc monoclonal antibody.
This work was supported by the Collaborative Research Center (Sonderforschungbereich) 938 (K.S.).
Authorship
Contribution: T.D. performed experiments, designed research, analyzed and interpreted data, and wrote the manuscript; A.K. performed experiments and analyzed and interpreted data; J.B. and K.T. performed experiments; A.L. contributed vital new reagents; M.S. and A.E. designed research; and K.S. designed research, performed experiments, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for J.B. is the Department of Gastroenterology, Medical Clinic I, Technical University Dresden, Dresden, Germany.
Correspondence: Knut Schäkel, Dr med, Department of Dermatology, Heidelberg University Hospital, Voßstraße 2, 69115 Heidelberg, Germany; e-mail: knut.schaekel@med.uni-heidelberg.de.