Key Points
Chemokine (C-C motif) ligand 28 (CCL28) is a novel growth factor for human hematopoietic stem and progenitor cells.
CCL28 supports the in vitro and in vivo functional integrity of cultured primitive hematopoietic cells.
In an attempt to discover novel growth factors for hematopoietic stem and progenitor cells (HSPCs), we have assessed cytokine responses of cord blood (CB)–derived CD34+ cells in a high-content growth factor screen. We identify the immunoregulatory chemokine (C-C motif) ligand 28 (CCL28) as a novel growth factor that directly stimulates proliferation of primitive hematopoietic cells from different ontogenetic origins. CCL28 enhances the functional progenitor cell content of cultured cells by stimulating cell cycling and induces gene expression changes associated with survival. Importantly, addition of CCL28 to cultures of purified putative hematopoietic stem cells (HSCs) significantly increases the ability of the cells to long-term repopulate immunodeficient mice compared with equivalent input numbers of fresh cells. Together, our findings identify CCL28 as a potent growth-promoting factor with the ability to support the in vitro and in vivo functional properties of cultured human hematopoietic cells.
Introduction
Extrinsic signaling molecules have been widely examined for their potential to support hematopoietic stem cells (HSCs) ex vivo. However, few growth factors have been identified that maintain the primitive properties of HSCs.1,2 Recently, several proteins or small molecules were shown to increase the numbers of cultured human HSPCs, but the majority of these rely on the basic support of at least 3 different cytokines,3,,,,-8 some of which may promote differentiation at the expense of HSC maintenance.1,2,9 Thus, current culture conditions for HSPCs should be improved by novel HSC-supportive factors. Using a systematic screening approach, we have identified CCL28 as a promising new growth factor that preserves the functional integrity of human HSPCs.
Study design
Culture conditions and progenitor cell assays
HSPCs were cultured in serum-free expansion medium (StemCell Technologies) supplemented with stem cell factor (SCF) at 10 ng/mL (S10) or 100 ng/mL (S100). For screening (n = 276) and validation (n = 36), recombinant cytokines (Peprotech) were used at 100 ng/mL. Colony-forming cell (CFC) assays were established according to manufacturer’s instructions (StemCell Technologies). Long-term culture-initiating cell (LTC-IC) assays are described elsewhere.10 For details, see supplemental Methods. Human CB from umbilical cords at the end of full-term deliveries was obtained from the Department of Obstetrics in Lund, Sweden, with the informed consent of the mothers, or it was purchased from the Anthony Nolan Cell Therapy Centre (London, UK). Human bone marrow (BM) aspirates were obtained from the posterior iliac crest of fully informed healthy donors from the Department of Hematology in Lund. This study was conducted in accordance with the Declaration of Helsinki.
See supplemental Methods for microarray (GEO accession number: GSE45136), cell cycle, and apoptosis assays.
Xenotransplantation
NOD.Cg-PrkdcscidIl2rgtm1Wjl/SzJ mice (NSG; Jackson Laboratory) were used for animal experiments, as approved by the Lund/Malmö Ethical Committee. For details, see supplemental Methods on the Blood Web site.
Statistical analysis
Statistical significance was calculated using paired Student t test. Unless otherwise stated, error bars indicate standard error of the mean (SEM).
Results and discussion
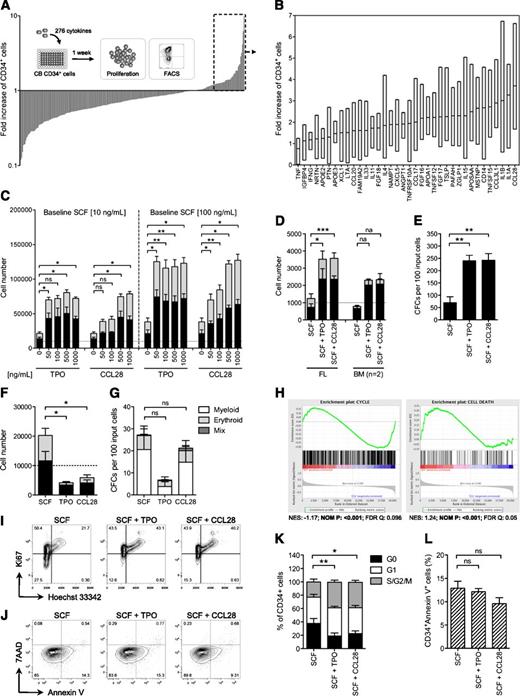
To discover novel growth factors for HSPCs, we designed a screening assay for expansion of CB-derived CD34+ cells based on high-throughput fluorescence-activated cell sorting (FACS) analysis of CD34 expression after a 7-day culture period. Given the high proliferative potential of CB progenitors,11,-13 we hypothesized that the power of each factor would be best distinguished in low concentrations of SCF, which promotes survival without provoking extensive proliferation.14,15 A concentration of 10 ng/mL SCF was sufficient to maintain survival and induce strong synergistic effects on CD34+ cell expansion together with the known HSC-supportive factor thrombopoietin (TPO)16 (supplemental Figure 1A). Thus, we used this condition to systematically screen 276 human growth factors for their potential to expand CD34+ cells (Figure 1A). On the basis of 2 independent screens (supplemental Table 1E) and 3 consecutive validation experiments (Figure 1B; supplemental Figure 1B), CCL28 showed the most prominent and consistent expansion of CD34+ cells and was therefore selected for further investigation.
A high-content growth factor screen in human CB progenitors identifies CCL28 as potent stimulator of HSPC proliferation. (A) Outcome of the primary screen. Proliferation of cells cultured in S10 was set to 1 and relative fold increase of CD34+ cells was determined after 7 days of culture. The mean values from 2 screens are shown. The experimental design of the primary screen is shown as an overlay. (B) Outcome of the validation. Plotted are screening and validation results from 36 selected candidate factors (n = 4). Shown are floating bars from the minimum to the maximum, the line indicates the mean. (C) CB CD34+ cells were cultured in S10 or S100 together, with increasing concentrations of TPO or CCL28, and analyzed for proliferation and CD34 expression by FACS at day 7 (n = 3-4). After titration, CCL28 was used at 500 ng/mL. (D) FL- and BM-derived CD34+ cells were cultured in S10 and analyzed for proliferation and CD34 expression by FACS at day 7 (n = 2-3). Error bars represent SD. (E) Numbers of total CFCs per an equivalent of 100 input CB CD34+ cells at day 0 (n = 3). (F-G) Proliferation (F; n = 7) and CFC (G; n = 5) potential of CB progenitors cultured in single cytokine stimulation for 7 days. (H) Gene set enrichment analysis results for cell cycle and cell death signatures in SCF vs SCF + CCL28 conditions. NES, normalized enrichment score; NOM P, nominal P value; FDR, false discovery rate. (I-J) Representative cell cycle (I) and apoptosis (J) FACS plots of CD34+-gated cells. (K-L) Quantification of cell cycle (K) and apoptosis (L) data (n = 5). The dashed lines in figures (C), (D), and (F) indicate input cell numbers; black and gray bars represent CD34+ and CD34– cells, respectively. *P < .05; **P < .01; ***P < .01; ns, not significant; na, not applicable.
A high-content growth factor screen in human CB progenitors identifies CCL28 as potent stimulator of HSPC proliferation. (A) Outcome of the primary screen. Proliferation of cells cultured in S10 was set to 1 and relative fold increase of CD34+ cells was determined after 7 days of culture. The mean values from 2 screens are shown. The experimental design of the primary screen is shown as an overlay. (B) Outcome of the validation. Plotted are screening and validation results from 36 selected candidate factors (n = 4). Shown are floating bars from the minimum to the maximum, the line indicates the mean. (C) CB CD34+ cells were cultured in S10 or S100 together, with increasing concentrations of TPO or CCL28, and analyzed for proliferation and CD34 expression by FACS at day 7 (n = 3-4). After titration, CCL28 was used at 500 ng/mL. (D) FL- and BM-derived CD34+ cells were cultured in S10 and analyzed for proliferation and CD34 expression by FACS at day 7 (n = 2-3). Error bars represent SD. (E) Numbers of total CFCs per an equivalent of 100 input CB CD34+ cells at day 0 (n = 3). (F-G) Proliferation (F; n = 7) and CFC (G; n = 5) potential of CB progenitors cultured in single cytokine stimulation for 7 days. (H) Gene set enrichment analysis results for cell cycle and cell death signatures in SCF vs SCF + CCL28 conditions. NES, normalized enrichment score; NOM P, nominal P value; FDR, false discovery rate. (I-J) Representative cell cycle (I) and apoptosis (J) FACS plots of CD34+-gated cells. (K-L) Quantification of cell cycle (K) and apoptosis (L) data (n = 5). The dashed lines in figures (C), (D), and (F) indicate input cell numbers; black and gray bars represent CD34+ and CD34– cells, respectively. *P < .05; **P < .01; ***P < .01; ns, not significant; na, not applicable.
To determine the contexts in which CCL28 stimulates HSPCs, we first evaluated the effect of increasing CCL28 doses at different SCF concentrations. We observed a dose-dependent increase in CD34+ cell numbers in response to stimulation with 50 to 1000 ng/mL CCL28 (Figure 1C; supplemental Figure 3B). At 500 ng/mL, the effect of CCL28 reached saturation and yielded similar synergistic effects on CD34+ cell expansion as TPO (Figure 1C; supplemental Figure 2). BM– and fetal liver (FL)-derived CD34+ cells were stimulated in a similar manner (Figure 1D). CFC assays showed that CCL28, similarly to TPO, induced a robust expansion of functional progenitor cells together with SCF (Figure 1E and supplemental Figure 3A). CCL28 also promoted growth, but to a lesser extent, in combination with TPO or fms-like tyrosine kinase 3 ligand (FLT3L) (supplemental Figure 3C-D). When it was added alone, CCL28 failed to induce a net proliferation of CD34+ cells but was sufficient to maintain progenitor activity (Figure 1F-G), suggesting that it provides crucial survival signals for primitive hematopoietic cells.
To understand the basis of the cellular response to CCL28 stimulation, we performed transcriptional profiling and gene set enrichment analysis.17 Treatment with either CCL28 (Figure 1H) or TPO (supplemental Figure 4) caused a significant enrichment of genes correlating with the GO term cell cycle. Genes associated with cell viability were significantly enriched in CCL28-stimulated cells but not in cells treated with TPO (Figure 1H; supplement Figure 4). The gene expression signatures translated into effects on cell cycling with higher frequencies of dividing cells after CCL28 and TPO stimulation (Figure 1I,K). Moreover, we observed a trend toward decreased apoptosis in CCL28-stimulated cells (Figure 1J,L). Thus, CCL28 supports proliferation of hematopoietic progenitors by stimulating cell cycling and by suppressing apoptosis.
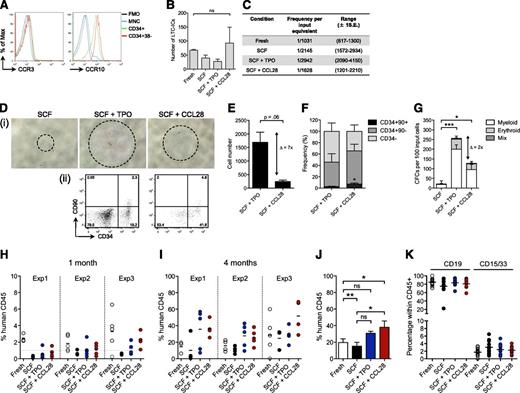
CCL28 signals through the 2 cell surface receptors CCR3 and CCR10.18,19 We detected CCR10, but not CCR3, expression in the vast majority of CD34+ cells and in the HSC-enriched CD34+CD38– population (Figure 2A), suggesting that CCL28 operates through CCR10 on the earliest hematopoietic progenitors. To assess whether CCL28 indeed supports the most immature fraction of HSPCs, we performed LTC-IC assays, which showed preserved LTC-IC activity of CCL28-treated cultures, compared with starting equivalents of fresh cells (Figure 2B-C). These results prompted us to investigate the impact of CCL28 treatment on growth and engraftment capacity of CB-derived putative human HSCs (CD34hiCD38loCD90+CD45RA– cells).20 As indicated by receptor expression, CCL28 stimulated putative HSCs in a direct manner, demonstrated by increased cell numbers and progenitor activity compared with SCF-treated cultures (Figure 2D-G). Although TPO treatment outranged CCL28 stimulation with regard to overall proliferation potential, CCL28 maintained a higher frequency of the most primitive cell fractions (Figure 2F). In addition, transplantation of CCL28-stimulated HSCs into NSG mice21 yielded 62% engrafted recipients 4 weeks post transplant, whereas only 9% and 36% of the mice transplanted with SCF- and TPO-treated cells, respectively, displayed human chimerism >1% (Figure 2H; supplemental Table 2). Compared with equivalent numbers of fresh cells, CCL28-treated cells showed slightly lower short-term engraftment, indicating either a subtle loss of short-term HSCs or delayed engraftment kinetics of the cultured cells (supplemental Figure 5). By contrast, CCL28 treatment significantly improved long-term multilineage reconstitution levels compared with both fresh cells and cells cultured with SCF alone, demonstrating that the combined effect of SCF and CCL28 supports a net increase of long-term repopulating activity (Figure 2I-K). TPO-treated cells showed similar engraftment levels as CCL28, although not significantly increased, compared with fresh cells. Collectively, these findings implicate CCL28 as a new, potent growth factor that supports the functional integrity of ex vivo–cultured HSPCs, placing it in a unique category of cytokines with HSC-supportive functions such as SCF and TPO.
CCL28 directly stimulates putative human HSCs and improves the long-term repopulating activity. (A) Representative histogram plots for cell surface expression of CCR3 and CCR10 in different fractions of CB (n = 5). FMO, fluorescence minus one; MNC, mononuclear cells. (B-C) CD34-enriched CB cells were plated in limiting dilutions either at day 0 or day 7 after culture in S10 and the indicated cytokines. Shown are LTC-IC numbers (B) and frequencies (C) as calculated by L-Calc (n = 3). (D-F) CB CD34hiCD38loCD90+CD45RA– cells were cultured in S10 and the respective cytokines and monitored for proliferation and progenitor activity. (D) Representative bright-field microscopy images (i) and FACS plots (ii) of the progeny from 1 × 102 CD34hiCD38loCD90+CD45RA– cells at day 10. (E-F) Total cell number (E) and frequency (F) of CD34–, CD34+CD90–, and CD34+CD90+ cells as determined by FACS analysis at days 10 to 13 (n = 3). (G) Numbers of differential CFCs per an equivalent of 100 input CD34hiCD38loCD90+CD45RA– cells at day 0 (n = 4). Statistical significance was calculated on total CFCs. Delta (Δ) indicates the difference between TPO and CCL28 treatment. (H-K) CD34hiCD38loCD90+CD45RA– cells were transplanted either directly or after 7 days of culture with the indicated cytokines into sublethally irradiated NSG recipients. Engraftment levels of human cells in peripheral blood after 1 (H) and 4 (I) months are shown. The dashed line in (H) marks the 1% cutoff for positive engraftment. Each data point represents an individual mouse; shown is data from 3 independent experiments with 3 to 5 recipients per group. Experiment 1: 1 × 103 IEM; experiments 2 and 3: 2 × 103 input equivalents per mouse. (J) Collated data showing recipient means across all experiments. (K) Lineage distribution in peripheral blood of NSG recipients 4 months post transplantation. *P < .05; **P < .01, ***P < .001.
CCL28 directly stimulates putative human HSCs and improves the long-term repopulating activity. (A) Representative histogram plots for cell surface expression of CCR3 and CCR10 in different fractions of CB (n = 5). FMO, fluorescence minus one; MNC, mononuclear cells. (B-C) CD34-enriched CB cells were plated in limiting dilutions either at day 0 or day 7 after culture in S10 and the indicated cytokines. Shown are LTC-IC numbers (B) and frequencies (C) as calculated by L-Calc (n = 3). (D-F) CB CD34hiCD38loCD90+CD45RA– cells were cultured in S10 and the respective cytokines and monitored for proliferation and progenitor activity. (D) Representative bright-field microscopy images (i) and FACS plots (ii) of the progeny from 1 × 102 CD34hiCD38loCD90+CD45RA– cells at day 10. (E-F) Total cell number (E) and frequency (F) of CD34–, CD34+CD90–, and CD34+CD90+ cells as determined by FACS analysis at days 10 to 13 (n = 3). (G) Numbers of differential CFCs per an equivalent of 100 input CD34hiCD38loCD90+CD45RA– cells at day 0 (n = 4). Statistical significance was calculated on total CFCs. Delta (Δ) indicates the difference between TPO and CCL28 treatment. (H-K) CD34hiCD38loCD90+CD45RA– cells were transplanted either directly or after 7 days of culture with the indicated cytokines into sublethally irradiated NSG recipients. Engraftment levels of human cells in peripheral blood after 1 (H) and 4 (I) months are shown. The dashed line in (H) marks the 1% cutoff for positive engraftment. Each data point represents an individual mouse; shown is data from 3 independent experiments with 3 to 5 recipients per group. Experiment 1: 1 × 103 IEM; experiments 2 and 3: 2 × 103 input equivalents per mouse. (J) Collated data showing recipient means across all experiments. (K) Lineage distribution in peripheral blood of NSG recipients 4 months post transplantation. *P < .05; **P < .01, ***P < .001.
To assess how CCL28 affects HSPCs in the context of a rich cytokine cocktail, we added it in combination with SCF, TPO, and FLT3L (STF) to cultures of CB CD34+ cells. In general, STF conditions supported CFCs and led to elevated numbers of LTC-ICs compared with fresh cells, whereas NSG engraftment levels were markedly reduced (supplemental Figure 6), indicating a strong proliferative drive, but poor maintenance, of the most primitive cells. The addition of CCL28 to these conditions enhanced the output of early progenitors with mixed CFC potential, but did not rescue the adverse effects of STF stimulation on NSG reconstitution ability (supplemental Figure 6). These findings illustrate the context-dependent function of growth factors and support the notion that multiple cytokine stimulation promotes proliferation at the expense of HSC activity.9,22 Thus, the further assessment of CCL28 to improve ex vivo culture conditions for HSPCs should be conducted in a systematic manner with other growth factor contexts and/or recently discovered molecules for HSC expansion.3,,-6,10,23
Finally, given the presence of CCL28 in the BM microenvironment24 (supplemental Figure 7), the potential role of CCL28 in regulating HSPCs in vivo as a niche-secreted factor under both normal and malignant25 conditions represents another interesting topic for future studies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Johan Richter, Justyna Rak, and Ann-Margreth Carlsson for providing human samples, and Lena Persson Feld for expert animal care.
This work was supported by grants from the Swedish Research Council, the Swedish Cancer Foundation, and the Swedish Pediatric Cancer Foundation (to J.L.).
Authorship
Contribution: C.K. and J.L. designed the study; C.K., A.B., N.M., G.K., and R.G. performed research; S.S. and M.M. performed microarray data analysis; T.E. provided scientific expertise; and C.K. and J.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jonas Larsson, Molecular Medicine and Gene Therapy, BMC A12, 221 84, Lund, Sweden; e-mail: jonas.larsson@med.lu.se.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal