Abstract
Human endoglin is an RGD-containing transmembrane glycoprotein identified in vascular endothelial cells. Although endoglin is essential for angiogenesis and its expression is up-regulated in inflammation and at sites of leukocyte extravasation, its role in leukocyte trafficking is unknown. This function was tested in endoglin heterozygous mice (Eng+/−) and their wild-type siblings Eng+/+ treated with carrageenan or LPS as inflammatory agents. Both stimuli showed that inflammation-induced leukocyte transendothelial migration to peritoneum or lungs was significantly lower in Eng+/− than in Eng+/+ mice. Leukocyte transmigration through cell monolayers of endoglin transfectants was clearly enhanced in the presence of endoglin. Coating transwells with the RGD-containing extracellular domain of endoglin, enhanced leukocyte transmigration, and this increased motility was inhibited by soluble endoglin. Leukocytes stimulated with CXCL12, a chemokine involved in inflammation, strongly adhered to endoglin-coated plates and to endoglin-expressing endothelial cells. This endoglin-dependent adhesion was abolished by soluble endoglin, RGD peptides, the anti-integrin α5β1 inhibitory antibody LIA1/2 and the chemokine receptor inhibitor AMD3100. These results demonstrate for the first time that endothelial endoglin interacts with leukocyte integrin α5β1 via its RGD motif, and this adhesion process is stimulated by the inflammatory chemokine CXCL12, suggesting a regulatory role for endoglin in transendothelial leukocyte trafficking.
Key Points
Endothelial endoglin has a regulatory role in leukocyte trafficking through vascular endothelia.
Leukocytes and endothelial cells interact via integrin receptors and endoglin, being this cell adhesion process stimulated by inflammatory stimuli.
Introduction
The vascular endothelium controls the transit of white blood cells into and out of the bloodstream. The migration of leukocytes involves the adhesive interaction of cell surface receptors with ligands expressed on endothelial cells in a process regulated by inflammatory stimuli. Stromal-derived factor 1 (SDF1α), renamed CXCL12, is a potent chemoattractant for a variety of cells including lymphocytes, monocytes, dendritic cells, and hematopoietic stem cells.1 CXCL12 and its receptor CXCR4 play relevant roles in immune and inflammatory responses, including leukocyte migration and recruitment, as well as integrin-dependent adhesion and transendothelial migration.1 CXCL12 is a critical activator of endothelial progenitors by inducing a proangiogenic phenotype and increasing migration and rolling mediated by α4 and αM integrin subunits.2
The process of leukocyte migration through the endothelial cell monolayer involves an interaction between leukocytes' integrins and endothelial-cell receptors, both acting as adhesion molecules. Integrins most relevant to leukocytes belong to the β1-integrin and the β2-integrin subfamilies. Classic chemoattractants and chemokines are the most powerful physiologic activators of integrin-mediated adhesion.3 In particular, chemokines can rapidly regulate leukocyte adhesion in a cell-specific manner by increasing both integrin affinity and valency.3 Nowadays, there is an active area of research trying to understand the molecular basis by which leukocyte integrins are involved in adhesion and transendothelial trafficking.
Human endothelial cells display on the plasma membrane an RGD-containing glycoprotein of 180-kDa called endoglin, alias CD105, which plays a critical physiologic role in the cardiovascular system.4,5 Endoglin is an auxiliary receptor for the transforming growth factor β (TGF-β) family of proteins that is essential for angiogenesis.5,6 Indeed, endoglin knockdown (Eng−/−) mouse embryos die because of vascular and cardiac abnormalities7 and endothelial cells from Eng+/− mice show reduced proliferation and migration and impaired blood vessel formation in vitro and in vivo.5,8 Furthermore, mutations in the human endoglin gene, that lead to endoglin haploinsufficiency, are responsible for hereditary hemorragic telangectasia (HHT) type 1, characterized by telangiectases in skin and mucosa, arteriovenous malformations in several organs, as well as nose and gastrointestinal bleeding.9 Shedding of a soluble form of endoglin, produced by MMP-1410 is involved in angiogenesis5,11 and plasma levels of soluble endoglin are markedly increased in preeclampsia, a disease associated with vascular hypertension and endothelial dysfunction.12 Structurally, the extracellular region of endoglin contains 2 distinct domains: (1) the NH2-terminal orphan domain involved in binding to TGF-β family members13-15 ; and (2) a zona pellucida (ZP) juxtamembrane domain containing ∼ 260 amino acid residues subdivided in ZP-N and ZP-C subdomains.13 Human endoglin displays an RGD motif within the ZP-N subdomain.4,13 The RGD tripeptide is a prototypic member of a family of motifs involved in integrin-based interactions with extracellular matrix (ECM) proteins, including fibronectin, fibrinogen, prothrombin, tenascin, thrombospondin, vitronectin, or von Willebrand factor.16 Members of the RGD family of related peptides have in common the presence of an acidic residue (D or E) that is critically involved in coordinating a Mg2+ cation bound to the MIDAS motif in the I domain of integrin β subunits,17 and this acidic residue is conserved among mammalian endoglins. Interestingly, the homologous sequence of the human RGD in mouse and pig endoglins is TDD, a motif that has also been shown in the disintegrin domain of murine ADAM-15 to specifically interact with integrins.18 These data suggest the involvement of endoglin in integrin binding and early studies postulated the implication of human endoglin in the binding to RGD-specific integrins,19,20 but no experimental basis for this hypothesis has been provided.
Several lines of evidence support a role for endoglin in leukocyte trafficking. First, endoglin expression is markedly up-regulated in endothelial cells of inflamed tissues with an associated inflammatory cell infiltrate.21 Second, endoglin is up-regulated in the postischemic kidney and endoglin-haploinsufficient mice are protected from renal ischemia-reperfusion injury, because of a reduction of cellular inflammatory responses.22 Moreover, arterial, venous, and capillary endothelia in lymphoid organs are highly reactive with anti-endoglin antibodies and a marked staining pattern is observed in high endothelial venules.23 Although endoglin is present throughout the vascular endothelium, its expression is stronger in capillaries, where most leukocyte infiltration to organs occurs, than in veins or arteries,24 suggesting a role for endothelial endoglin in lymphocyte trafficking and transmigration. The purpose of this study is to assess this hypothesis. Our results demonstrate a decreased leukocyte trafficking in endoglin-deficient mice, and that endothelial endoglin interacts with leukocyte integrin α5β1 via its RGD motif. This adhesion process is stimulated by the inflammatory chemokine CXCL12, suggesting a regulatory role for endoglin in transendothelial leukocyte trafficking.
Methods
Mice, in vivo treatments, and tissue staining
Mice were used in experimental protocols according with the regulations of the following institutions: Conseil de l′Europe (Directive 86/609/CEE published in the Oficial Daily No. L358/1-358/6, 18 December 1986) and Spanish Government (Real Decreto 223/1988 published in Boletín Oficial del Estado No. 67, pp. 8509-8512, 18 March 1988 and Disposición General No. 25 805 published in Boletín Oficial del Estado No. 256, pp. 31 349-31 362, 28 October 1990). Endoglin heterozygous (Eng+/−) mice25 were backcrossed onto the C57BL/6 (B6) background and genotyped as described.8 Inflammation of the peritoneum was achieved with carrageenan (intraperitoneal single injection; 1.5 μg/g body weight). Systemic inflammation was induced by a single intraperitoneal injection of lipopolysaccharide (LPS, 250 μg/g body weight). Lavages of the peritoneal cavity and tissue samples collection were performed at 4 and 24 hours after induction of inflammation. Blood samples were obtained from the tail tip into heparinized tubes. Total leukocytes and leukocyte composition in blood and peritoneal samples were analyzed by flow cytometry. Lung samples of powdered tissue were processed and myeloperoxidase (MPO) activity was measured following the Bradley method modified by Mullane.22 Tissue sections (3 μm) from lung were stained with hematoxylin and eosin or with the monoclonal anti-CD68 (Dako) antibody, followed by the secondary antibody Alexa488 anti–mouse IgG (Life Technologies).
Cells and stable transfectants
Human B and T lymphocytes were selected from buffy coats using lymphocyte specific MACS MicroBeads and the AutoMACS equipment (Miltenyi Biotec). The human cell lines Nalm6, Jurkat, Peer, U937, THP-1 and K562, B and T lymphocytes, and the mouse cell line SP2 were cultured in RPMI 1640 supplemented with 10% heat inactivated fetal calf serum (FCS). Human umbilical vein endothelial cells (HUVECs) were grown in EBM-2 medium, supplemented by EGM-2 SingleQuots (Lonza). Blood outgrowth endothelial cells (BOECs) were grown using 50 mL peripheral blood from healthy subjects or HHT patients as described.26 The generation of human endoglin transfectants in the rat myoblast cell line (L6E9-Mock and L6E9-E) has been described.27 The tetracycline-inducible bovine endothelial GM7372-EL and GM7372-Mock cells were cultured in DMEM medium.28 For induction of human endoglin expression in endothelial GM7372-EL cells,28 500 ng/mL of doxycycline (Dox; Sigma-Aldrich) was used in the culture media. The mouse endothelial cell line MS1 was cultured in DMEM with 10% FCS.
Antibodies and other reagents
Mouse monoclonal antibodies (mAbs) specific for human endoglin (P4A4), HLA-I (W6/32), β1 integrin (TS2/16; activating), β1 integrin (LIA1/2; inhibiting), CD31 (HC1/6), CD9 (VJ1/120), E-selectin (TEA2/1), VCAM (4B9), ICAM-1 (HU5/3), and rat MHC class I (OX18), and goat antibodies to CD146 (AF932; R&D) were used for flow cytometry or immunofluorescence microscopy. AMD3100 (Sigma-Aldrich) was used as inhibitor of the CXCR4/CXCL12 pathway. Human (rhEndoglin; Eng) and mouse (endoglin-Fc chimera; mEng) soluble endoglin, TNF-α and CXCL12 were from R&D Systems. The tripeptide RGD (Arg-Gly-Asp) was from Sigma-Aldrich and the pentapeptides GRGDS, SDGRG (DGR; negative control), the human endoglin-derived DRGDK (RGDK), and the mouse endoglin derived DTDDH (TDD) were synthesized in the Centro de Investigaciones Biologicas.
Flow cytometry and immunofluorescence microscopy
Cells were incubated with the primary antibody for 60 minutes at 4°C followed by Alexa Fluor secondary antibody (Invitrogen) for 30 minutes at 4°C. Fluorescence was measured in a Coulter Epics XL flow cytometer (Beckman Coulter). In transmigration assays, cell counting was performed using flow cytometry. Immunofluorescence on HUVECs was performed on chamber slides (Millipore). HUVECs were fixed and incubated with primary antibodies, followed by Alexa Fluor 488 secondary antibodies. Samples were observed using fluorescence confocal microscopy (SP2, Leica) and fluorescence intensity was measured with LAS-AF Lite Version 2.4.1 software (Leica).
Transwell migration assays
Transwell assays were performed using Transwell 24-well (Costar, Corning). Polycarbonate membranes were coated with rhEndoglin, CXCL12 or BSA. Alternatively, membranes were cultured until confluence (∼ 48 hours) with myoblast transfectants (L6E9-M and L6E9-E),27 endothelial transfectants (GM7372-M and GM7372-L),28 or endothelial cells (HUVECs, BOECs, MS1). To test the migration rate, different hematopoietic cell types in suspension were starved for 3 hours. Each transwell was seeded on top with 75 000 cells and RPMI medium 0.1% BSA and 100 ng/mL CXCL12 was added the lower part of the chambers.29,30 Transmigration was performed for 2 hours at 37°C. The net yield of transmigrated cells was within the 10%-42% range. Photographs of leukocyte adhesion were taken by confocal microscopy (SP5, Leica).
Static and flow chamber adhesion assays
Ninety-six–well plates (Falcon, Becton Dickinson) were coated with rhEndoglin 5 μg/mL, CXCL12 175 ng/mL, 320 ng/mL, or 500 ng/mL, and 1% BSA. Wells were blocked with 0.5% BSA in RPMI at 37°C. Cell adhesion was also performed on HUVEC monolayers incubated or not with TNF-α (5 ng/mL) for 1 hour. Cells in suspension were stained by a fluorescence marker (CFSE, Molecular Probes, Invitrogen). When necessary, cells were incubated with 10 μg/mL of mAbs TS2/16 or LIA1/2 or with 200 μM MnCl2 (Merck), a global activator of integrin activity.31 For competition experiments with rhEndoglin (Eng) or mEng, inhibitory effects were observed within the concentration range of 50 ng/mL-1 μg/mL. Cells were lysed and quantification of substrate adherence was carried out using a fluorescent analyzer (Varioskan, Thermo-Fisher Scientific). The images of cell adhesion were taken by the camera Olympus E-330 assembled on a light microscope (Axiovert 25, Zeiss). Flow chamber adhesion assays were carried out as described.32 Petri dishes were coated with rhEndoglin, CXCL12 or BSA. Cells were infused and allowed to contact the coating for 10 minutes before flow rate was adjusted to 1 dyne/cm2 (0.2 mL/min) and then increased at 2, 4, 6, 8, and 10 dyne/cm2. The range 1 to 10 dynes/cm2 is justified because under physiologic conditions shear stress vary from 0.4 dyne/cm2 (small veins) to 20 dyne/cm2 (peak flow in abdominal aorta).
Statistical analysis
All values are expressed as mean ± standard error of the mean (SEM). All multiple comparison data have been analyzed using 1-way ANOVA with posthoc Bonferroni and Scheffe tests. Direct group-group comparisons were carried out using independent student t tests with prior Levene tests for equality of variances. P < .01 was considered statistically significant, and P < .005 was considered highly statistically significant.
Results
Effect of endoglin deficiency on leukocyte infiltration during inflammation
Leukocyte infiltration was tested in Eng+/− and Eng+/+ mice treated intraperitoneally with carrageenan, a chemical agent that stimulates the transmigration of leukocytes to the peritoneal cavity. Indeed, Eng+/− and Eng+/+ mice showed a marked increase in the number of leukocytes into the peritoneum 24 hours after injection of carrageenan. This increase was significantly lower in Eng+/− than in Eng+/+ mice and was observed for neutrophils, lymphocytes, monocytes, and basophiles, but not for the small population of eosinophiles (Figure 1A). As expected, similar differences between Eng+/− and Eng+/+ mice were also observed in the number of total leukocytes infiltrated toward the peritoneum (Figure 1B left panel). This was accompanied by a decrease in total leukocytes in blood, that was lower in Eng+/− than in Eng+/+ mice (Figure 1B right panel). Furthermore, a systemic inflammation was induced by a single intraperitoneal injection of lipopolysaccharide (LPS) in Eng+/− and Eng+/+ mice. After 4 hours of induction of systemic inflammation with LPS, the myeloperoxidase (MPO) activity, used as a quantitative assessment of neutrophil/macrophage infiltration (Figure 1C) and the presence of the macrophage marker CD68 detected by specific immunofluorescence (Figure 1D) in lungs from Eng+/+ and Eng+/− mice, were evaluated. Both, MPO activity and CD68 staining, as detected by specific immunofluorescence, showed a marked neutrophil/macrophage infiltration in Eng+/+ that was much lower in Eng+/− mice. These results suggest that endoglin haploinsufficiency leads to a lower endothelial transmigration rate to the inflamed tissues.
Endothelial leukocyte transmigration in endoglin heterozygous mice treated with inflammatory stimuli. (A-B) Total leukocyte endothelial transmigration was monitored in carrageenan-treated and untreated Eng+/+ and Eng+/− mice. Subpopulations of leukocytes were monitored in the peritoneal fluid (A). Total leukocyte count (TLC) was measured in peritoneal fluid and blood (B). Data are the average of 3 experiments with 5 mice per experiment and group. *P < .05, respect to untreated animals; δP < .05, respect to carrageenan-treated Eng+/+ mice; #P < .05, respect to untreated Eng+/+ mice. (C-D). Transmigration in endoglin heterozygous mice injected with LPS. Myeloperoxidase (MPO) activity (C) and immunohistochemistry for the macrophage marker CD68 (D) in lung 4 hours after induction of systemic inflammation with LPS (25 mg/kg body weight) in Eng+/+ and Eng+/− mice. (C) Enzymatic activity values are represented as MPO/min/10 μg protein in lung tissues from untreated (white bars) or LPS-treated (green bars, n = 5) animals (white bars, n = 5). Values are expressed as mean ± SEM of 4 experiments with 5 mice per experiment and group. *P < .02, untreated versus LPS-treated; δP < .05, respect to LPS-treated Eng+/+ mice. (D) Immunofluorescence staining with anti-CD68 in lung sections (3 μm) from the same mice as in panel C). Representative photographs are shown. Magnification, 400×.
Endothelial leukocyte transmigration in endoglin heterozygous mice treated with inflammatory stimuli. (A-B) Total leukocyte endothelial transmigration was monitored in carrageenan-treated and untreated Eng+/+ and Eng+/− mice. Subpopulations of leukocytes were monitored in the peritoneal fluid (A). Total leukocyte count (TLC) was measured in peritoneal fluid and blood (B). Data are the average of 3 experiments with 5 mice per experiment and group. *P < .05, respect to untreated animals; δP < .05, respect to carrageenan-treated Eng+/+ mice; #P < .05, respect to untreated Eng+/+ mice. (C-D). Transmigration in endoglin heterozygous mice injected with LPS. Myeloperoxidase (MPO) activity (C) and immunohistochemistry for the macrophage marker CD68 (D) in lung 4 hours after induction of systemic inflammation with LPS (25 mg/kg body weight) in Eng+/+ and Eng+/− mice. (C) Enzymatic activity values are represented as MPO/min/10 μg protein in lung tissues from untreated (white bars) or LPS-treated (green bars, n = 5) animals (white bars, n = 5). Values are expressed as mean ± SEM of 4 experiments with 5 mice per experiment and group. *P < .02, untreated versus LPS-treated; δP < .05, respect to LPS-treated Eng+/+ mice. (D) Immunofluorescence staining with anti-CD68 in lung sections (3 μm) from the same mice as in panel C). Representative photographs are shown. Magnification, 400×.
Effect of endoglin expression on leukocyte transmigration in vitro
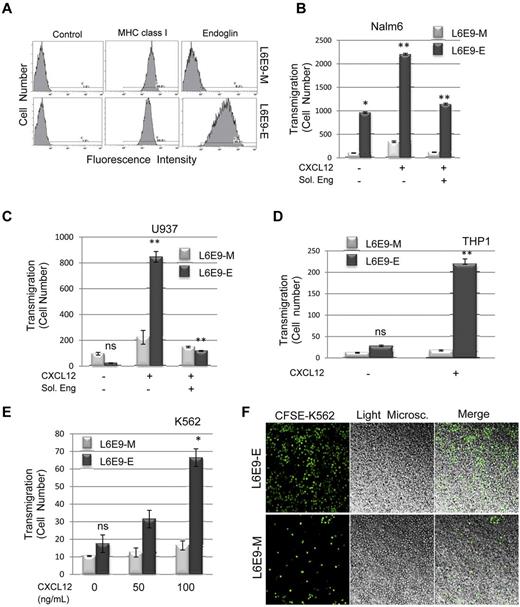
To investigate whether endoglin plays an active role in vascular endothelium during leukocyte transmigration, transwell assays with endoglin transfectants were performed. Myoblast transfectants expressing human endoglin (L6E9-E) grow in monolayers, somehow mimicking the vessels' endothelium, and allow the study of the individual contribution of endoglin.27 As shown in Figure 2A, human endoglin is highly expressed on the cell surface of L6E9-E transfectants compared with mock transfectants (L6E9-M), as determined by fluorescence flow cytometry. Transmigration of the B-cell line Nalm6, the monocytic cell lines U937 and THP-1, as well as the erythroleukemic line K562 was markedly increased through L6E9-E transfectants compared with mock transfectants (L6E9-M) in the presence of CXCL12, a chemokine induced by proinflammatory stimuli, such as LPS (Figure 2B-E). This response was significantly reduced in the presence of the ectodomain of endoglin when assayed in Nalm6 (Figure 2B, supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and U937 cells (Figure 2C). Soluble endoglin inhibited transmigration from 50 ng/mL to 5 μg/mL (data not shown) and using a constant concentration of 1 μg/mL, a time-dependent inhibition was observed (supplemental Figure 1), supporting the involvement of the extracellular region of endoglin. Of note, an increased endoglin-dependent migratory response was also observed in absence of the chemokine with Nalm6 cells (Figure 2B). The endoglin-dependent response with the erythroleukemic cell line K562 (Figure 2E) showed low transmigratory levels, in agreement with the poor motility of this cell lineage. Interestingly, examination of the transwells after the transmigration assay showed that many of K562 cells remained bound to the monolayer of endoglin transfectants, whereas very few cells were detected bound to mock transfectants (Figure 2F). Similar results were obtained with Nalm6 cells (data not shown), suggesting that the endoglin-dependent transmigration response involves a cell adhesion step.
Leukocyte trasmigration through endoglin expressing transfectants. (A) Flow cytometry analysis of endoglin transfectants. Rat myoblast transfectants expressing human endoglin (L6E9-E) and the corresponding mock transfectants (L6E9-M) were analyzed by immunofluorescence flow cytometry using mAbs P4A4 (anti-endoglin), OX18 (anti-MHC class I antigen), as a positive control and X63 (left panels) as a negative control. (B-E) Transmigration assays. Transwell experiments were performed using 0.5-μm pore membranes previously covered with a confluent monolayer of myoblast transfectants (L6E9-M or L6E9-E). To test the migration rate, Nalm6, U937, THP-1, or K562 cells were seeded on top of the transwell and 100 ng/mL CXCL12 (unless stated otherwise) was added to the bottom of chambers. After incubation for 2 hours at 37°C, an aliquot (100 μL) of transmigrated cells was counted by flow cytometry. There is a significant increased transmigration through L6E9-E versus L6E9-M (B-E) that is clearly inhibited in the presence of soluble endoglin (B-C). The transmigration of K562 is much lower than that of Nalm6, U937, or THP-1 cells. Each bar represents the mean value of 6 (B) or 3 (C-D) assays each performed in triplicates (**P < .005; *P < .01; ns, not significant). (F) Confocal analysis of transwells after transmigration. K562 cells were labeled with CFSE, loaded in the wells and subjected to transmigration assays through L6E9-E or L6E9-M transfectants as in panels B and D. Then, remaining cells on transwells were visualized by confocal microscopy. CFSE-labeled cells emitting green light are on the left, monolayers of L6E9 transfectants and K562 transmigrating cells visualized by light microscopy are in the middle, and the merged figures are on the right. Magnification, ×200.
Leukocyte trasmigration through endoglin expressing transfectants. (A) Flow cytometry analysis of endoglin transfectants. Rat myoblast transfectants expressing human endoglin (L6E9-E) and the corresponding mock transfectants (L6E9-M) were analyzed by immunofluorescence flow cytometry using mAbs P4A4 (anti-endoglin), OX18 (anti-MHC class I antigen), as a positive control and X63 (left panels) as a negative control. (B-E) Transmigration assays. Transwell experiments were performed using 0.5-μm pore membranes previously covered with a confluent monolayer of myoblast transfectants (L6E9-M or L6E9-E). To test the migration rate, Nalm6, U937, THP-1, or K562 cells were seeded on top of the transwell and 100 ng/mL CXCL12 (unless stated otherwise) was added to the bottom of chambers. After incubation for 2 hours at 37°C, an aliquot (100 μL) of transmigrated cells was counted by flow cytometry. There is a significant increased transmigration through L6E9-E versus L6E9-M (B-E) that is clearly inhibited in the presence of soluble endoglin (B-C). The transmigration of K562 is much lower than that of Nalm6, U937, or THP-1 cells. Each bar represents the mean value of 6 (B) or 3 (C-D) assays each performed in triplicates (**P < .005; *P < .01; ns, not significant). (F) Confocal analysis of transwells after transmigration. K562 cells were labeled with CFSE, loaded in the wells and subjected to transmigration assays through L6E9-E or L6E9-M transfectants as in panels B and D. Then, remaining cells on transwells were visualized by confocal microscopy. CFSE-labeled cells emitting green light are on the left, monolayers of L6E9 transfectants and K562 transmigrating cells visualized by light microscopy are in the middle, and the merged figures are on the right. Magnification, ×200.
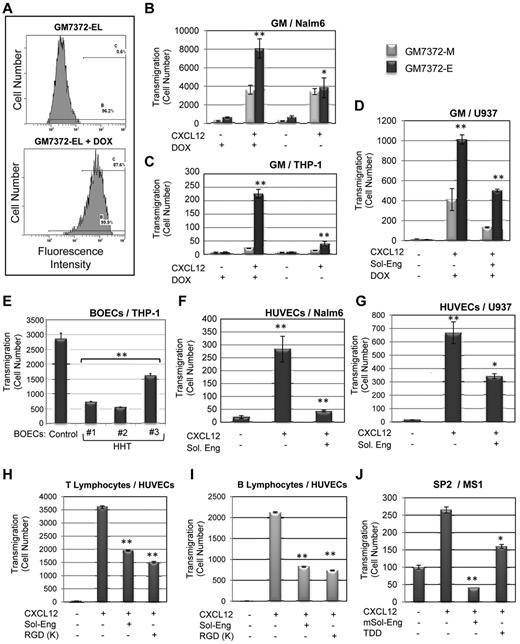
A comparative analysis among different chemokines in leukocyte transmigration through HUVECs was carried out. Under our experimental conditions CXCL12 induced a marked increased transmigration, whereas other chemokines tested (CXCL2, CXCL5, and CXCL20) showed a much weaker effect (supplemental Figure 2). Based on these data and the fact that CXCR4 is the only chemokine receptor with ubiquitous expression in leukocytes and is involved in integrin activation,1 we selected CXCL12 for the following transmigration experiments. To further assess the role of endothelial endoglin in leukocyte transmigration, 3 different models of endothelial cells were used. First, a doxycycline-inducible bovine endothelial cell line, GM7372-EL was studied.28 These cells have been engineered to express human endoglin in the presence of doxycycline (Dox), as evidenced by fluorescence flow cytometry (Figure 3A). As shown in Figure 3B through D, transmigration of Nalm6, THP-1, and U937 cells through the GM7372-EL monolayer was markedly increased on endoglin overexpression with Dox compared with mock transfectants (GM7372-M). This response was significantly reduced in the presence of the ectodomain of endoglin when assayed in U937 cells (Figure 3D), confirming the involvement of the extracellular region of endoglin. Second, transmigration studies with blood outgrowth endothelial cells (BOECs) from HHT patients who show an endoglin deficient expression26 were carried out. As shown in Figure 3E, on CXCL12 stimulation, transmigration levels of THP-1 cells through HHT BOECs showed a decrease with respect to healthy controls, suggesting a critical role for endoglin in this process. Next, similar transwell assays were performed using HUVECs (Figure 3F-I). Transmigration activity of Nalm6 cells, U937 cells, and T and B lymphocytes through HUVECs was clearly increased on CXCL12 stimulation and this increase was markedly reduced in the presence of soluble endoglin (Figure 3F-I) or the endoglin-derived peptide RGDK (Figure 3H-I). Interestingly, transmigration activity of mouse SP2 cells through murine MS1 endothelial cells was also increased on CXCL12 stimulation and this increase was inhibited in the presence of mouse soluble endoglin or the murine endoglin-derived peptide TDD (Figure 3J).
Leukocyte transmigration through endothelial cells. (A-D) Endoglin overexpressing endothelial cells. (A) Flow cytometry analysis of endoglin transfectants. The tetracycline-inducible bovine endothelial cell line GM7372-EL, engineered to express human endoglin were incubated or not with Dox for 24 hours. Flow cytometry analysis was carried out on the Dox inducible green fluorescence protein (GFP) used here as a marker of human endoglin expression. (B-D) Transmigration assays. Transwell experiments were performed using confluent monolayers of bovine endothelial cell lines GM7372-EL and GM7372-M. To test the migration rate, Nalm6 (B), THP-1 (C), or U937 (D) cells were seeded on top of the transwell and 100 ng/mL CXCL12 was added to the bottom of chambers, unless otherwise stated. Soluble endoglin (Sol-Eng) was added to U937 cells, as indicated (D). After incubation for 2 hours at 37°C, transmigrated cells were counted by flow cytometry. There is a significant increased transmigration through GM7372-EL versus GM7372-M monolayers. Each bar represents the mean value of 3 assays each performed in triplicates. **P < .005; *P < .01. (E-I) Primary cultures of endothelial cells. (E) Blood outgrowth endothelial cells (BOECs). Confluent monolayers from healthy subjects (Control) or 3 different HHT patients (Nos. 1, 2, and 3) were subjected to transmigration assays using THP-1 cells as in previous figures. Endothelial cells from HHT No. 1 correspond to an HHT1 patient with a pathogenic mutation (R171X) in the endoglin gene and endothelial cells from HHT no. 2 and no. 3 show decreased expression of endoglin protein.28 There is a significant decreased transmigration through HHT versus control monolayers of BOECs. (F-I) Transmigration through human umbilical vein endothelial cells (HUVECs). Confluent monolayers of HUVECs were tested for the migration rate of Nalm6 (F), U937 (G), T lymphocytes (H), and B lymphocytes (I) in the presence or absence of 100 ng/mL CXCL12 at the bottom of chambers. Soluble endoglin (Sol-Eng) at 1 μg/mL or the human endoglin-derived RGDK peptide were added, as indicated. (J) Transmigration of murine SP2 cells through mouse MS1 endothelial cells was carried out as above, but in the presence of mouse soluble endoglin (mSol-Eng) at 1 μg/mL or mouse endoglin-derived TDD peptide, as indicated. After incubation for 2 hours at 37°C, transmigrated cells were counted by flow cytometry. In the presence of CXCL12 there is a marked increased transmigration of human Nalm6 and U937 cells as well as T and B lymphocytes through HUVECs that is inhibited in the presence of soluble endoglin. A similar behavior was observed with murine SP2 cells migrating through MS1 mouse endothelial cells. Each bar represents the mean value of 3 experiments, each performed in triplicates. **P < .005; *P < .01.
Leukocyte transmigration through endothelial cells. (A-D) Endoglin overexpressing endothelial cells. (A) Flow cytometry analysis of endoglin transfectants. The tetracycline-inducible bovine endothelial cell line GM7372-EL, engineered to express human endoglin were incubated or not with Dox for 24 hours. Flow cytometry analysis was carried out on the Dox inducible green fluorescence protein (GFP) used here as a marker of human endoglin expression. (B-D) Transmigration assays. Transwell experiments were performed using confluent monolayers of bovine endothelial cell lines GM7372-EL and GM7372-M. To test the migration rate, Nalm6 (B), THP-1 (C), or U937 (D) cells were seeded on top of the transwell and 100 ng/mL CXCL12 was added to the bottom of chambers, unless otherwise stated. Soluble endoglin (Sol-Eng) was added to U937 cells, as indicated (D). After incubation for 2 hours at 37°C, transmigrated cells were counted by flow cytometry. There is a significant increased transmigration through GM7372-EL versus GM7372-M monolayers. Each bar represents the mean value of 3 assays each performed in triplicates. **P < .005; *P < .01. (E-I) Primary cultures of endothelial cells. (E) Blood outgrowth endothelial cells (BOECs). Confluent monolayers from healthy subjects (Control) or 3 different HHT patients (Nos. 1, 2, and 3) were subjected to transmigration assays using THP-1 cells as in previous figures. Endothelial cells from HHT No. 1 correspond to an HHT1 patient with a pathogenic mutation (R171X) in the endoglin gene and endothelial cells from HHT no. 2 and no. 3 show decreased expression of endoglin protein.28 There is a significant decreased transmigration through HHT versus control monolayers of BOECs. (F-I) Transmigration through human umbilical vein endothelial cells (HUVECs). Confluent monolayers of HUVECs were tested for the migration rate of Nalm6 (F), U937 (G), T lymphocytes (H), and B lymphocytes (I) in the presence or absence of 100 ng/mL CXCL12 at the bottom of chambers. Soluble endoglin (Sol-Eng) at 1 μg/mL or the human endoglin-derived RGDK peptide were added, as indicated. (J) Transmigration of murine SP2 cells through mouse MS1 endothelial cells was carried out as above, but in the presence of mouse soluble endoglin (mSol-Eng) at 1 μg/mL or mouse endoglin-derived TDD peptide, as indicated. After incubation for 2 hours at 37°C, transmigrated cells were counted by flow cytometry. In the presence of CXCL12 there is a marked increased transmigration of human Nalm6 and U937 cells as well as T and B lymphocytes through HUVECs that is inhibited in the presence of soluble endoglin. A similar behavior was observed with murine SP2 cells migrating through MS1 mouse endothelial cells. Each bar represents the mean value of 3 experiments, each performed in triplicates. **P < .005; *P < .01.
Endoglin is involved in leukocyte adhesion during transmigration
Several lines of evidence suggested the involvement of endoglin in the leukocyte adhesion step during transmigration: (1) the chemokine CXCL2 used in the transmigration experiments is an activator of the integrin family, including the α5β1 member present in leukocytes that recognizes ligands through the RGD motif; (2) the extracellular domain of endoglin contains an RGD motif and an RGD-containing soluble form of endoglin is able to inhibit leukocyte transmigration through endoglin expressing adherent cells (Figures 2–3); and (3) an increased cell adhesion to endoglin expressing adherent cells was observed in transmigration experiments (Figure 2F). Furthermore, the endoglin-dependent leukocyte transmigration through endoglin expressing adherent cells was inhibited by the RGD peptide (supplemental Figure 3) and by the chemokine receptor (CXCR4) inhibitor AMD3100 (data not shown), whereas it was increased in the presence of MnCl2 (data not shown), an activator of integrin activity.
To better define the role of endoglin in transmigration, transwells were coated with endoglin (instead of cell monolayers) in the absence or presence of CXCL12. Transmigration of Nalm6 cells through endoglin-coated membranes was higher than that of BSA-coated membranes, whereas transmigration through membranes simultaneously coated with endoglin and CXCL12 was negligible (Figure 4A bottom row, B). Compatible with these findings, the number of Nalm6 cells retained in endoglin-coated transwells was higher than in BSA-coated membranes, whereas membranes simultaneously coated with endoglin and CXCL12 showed a marked increase of bound cells well above the other coating conditions (Figure 4A top row, B). This strong adhesion is probably because of the combined action of CXCL12 that activates leukocyte integrins, followed by the binding of these integrins to endoglin, not allowing leukocyte transmigration. Next, adhesion of B lymphocytes to HUVECs was carried out on endothelial activation with TNF-α as an inflammatory stimulus and activator of leukocyte adhesion. Treatment with TNF-α showed a strong increase in lymphocyte binding to HUVECs33 that was inhibited at a similar extent by soluble endoglin or antibodies to E-selectin, an endothelial receptor critically involved in leukocyte adhesion (Figure 4C-D). The inhibitory action of soluble endoglin and anti–E-selectin antibodies was not additive (Figure 4D), a finding compatible with the similar inhibitory capacity of these 2 molecules in migratory studies of B lymphocytes through HUVECs (Figure 4E). Taken together, these results strongly suggest the involvement of endoglin in cell adhesion. The effect of TNF-α on endothelial endoglin was also studied at the expression and localization levels. As expected, TNF-α markedly up-regulated the expression of VCAM, ICAM-1, and CD146 in HUVECs,33,34 although moderately increasing CD31 and E-selectin. By contrast, TNF-α partly decreased endoglin expression, leaving CD9 levels unaffected (supplemental Figure 4). Immunofluorescence microscopy studies revealed a clear increase in the levels of endoglin at cell-cell contacts (Figure 4F-G; supplemental Videos 2-3). This change in the subcellular distribution of endoglin is compatible with an active role of endoglin during leukocyte extravasation that occurs mainly through the cell-cell contacts of endothelia. In the same experiment, CD146 was also redistributed to cell-cell contacts in TNF-α–treated cells as reported,34 to promote the transendothelial migration of leukocytes together with CD9, CD31, ICAM-1, and VCAM, which also localize to endothelial cell-cell contacts (supplemental Figures 4-5).
Role of endoglin in leukocyte transmigration. (A-B) Transmigration through endoglin coated membranes. (A) Transwell experiments were performed using 0.5-μm pore membranes previously coated with 1% BSA, 5 μg/mL soluble endoglin or 5 μg/mL soluble endoglin plus 175 ng/mL CXCL12, as indicated. Nalm6 cells were seeded on top of the transwell and 100 ng/mL CXCL12 was added to lower chambers. After incubation for 2 hours at 37°C, transmigrated cells in the bottom chamber were examined by inverse light microscopy (bottom row; magnification ×200) and cells that remained bound to the transwell were stained with propidium iodide (red) and visualized by confocal microscopy (top row; magnification, ×100). (B) Quantification of cells bound to the transwell (red bars) and cells transmigrated (gray bars) was carried out by measuring the surface area of the cells using ImageJ Version 1.46 software. Percentages of the cell signal respect to the total area are indicated. (C-D) Lymphocyte adhesion to HUVECs treated with TNF-α. Confluent monolayers of HUVECs were activated or not with TNF-α for 1 day. Then, culture medium was removed and HUVECs were tested for the adhesion of CFSE-labeled B lymphocytes in the presence CXCL12, soluble endoglin (Eng) or anti–E-selectin mAb (α-Sel), as indicated. (C) After incubation for 1 hour at 37°C, lymphocytes (green) bound to HUVECs were visualized by confocal microscopy (magnification, ×100). (D) Cells in plates were lysed and quantification of bound lymphocytes in the absence (white bars) or in the presence (gray bars) of TNF-α was carried out using a fluorescent analyzer. (E) Lymphocyte transmigration through HUVECs. Confluent monolayers of HUVECs were tested for the migration rate of B lymphocytes in the presence or absence of CXCL12 at the bottom of chambers. Soluble endoglin (Sol-Eng) or anti–E-selectin mAb (α-selectin) were added, as indicated. After incubation for 2 hours at 37°C, transmigrated cells were counted by flow cytometry. (F-G) Immunofluorescence of HUVECs treated with TNF-α. Monolayers of HUVECs were treated or not with TNF-α and incubated with P4A4 mAb (anti-endoglin), followed by the secondary antibody Alexa Fluor 488 anti–mouse IgG. (F) Samples were analyzed using fluorescence confocal microscopy (SP2, Leica). Representative photographs are shown (top 2 rows). The lower row shows the intensity of endoglin staining according to the color scale. (0-200) A diagram of an individual cell (dashed line) is drawn over the pictures. (G) The fluorescence intensity of the whole cell and the cell-cell contact sites was measured with LAS-AF Lite Version 2.4.1 software (Leica). Each value represents the mean average of 60 measurements. The statistical significance of treated versus untreated samples is indicated (**P < .005; *P < .01).
Role of endoglin in leukocyte transmigration. (A-B) Transmigration through endoglin coated membranes. (A) Transwell experiments were performed using 0.5-μm pore membranes previously coated with 1% BSA, 5 μg/mL soluble endoglin or 5 μg/mL soluble endoglin plus 175 ng/mL CXCL12, as indicated. Nalm6 cells were seeded on top of the transwell and 100 ng/mL CXCL12 was added to lower chambers. After incubation for 2 hours at 37°C, transmigrated cells in the bottom chamber were examined by inverse light microscopy (bottom row; magnification ×200) and cells that remained bound to the transwell were stained with propidium iodide (red) and visualized by confocal microscopy (top row; magnification, ×100). (B) Quantification of cells bound to the transwell (red bars) and cells transmigrated (gray bars) was carried out by measuring the surface area of the cells using ImageJ Version 1.46 software. Percentages of the cell signal respect to the total area are indicated. (C-D) Lymphocyte adhesion to HUVECs treated with TNF-α. Confluent monolayers of HUVECs were activated or not with TNF-α for 1 day. Then, culture medium was removed and HUVECs were tested for the adhesion of CFSE-labeled B lymphocytes in the presence CXCL12, soluble endoglin (Eng) or anti–E-selectin mAb (α-Sel), as indicated. (C) After incubation for 1 hour at 37°C, lymphocytes (green) bound to HUVECs were visualized by confocal microscopy (magnification, ×100). (D) Cells in plates were lysed and quantification of bound lymphocytes in the absence (white bars) or in the presence (gray bars) of TNF-α was carried out using a fluorescent analyzer. (E) Lymphocyte transmigration through HUVECs. Confluent monolayers of HUVECs were tested for the migration rate of B lymphocytes in the presence or absence of CXCL12 at the bottom of chambers. Soluble endoglin (Sol-Eng) or anti–E-selectin mAb (α-selectin) were added, as indicated. After incubation for 2 hours at 37°C, transmigrated cells were counted by flow cytometry. (F-G) Immunofluorescence of HUVECs treated with TNF-α. Monolayers of HUVECs were treated or not with TNF-α and incubated with P4A4 mAb (anti-endoglin), followed by the secondary antibody Alexa Fluor 488 anti–mouse IgG. (F) Samples were analyzed using fluorescence confocal microscopy (SP2, Leica). Representative photographs are shown (top 2 rows). The lower row shows the intensity of endoglin staining according to the color scale. (0-200) A diagram of an individual cell (dashed line) is drawn over the pictures. (G) The fluorescence intensity of the whole cell and the cell-cell contact sites was measured with LAS-AF Lite Version 2.4.1 software (Leica). Each value represents the mean average of 60 measurements. The statistical significance of treated versus untreated samples is indicated (**P < .005; *P < .01).
Endoglin is involved in integrin-mediated leukocyte adhesion
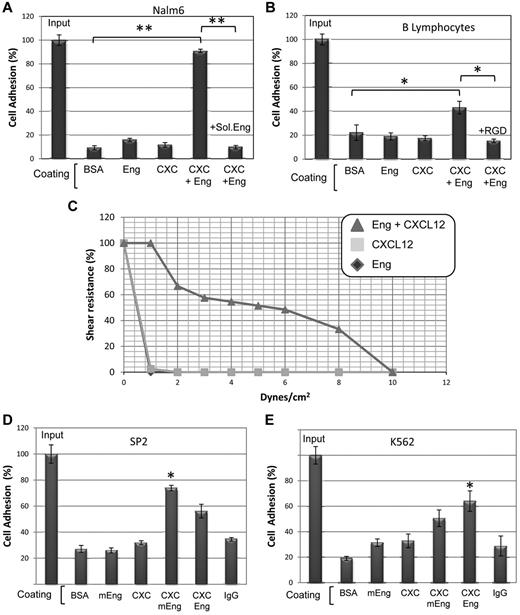
To further assess whether endoglin is involved in cell adhesion we performed static adhesion assays. As shown in Figure 5A, binding of the pre-B cell line Nalm6 to endoglin was slightly higher than that to BSA or CXCL12-coated plates. Interestingly, plates simultaneously coated with endoglin and CXCL12, showed a strong increase of cell binding that was abolished in the presence of soluble endoglin. Similar studies using B lymphocytes (Figure 5B) or U937, Peer, and Jurkat cells (data not shown) also showed a significantly higher adhesion to endoglin/CXCL12 than that to endoglin or CXCL12, and this endoglin-dependent adhesion was inhibited in the presence of the RGD peptide (Figure 5B). The strength of endoglin-dependent binding was next analyzed by cell adhesion assays under flow using a physiologic shear stress of 1 to 10 dynes/cm2 (Figure 5C). Nalm6 cells bound to plates simultaneously coated with endoglin and CXCL12, showed a strong adhesive capacity that was resistant up to 8 dynes/cm2 (supplemental Video 1), whereas cells bound to either CXCL12 or endoglin showed a much weaker resistance to flow. Similar flow chamber studies with Jurkat, Peer, and U937 cells also showed an endoglin-dependent resistance to flow (data not shown). The possible involvement of murine endoglin in adhesion was next studied. Adhesion of the mouse B cell line SP2 to plates simultaneously coated with murine endoglin and CXCL12, showed a clear increase that was higher than that supported by human endoglin/CXCL12, suggesting species specificity. Conversely, binding of human K562 cells to murine endoglin/CXCL12 showed an increase that was lower than that induced by human endoglin/CXCL12 (Figure 5D-E).
Role of endoglin in static and flow cell adhesion assays. (A,B,D,E) Static cell adhesion. Plates were coated with BSA, human endoglin (Eng), mouse endoglin (mEng; Fc fusion construct), CXCL12 (CXC) or human IgG (used as control of the Fc part of mEng fusion protein), as indicated. Human Nalm6 cells (A), B lymphocytes (B) and K562 cells (E), as well as murine SP2 cells (D) were labeled with CFSE, loaded in the wells (1 × 105 cells/well) and incubated with or without soluble endoglin or the RGD tripeptide, as indicated for 1h at 37°C. Bound cells were lysed and quantification was carried out using a fluorescent analyzer. Results are shown as percentage respect to total cell input (100%). Each assay was performed in triplicate and the SEM is indicated. (*P < .01; **P < .005). (C) Flow adhesion assay. Coated plates were incorporated as the lower wall of a flow chamber and mounted on an inverted microscope equipped with a monochromatic camera. Nalm6 cells were infused and allowed to bind to the substrate before flow rate was adjusted to 1 dyne/cm2 and then increased every 30 seconds. The percentages of cells that remained bound are represented in the vertical axis. A representative video of Nalm6 cells bound to Eng/CXCL12 under increasing flow rates is available online (see supplemental Video 1).
Role of endoglin in static and flow cell adhesion assays. (A,B,D,E) Static cell adhesion. Plates were coated with BSA, human endoglin (Eng), mouse endoglin (mEng; Fc fusion construct), CXCL12 (CXC) or human IgG (used as control of the Fc part of mEng fusion protein), as indicated. Human Nalm6 cells (A), B lymphocytes (B) and K562 cells (E), as well as murine SP2 cells (D) were labeled with CFSE, loaded in the wells (1 × 105 cells/well) and incubated with or without soluble endoglin or the RGD tripeptide, as indicated for 1h at 37°C. Bound cells were lysed and quantification was carried out using a fluorescent analyzer. Results are shown as percentage respect to total cell input (100%). Each assay was performed in triplicate and the SEM is indicated. (*P < .01; **P < .005). (C) Flow adhesion assay. Coated plates were incorporated as the lower wall of a flow chamber and mounted on an inverted microscope equipped with a monochromatic camera. Nalm6 cells were infused and allowed to bind to the substrate before flow rate was adjusted to 1 dyne/cm2 and then increased every 30 seconds. The percentages of cells that remained bound are represented in the vertical axis. A representative video of Nalm6 cells bound to Eng/CXCL12 under increasing flow rates is available online (see supplemental Video 1).
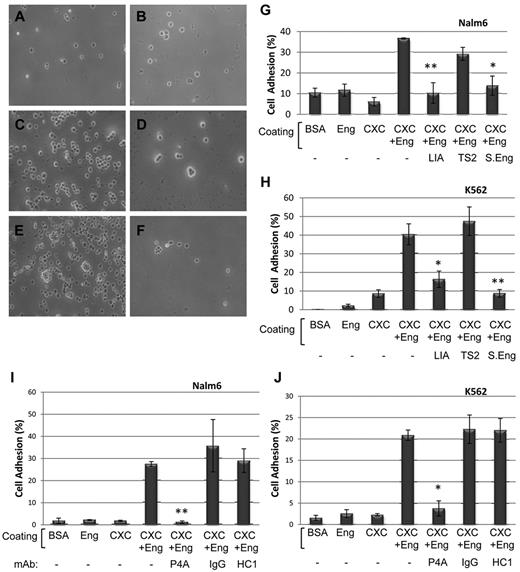
Leukocytes express the integrin α5β1, which is able to interact with ECM proteins via the RGD motif.35 To assess the involvement of β1 integrins in the endoglin-mediated cell adhesion, 2 specific antibodies were used. Thus, binding of Nalm6 cells to plates simultaneously coated with endoglin and CXCL12 (Figure 6A-G), was clearly inhibited in the presence of the blocking anti-β1 mAb LIA1/2 (Figure 6D-G), or soluble endoglin (Figure 6F-G). By contrast, the anti-β1 mAb TS2/16, a well known activator of β1 integrins, did not show any inhibitory effect (Figure 6E-G). Similar results were observed with K562 cells (Figure 6H). In addition, endoglin-dependent cell adhesion was abolished in the presence of AMD3100, an inhibitor of the CXCL12/CXCR4 pathway (data not shown), supporting the critical role of CXCL12-dependent integrin activation in these assays. Next, the effect of anti-endoglin antibodies on cell adhesion was tested. The anti-endoglin mAb P4A4 specifically inhibited the endoglin-dependent adhesion of Nalm6 and K562 cells (Figure 6I-J), whereas no significant effect was observed with an anti-CD31 mAb (HC1/6) or an isotype matched mAb. These results support the involvement of β1 integrins in the endoglin-dependent adhesion. Further support for this hypothesis was obtained from adhesion experiments in the presence of several RGD peptides. Thus, the endoglin-dependent adhesion of Nalm6 and K562 cells was completely inhibited by the tripeptide RGD and the pentapeptides GRGDS (derived from fibronectin) and DRGDK (derived from endoglin), whereas no inhibitory effect was observed in the presence of SDGRG (containing the same amino acids as GRGDS, but in reverse order; data not shown). This is in agreement with the inhibitory role of the RGD peptide in endoglin-dependent transmigration (supplemental Figure 3) and cell adhesion (Figure 5B).
Involvement of integrins in endoglin-dependent cell adhesion. (A-F) Light microscopy analysis of adherent cells. Plates were coated with BSA (A), endoglin (B) or CXCL12 plus endoglin (C-F). Nalm6 cells were incubated for 1 hour at 37°C in the absence (A-B) or in the presence of the inhibitory anti-β1 integrins mAb LIA1/2 (D), the activator anti-β1 integrins mAb TS2/16 (E) or soluble endoglin (F). Bound cells were visualized by inverse light microscopy. Magnification, ×200. (G-J) Quantification analysis. Plates were coated with BSA, endoglin, CXCL12, or endoglin plus CXCL12, as indicated. Bound Nalm6 cells, previously labeled with CFSE, from the same experiment in panels A through F were lysed and quantification was carried out (G). A representative binding experiment with K562 cells is also shown (H). (I-J) Role of anti-endoglin antibodies in cell adhesion assays. Nalm6 (I) and K562 (J) cells were labeled with CFSE, loaded in the wells and incubated with or without mAb P4A4 (anti-endoglin), mAb HC1/6 (anti-CD31), or an irrelevant IgG2b mAb (control matched), as indicated for 1 hour at 37°C. Bound cells were lysed and quantification was carried out using a fluorescent analyzer. Results are shown as percentage respect to total input (100%). Each assay was performed in triplicate and the SEM is indicated. **P < .005; *P < .01. CXC indicates CXCL12; LIA, mAb LIA1/2; TS2, mAb TS2/16; S.Eng, soluble endoglin; HC1, mAb HC1/6; and IgG, IgG2b.
Involvement of integrins in endoglin-dependent cell adhesion. (A-F) Light microscopy analysis of adherent cells. Plates were coated with BSA (A), endoglin (B) or CXCL12 plus endoglin (C-F). Nalm6 cells were incubated for 1 hour at 37°C in the absence (A-B) or in the presence of the inhibitory anti-β1 integrins mAb LIA1/2 (D), the activator anti-β1 integrins mAb TS2/16 (E) or soluble endoglin (F). Bound cells were visualized by inverse light microscopy. Magnification, ×200. (G-J) Quantification analysis. Plates were coated with BSA, endoglin, CXCL12, or endoglin plus CXCL12, as indicated. Bound Nalm6 cells, previously labeled with CFSE, from the same experiment in panels A through F were lysed and quantification was carried out (G). A representative binding experiment with K562 cells is also shown (H). (I-J) Role of anti-endoglin antibodies in cell adhesion assays. Nalm6 (I) and K562 (J) cells were labeled with CFSE, loaded in the wells and incubated with or without mAb P4A4 (anti-endoglin), mAb HC1/6 (anti-CD31), or an irrelevant IgG2b mAb (control matched), as indicated for 1 hour at 37°C. Bound cells were lysed and quantification was carried out using a fluorescent analyzer. Results are shown as percentage respect to total input (100%). Each assay was performed in triplicate and the SEM is indicated. **P < .005; *P < .01. CXC indicates CXCL12; LIA, mAb LIA1/2; TS2, mAb TS2/16; S.Eng, soluble endoglin; HC1, mAb HC1/6; and IgG, IgG2b.
Discussion
Our data support the involvement of endoglin in lymphocyte trafficking through endothelia, a process that is stimulated by inflammatory events (Figure 7). The active role played by endoglin in this process is compatible with: (1) the high expression of endoglin in high endothelial venules,23 characterized by facilitating circulating lymphocytes to directly enter lymph nodes36 ; (2) the increased expression of endoglin in endothelia on inflammatory stimuli21,37 and during neoangiogenesis5,38 ; and (3) the impaired recruitment of HHT1 mononuclear cells to the ischemic heart in a mouse model.39 Our results suggest that the mechanism by which endothelial endoglin contributes to leukocyte extravasation involves intercellular adhesion mediated by the RGD motif present on the ectodomain of endoglin. The RGD and RGD-like motifs are present in a large number of ECM proteins and are specifically recognized by many integrins, receptors that mediate attachments between a cell and other cells or the ECM.16,35 The interaction between integrins and RGD motifs modulates processes, such as cell migration, adhesion, and angiogenesis.35,40,41 Indeed, synthetic RGD peptides have been found to be selective and potent inhibitors of angiogenesis and are promising tools for pathologic angiogenesis, thrombosis as well as drug therapy and imaging of inflammatory-related diseases. Interestingly, membrane bound endoglin shows proangiogenic activity,5,7 whereas RGD-containing soluble endoglin displays an anti-angiogenic function,10,14 strongly suggesting that the angiogenesis related role of endoglin may involve its RGD motif. Of note, an abnormal angiogenesis has been postulated as an underlying pathogenic mechanism in HHT1, an autosomal dominant disorder characterized by arteriovenous malformations and telangiectases-associated hemorrhagic events.7 Indeed, anti-angiogenic therapy, including thalidomide or bevacizumab, is currently used to treat HHT patients.13 Interestingly, it has been reported that infiltrating leukocytes can modulate the angiogenic response.42 Thus, the decreased leukocyte transmigration through HHT endothelial cells (Figure 3E) or on inflammation in an animal model of HHT1 (Figure 1), suggest that endoglin haploinsufficiency in HHT1 patients may lead to an impaired leukocyte trafficking as the basis of an abnormal angiogenesis. Further studies are needed to assess whether leukocyte trafficking, via the endoglin RGD motif, contributes to HHT1 pathogenesis.
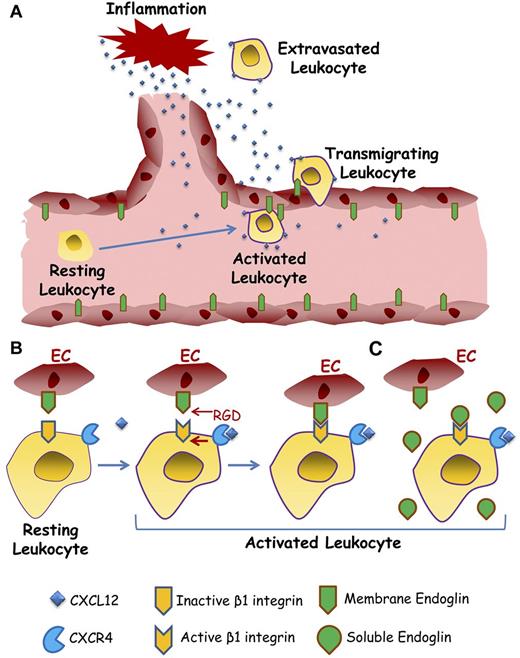
Role of endothelial endoglin in leukocyte extravasation. A schematic diagram shows a hypothetical model for leukocyte transmigration through the vessel endothelium. (A) On inflammatory stimuli, different soluble factors are released, including the chemokine CXCL12, leading to activation and endoglin-dependent extravasation of leukocytes. (B) The leukocyte's transmigration process involves the binding of CXCL12 to its receptor CXCR4, which in turn activates the β1 integrins. Once activated, β1 integrin binds to the RGD motif of endoglin present on the endothelial cell (EC) surface, allowing the extravasation and migration of leukocytes to the inflammatory site. (C) Soluble endoglin competes for the binding between leukocyte β1 integrin and endothelial membrane anchored endoglin, thus interfering with leukocyte adhesion and transmigration. The dimeric nature of endoglin has been omitted for simplification.
Role of endothelial endoglin in leukocyte extravasation. A schematic diagram shows a hypothetical model for leukocyte transmigration through the vessel endothelium. (A) On inflammatory stimuli, different soluble factors are released, including the chemokine CXCL12, leading to activation and endoglin-dependent extravasation of leukocytes. (B) The leukocyte's transmigration process involves the binding of CXCL12 to its receptor CXCR4, which in turn activates the β1 integrins. Once activated, β1 integrin binds to the RGD motif of endoglin present on the endothelial cell (EC) surface, allowing the extravasation and migration of leukocytes to the inflammatory site. (C) Soluble endoglin competes for the binding between leukocyte β1 integrin and endothelial membrane anchored endoglin, thus interfering with leukocyte adhesion and transmigration. The dimeric nature of endoglin has been omitted for simplification.
Although the RGD sequence has been widely studied in a large number of ECM proteins, only a few cell surface proteins containing an active RGD-like motif have been reported, namely members of the ADAM (a disintegrin and metalloproteinase) family of proteins.43 The presence of this putative integrin binding motif in human endoglin is located in an exposed area of the protein, thus compatible with receptor binding.4 The RGD motif of endoglin is present in primates but not in rodents (supplemental Figure 6), suggesting that this tripeptide may represent a recent adaptation. However, it should be noted that not only RGD itself but also other RGD-related sequences are recognized by integrins35 and the presence of an acidic residue (D or E), critical in the binding to integrins,17 is conserved among mammalian endoglins, including the tripeptide TDD present in mouse and pig (TDD; supplemental Figure 6). Interestingly, the TDD motif of mouse ADAM-15 specifically interacts with integrins,18 whereas the homologous sequence in human ADAM-15 is the prototypic RGD sequence,40 thus resembling the situation of mouse and human endoglin. Indeed, our data using murine leukocytes, endothelial cells, endoglin, and TDD peptides (Figures 3J and 5D-E), suggest that, similarly to human endoglin, mouse endoglin in endothelial cells is also involved in leukocyte adhesion and transmigration.
From the structural point of view, the RGD motif is contained within the ZP domain of endoglin's extracellular region.13 This ZP domain consists of a module of approximately 260 amino acids which is shared by a large family of extracellular proteins with widely diverse functions.44 The role of the ZP domains appears to be related to protein-protein interactions and protein polymerization, a function fully consistent with the role of the RGD-dependent interaction of endoglin with integrins described here.
To bind endoglin, integrins need to be activated by CXCL12-triggered inside-out signaling, as demonstrated in our adhesion and transmigration experiments. In agreement with this view, it has been shown that CXCL12/CXCR4 signaling route promotes integrin-dependent leukocyte adhesion and transmigration.1,45 The involvement of β1 integrins via RGD was demonstrated by the specific inhibition of the endoglin-dependent adhesion in the presence of different peptides containing the RGD core or the inhibitory anti-β1 antibody LIA1/2. By contrast, the activator anti-β1 antibody TS2/16 did not affect cell adhesion, as would be expected for integrins already active through CXCL12 signaling. Among the different cell types tested here, in our hands Nalm6 cells are the ones which yielded the strongest adhesion in static and flow assays when a coating was made with endoglin and CXCL2. Nonetheless, it should be noted that the optimal concentrations of CXCL12 for the different cell types differed probably because of the variable expression and/or sensitivity of the chemokine receptors, namely CXCR4, as well as the different expression pattern of surface integrins. Because integrin α5β1 binds to the RGD sequence in ECM proteins,35 and it is present in all hematopoietic cell lines tested here, notably is the only β1 integrin expressed on K562 cells, it can be postulated that this integrin is involved in the specific interaction with the RGD motif of endoglin. Based on these results, we postulate that on CXCL12 stimulation, the integrin α5β1 interacts with endoglin RGD motif triggering leukocyte adhesion and transmigration during the inflammation process (Figure 7A-B). Nonetheless, the interaction of endoglin with other integrin family members cannot be excluded.
Several lines of evidence support the link between integrins and endoglin functions: (1) endoglin overexpression modulates cellular adhesion and migration, biologic activities usually adscribed to integrins46 ; (2) on overexpression of endoglin, fibroblast transfectants tend to form clusters that are inhibited in the presence of anti-α5β1 integrin antibodies or by coating the culture plates with an RGD-containing 80 kDa fragment of fibronectin46 ; (3) experiments using RGD-labeled microspheres to induce and isolate RGD binding, integrin-rich sites of focal adhesion showed that endoglin regulates cell migration and focal adhesion composition via interaction through its cytoplasmic domain with the LIM domain-containing protein zyxin present in focal adhesion sites28 ; and (4) carcinoembryonic antigen (CEA)–binding of human pathogenic bacteria triggered de novo expression of endoglin, which, in turn changed focal adhesion composition, activating β1 integrins, inducing a dramatic increase in the ECM-binding capacity of the cells.47,48 Taken together, these data strongly suggest a close physical and functional association between endoglin and β1 integrins.
An interesting observation of this study is the ability of soluble endoglin to inhibit leukocyte adhesion (Figure 7C). This observation may contribute to understand the possible pathogenic role of the up-regulated levels of soluble endoglin in several pathologies, including preeclampsia and cancer.11,49 In preeclampsia, markedly increased circulating levels of soluble endoglin are detected 2 to 3 months before the onset of the disease. After the onset, the mean serum levels in women with preterm preeclampsia are 46.4 ng/mL compared with 9.8 ng/mL in controls.12 These in vivo concentrations are within the lower range of concentrations tested in our in vitro experiments (50 ng/mL-1 μg/mL), which showed an inhibitory activity on leukocyte adhesion and endothelial transmigration. The impact of this activity on vascular endothelia is probably related to the anti-angiogenic action reported for the pathogenic role in preeclampsia11 and for the inhibitory role in tumor angiogenesis10 of soluble endoglin, suggesting that this protein is a promising therapeutic target in these pathologies.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Drs Pedro Lastres (Department of Flow Cytometry, CIB-CSIC) and Maria T. Seisdedos (Department of Confocal and Multidimensional Microscopy, CIB-CSIC) for comments and technical assistance, Dr Michelle Letarte (The Hospital for Sick Children, Toronto, ON) for Eng+/− mice, Dr Calvin P. H. Vary (Maine Medical Center Research Institute, Scarborough, ME) for bovine endothelial cells, Drs Francisco Sanchez-Madrid and Maria Yañez-Mo for antibodies, Dr Paloma Sanchez-Mateos for helpful comments at early stages of this work, and Drs David Garcia and Consuelo Gonzalez-Manchon for technical advice.
This work was supported by grants from Ministerio de Economia y Competitividad of Spain (SAF2010-61 827 to C.B. and SAF2010-15 881 to J.M.L.-N.), Centro de Investigación Biomédica en Red de Enfermedades Raras (CIBERER to C.B.), and Red de Investigación Cooperativa en Enfermedades Renales (REDINDEN to J.M.L.-N.). CIBERER and REDINREN are initiatives of the Instituto de Salud Carlos III (ISCIII) of Spain supported by FEDER funds. E.R. is supported by the JAE-Doc Program of CSIC funded by FEDER.
Authorship
Contribution: E.R., J.M.L.-N., and C.B. designed the research, analyzed the data, and wrote the paper; E.R. performed in vitro assays; C.B. and J.M.L.-N. supervised the research and provided funding support; N.E. and A.D. performed the in vivo assays; and F.S.-R., L.M.B., C.C., F.J.B., and C.L. performed research and provided suggestions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Carmelo Bernabeu, Centro de Investigaciones Biologicas, CSIC, Ramiro de Maeztu 9, Madrid 28040, Spain; e-mail: bernabeu.c@cib.csic.es.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal