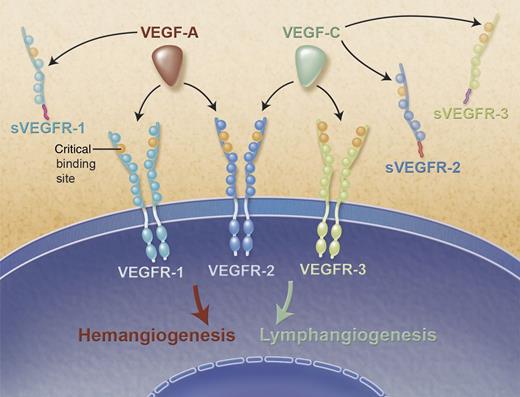
Soluble VEGF receptors trap VEGF-A and VEGF-C and inhibit hemangiogenesis and lymphangiogenesis. Schematic representation of VEGF-A and VEGF-C (red and green, respectively) and their cognate VEGF receptors. Membrane-bound VEGF receptor (VEGFR-1, VEGFR-2, and VEGFR-3) with its seven extracellular immunoglobulin-like domains represented as circles. Critical binding domains for VEGF-A and VEGF-C of each receptor are displayed in orange. Soluble VEGF receptors (sVEGFR-1, sVEGFR-2, and sVEGFR-3) composed of the first 6 Ig-like domains and the unique C-terminus tail from alternative splicing are represented in magenta, red, and purple for sVEGFR-1, sVEGFR-2, and sVEGFR-3, respectively. Professional illustration by Alice Y. Chen.
Soluble VEGF receptors trap VEGF-A and VEGF-C and inhibit hemangiogenesis and lymphangiogenesis. Schematic representation of VEGF-A and VEGF-C (red and green, respectively) and their cognate VEGF receptors. Membrane-bound VEGF receptor (VEGFR-1, VEGFR-2, and VEGFR-3) with its seven extracellular immunoglobulin-like domains represented as circles. Critical binding domains for VEGF-A and VEGF-C of each receptor are displayed in orange. Soluble VEGF receptors (sVEGFR-1, sVEGFR-2, and sVEGFR-3) composed of the first 6 Ig-like domains and the unique C-terminus tail from alternative splicing are represented in magenta, red, and purple for sVEGFR-1, sVEGFR-2, and sVEGFR-3, respectively. Professional illustration by Alice Y. Chen.
The field of angiogenesis was greatly enlightened by the discovery of soluble vascular endothelial growth factor receptor 1 (sVEGFR-1) in 1993 by Kendall and Thomas.2 sVEGFR-1, a potent endogenous antiangiogenic molecule, sequesters VEGF-A, VEGF-B, and Placental Growth Factor from the endothelial cells of neighboring blood vessels, stunting their growth. sVEGFR-1 has since gained wide importance not only as an angiogenesis regulator but also as a disease biomarker and has been extensively studied in the context of preeclampsia, chronic kidney disease, and cancer treatment response toxicity, to name a few conditions.3,-5 Its serum levels increase dramatically in preeclampsia before manifestation of clinical disease.3 A rise in sVEGFR-1 levels has also been demonstrated to be predictive of cardiovascular disarray associated with chronic kidney disease.4 In a population of patients with advanced rectal cancer, the presence of high plasma sVEGFR-1 levels was associated with a lower rate of adverse events but poorer treatment response when treated with anti–VEGF-A therapy.5 In the eye, sVEGFR-1 is critical for maintaining a cornea devoid of blood vessels, which is critical for optical clarity and imperative for optimal vision.6 Collectively, these data attest to the broad implications of such discoveries. The existence of a soluble splicing variant of vascular endothelial growth factor receptor 2 (sVEGFR-2) was described in 2009.7 It was shown to be a key regulator of lymphatic vessel development and growth by virtue of its ability to trap and inhibit VEGF-C. sVEGFR-2 is essential in maintaining the cornea alymphatic during development, following injury, and after corneal transplantation. Since its discovery, a number of studies have established its role in the pathophysiology of several conditions. It has been shown to play an important role in neuroblastoma progression and breast cancer metastasis.8,9 Neuroblastoma progression from a nonmetastatic to a metastatic state was associated with a decline in sVEGFR-2 production in tumor specimens, whereas it was unchanged in tumors without metastatic potential.8 In breast cancer, sVEGFR-2 overexpression decreased lymph node metastasis and increased survival in a murine metastatic mammary cancer model.9 More recently, circulating sVEGFR-2 levels have been studied in the context of preeclampsia, where it was found to be significantly decreased prior to disease presentation.10 It now holds a promising future as a potential biomarker in preeclampsia, trailing the steps of its predecessor, sVEGFR-1. The ramifications of such discoveries are often unpredictable but invariably fruitful and exciting. Here, Singh et al reveal the newest member of the VEGF family of molecules and highlight its significance in lymphatic growth regulation and as a therapeutic target for enhancing corneal transplant survival. Most importantly, this discovery catapults new and exciting prospects to the field of angiogenesis and all of its ramifications to the pathophysiology of so many maladies.
Conflict-of-interest disclosure: The author declares no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal