In this issue of Blood, Boyerinas and colleagues report about a novel mechanism of leukemia cell evasion from chemotherapy.1
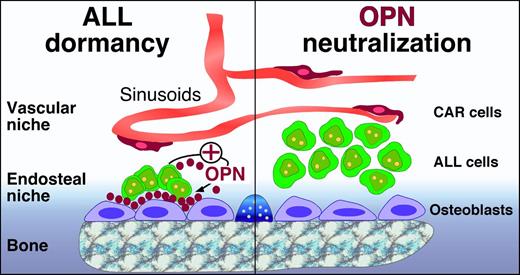
Potential role of OPN in ALL cell dormancy. In the marrow microenvironment, HPCs are maintained in perivascular and endosteal niches, which also can be occupied by ALL and other leukemia cells. In the osteoblast niches, osteoblasts and ALL cells secrete OPN, which becomes part of the extracellular matrix and builds a bridge between ALL cells and osteoblasts/the bone (left). Adherence of ALL cells to OPN via adhesion molecules causes ALL cell dormancy, which makes ALL cells less susceptible to cytotoxic drugs, such as Ara-C. ALL cell dormancy can be reversed by OPN neutralization (right), which causes an increase in dividing/cycling ALL cells. This reversal of ALL cell dormancy by OPN neutralization can potentially be used for “chemosensitization” to target dormant ALL cells that otherwise survive conventional polychemotherapy.
Potential role of OPN in ALL cell dormancy. In the marrow microenvironment, HPCs are maintained in perivascular and endosteal niches, which also can be occupied by ALL and other leukemia cells. In the osteoblast niches, osteoblasts and ALL cells secrete OPN, which becomes part of the extracellular matrix and builds a bridge between ALL cells and osteoblasts/the bone (left). Adherence of ALL cells to OPN via adhesion molecules causes ALL cell dormancy, which makes ALL cells less susceptible to cytotoxic drugs, such as Ara-C. ALL cell dormancy can be reversed by OPN neutralization (right), which causes an increase in dividing/cycling ALL cells. This reversal of ALL cell dormancy by OPN neutralization can potentially be used for “chemosensitization” to target dormant ALL cells that otherwise survive conventional polychemotherapy.
Acute lymphoblastic leukemia (ALL) cells hijack endosteal bone marrow niches by adherence to osteopontin (OPN), a highly acidic extracellular matrix protein. Normally, OPN is secreted by osteoblasts and functions as a bridge between bone and blood, ie, between the endosteal marrow niche and hematopoietic progenitor cells (HPCs). In these niches, HPCs remain quiescent because of OPN-suppressed proliferation, presumably to maintain and limit the size of the HPC pool.2,3 The authors hypothesized that ALL cells may use a similar OPN-dependent mechanism to occupy endosteal niches, where quiescent ALL cells can replace HPCs and survive conventional cytotoxic chemotherapy. The authors demonstrate that ALL cells adhere to OPN via integrins (α4β1, α5β1, α9β1) and, in addition, actively secrete OPN, thereby participating in the remodeling of the niche. In a xenograft mouse model, highest levels of OPN were seen around dormant ALL cells. Through this OPN-mediated mechanism, ALL cells survive in endosteal niches and can become the seed for minimal residual disease and relapses. Consequently, the authors demonstrate that OPN neutralization with antibodies awakens ALL cells in the ALL mouse model, causing an increase in dividing/cycling ALL cells, which in turn sensitized ALL cells to cytarabine (Ara-C) therapy.
During the last 2 decades, there has been substantial progress in identifying key cellular and molecular players in crosstalk between normal and malignant hematopoietic cells and the marrow microenvironment.4 At least 2 hematopoietic stem cell (HSC) bone marrow niches have been described: one endosteal and one perivascular (see figure). Specialized osteoblasts lining the bone surface form endosteal niches. On the other hand, sinusoidal endothelial cells and a small population of perivascular reticular cells with long processes, expressing high amounts of CXCL12 (called CXCL12-abundant reticular or CAR cells), create vascular niches, which are scattered throughout the marrow cavity. Earlier groundbreaking in vivo studies from the same group revealed that malignant B cells not only interact with stromal cells and molecules that are described within HSC niches but specifically invade, exploit, and disrupt HSC niches during malignant progression, thereby negatively affecting benign hematopoiesis.5,6
How can we stop leukemia cells from parasitizing in these niches and give them a taste of their own medicine? CXCR4, a chemokine receptor that tethers hematopoietic cells to CXCL12-secreting stromal cells, is the first therapeutic target for disrupting crosstalk between leukemia cells and the marrow microenvironment.7 Many clinical trials use CXCR4 antagonists (eg, plerixafor/AMD3100) for “chemosensitization” when given in combination with cytotoxic agents. These studies are either ongoing or are recently finished, and a first trial in acute myeloid leukemia has reported encouraging results.8 Targeting OPN in ALL, as introduced in this study,1 follows a similar concept: OPN neutralization releases ALL cells from their dormant state in the endosteal niches and makes them better accessible and receptive toward cytotoxic drugs that target cycling cells. As such, this study is in line with an ongoing important paradigm shift in cancer therapy, moving from cancer cells as the primary target of therapy toward new treatments that also interfere with protective tumor-microenvironment interactions.9 It is also tempting to think about analogies between OPN and “The Sleeping Beauty”: ALL cells are doomed to sleep in endosteal niches by OPN, but once this curse is lifted, life in the marrow awakens. The encouraging data about OPN neutralization in ALL in this study1 stir hope that, unlike in “The Sleeping Beauty”, it will take a much shorter time than 100 years until the reversion of leukemia cell dormancy in ALL can be clinically tested.
Conflict-of-interest disclosure: The author declares no competing financial interests.