Abstract
In immunoglobulin light chain amyloidosis a small, indolent plasma cell clone synthesizes light chains that cause devastating organ damage. Early diagnosis, based on prompt recognition of “red-flags” before advanced cardiomyopathy ensues, is essential for improving outcomes. Differentiation from other systemic amyloidoses may require advanced technologies. Prognosis depends on the extent of cardiac involvement, and cardiac biomarkers guide the choice of therapy. The protean clinical presentation requires individualized treatment. Close monitoring of clonal and organ response guides therapy changes and duration. Conventional or high-dose alkylator-based chemotherapy is effective in almost two-thirds of patients. Combinations of proteasome inhibitors, dexamethasone, and alkylators achieve high response rates, although controlled studies are needed. Risk-adapted stem cell transplant and consolidation with novel agents may be considered in selected patients. Immune-modulatory drugs are good options for refractory/relapsed patients. Novel agents and therapeutic targets are expected to be exploited, in an integrated, more effective and less toxic treatment strategy.
Introduction
The systemic amyloidoses comprise an increasing number of diseases characterized by multiorgan deposition of misfolded and aggregated autologous proteins as β-pleated sheet fibrils.1 Immunoglobulin light chain (AL) amyloidosis is the most common (incidence ∼10 patients per million per year)2 and the most severe because it often targets the heart.3 A small, usually indolent plasma cell (PC) clone synthesizes an unstable, misfolded light chain (LC), which is prone to aggregate and form amyloid fibrils. This process causes systemic toxicity and devastating organ dysfunction.4 Genetic characteristics of amyloid LCs have been associated with kidney5 and heart6 predilection, but mechanisms of tissue specificity and organ dysfunction are poorly understood. Over the past decade, effective regimens have been developed markedly improving survival,7,8 but the 25% to 30% early death rate has not changed, with patients dying within a few weeks of cardiac failure due to late diagnosis. Early diagnosis remains the, as yet, elusive key for improving the care of this dreadful but treatable disease.
When to suspect amyloidosis
The protean clinical features of AL amyloidosis reflect its systemic nature and are detailed in Figure 1. Combinations such as nephrotic syndrome and heart failure, simultaneous peripheral and autonomic neuropathy in nondiabetic patients, “left ventricular hypertrophy” on echocardiography without consistent electrocardiographic evidence or low limb lead voltages, hepatomegaly with normal imaging, or albuminuria in patients with MGUS or myeloma should raise suspicion of amyloidosis.
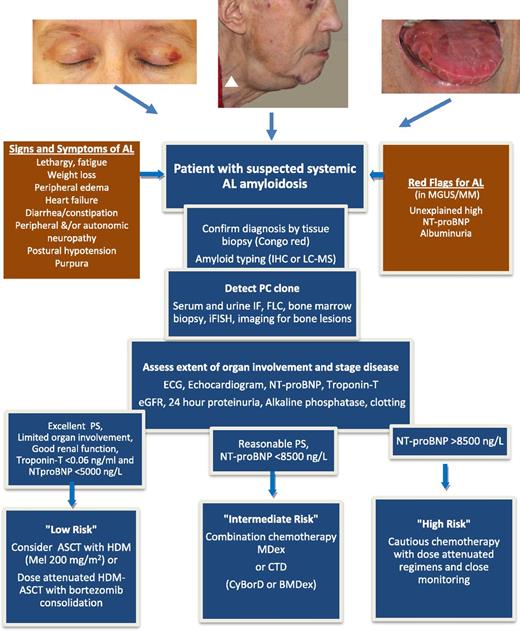
Pathway for a patient with suspected AL amyloidosis. Signs and symptoms derive from organ involvement: heart and kidney in 70% of patients each, liver in 17% of patients, soft tissues in 17% of patients, peripheral and autonomous nervous system in 15% of patients each, and gastrointestinal tract in 10% of patients. Clinical manifestations with head and neck purpura, unexplained submandibular swelling, signs of heart diastolic dysfunction such as jugular venous distention (white arrowhead), or stiff indented macroglossia, although found in no more than 15% of patients, are prototypic, but late manifestations of the disease. Symptoms and signs derive from advanced organ failure caused by the amyloid-forming process, whereas biomarkers of heart and kidney function (NT-proBNP and albuminuria) may allow identifying presymptomatic patients with initial, possibly reversible, amyloidotic organ damage. The diagnostic process involves the detection of the amyloid deposits and of the underlying PC clone. Considering the wide clinical heterogeneity of this disease, the assessment of the organ dysfunction is necessary in order to define the risk of chemotherapy. The flowchart represents the authors’ view on the current approach for management of a patient with AL amyloidosis. NT-proBNP cutoffs are not applicable in patients with end-stage renal failure. MDex represents the standard of care in most countries, and risk-adapted CTD is an alternative. Although increasingly used in clinical practice, the clinical benefit of adding bortezomib to dexamethasone and alkylating agents frontline remains unproven and is still being tested in controlled trials. ASCT with HMD, autologous stem cell transplantation with high-dose melphalan; BMDex, bortezomib, melphalan, dexamethasone; CTD, cyclophosphamide, thalidomide, dexamethasone; CyBorD, cyclophosphamide, bortezomib, dexamethasone; eGFR, estimated glomerular filtration rate; FLC, serum-free LC measurement; IF, immunofixation; iFISH, interphase fluorescence in situ hybridization; IHC, immunohistochemistry; LC-MS, laser capture microdissection and mass spectrometry; MDex, melphalan dexamethasone; MGUS/MM, monoclonal gammopathy of undetermined significance/multiple myeloma; NT-proBNP, amino-terminal pro–natriuretic peptide type-B; PS, performance status.
Pathway for a patient with suspected AL amyloidosis. Signs and symptoms derive from organ involvement: heart and kidney in 70% of patients each, liver in 17% of patients, soft tissues in 17% of patients, peripheral and autonomous nervous system in 15% of patients each, and gastrointestinal tract in 10% of patients. Clinical manifestations with head and neck purpura, unexplained submandibular swelling, signs of heart diastolic dysfunction such as jugular venous distention (white arrowhead), or stiff indented macroglossia, although found in no more than 15% of patients, are prototypic, but late manifestations of the disease. Symptoms and signs derive from advanced organ failure caused by the amyloid-forming process, whereas biomarkers of heart and kidney function (NT-proBNP and albuminuria) may allow identifying presymptomatic patients with initial, possibly reversible, amyloidotic organ damage. The diagnostic process involves the detection of the amyloid deposits and of the underlying PC clone. Considering the wide clinical heterogeneity of this disease, the assessment of the organ dysfunction is necessary in order to define the risk of chemotherapy. The flowchart represents the authors’ view on the current approach for management of a patient with AL amyloidosis. NT-proBNP cutoffs are not applicable in patients with end-stage renal failure. MDex represents the standard of care in most countries, and risk-adapted CTD is an alternative. Although increasingly used in clinical practice, the clinical benefit of adding bortezomib to dexamethasone and alkylating agents frontline remains unproven and is still being tested in controlled trials. ASCT with HMD, autologous stem cell transplantation with high-dose melphalan; BMDex, bortezomib, melphalan, dexamethasone; CTD, cyclophosphamide, thalidomide, dexamethasone; CyBorD, cyclophosphamide, bortezomib, dexamethasone; eGFR, estimated glomerular filtration rate; FLC, serum-free LC measurement; IF, immunofixation; iFISH, interphase fluorescence in situ hybridization; IHC, immunohistochemistry; LC-MS, laser capture microdissection and mass spectrometry; MDex, melphalan dexamethasone; MGUS/MM, monoclonal gammopathy of undetermined significance/multiple myeloma; NT-proBNP, amino-terminal pro–natriuretic peptide type-B; PS, performance status.
Is it possible to diagnose amyloidosis before the overt end-organ dysfunction ensues?
Clinical manifestations of AL amyloidosis reflect advanced organ damage. Early diagnosis requires switching from traditional symptoms- and signs-bound diagnostics to sensitive biomarkers signaling presymptomatic organ damage. The progressive, clinically silent involvement of heart and kidneys can be detected early by simple tests. The NT-proBNP is the most sensitive, although not specific, marker for amyloid cardiomyopathy. A concentration >332 ng/L has 100% sensitivity9 and even in asymptomatic patients with normal echocardiogram predicts the development of cardiac amyloidosis.10 Treatment at the asymptomatic stage may prevent irreversible organ damage, and better organ function will allow adequate treatment delivery.
Undetected advanced organ failure can ensue even during careful follow-up of individuals with MGUS, unless looked for specifically. We would recommend that all patients with MGUS and abnormal FLC ratio (who are at higher risk of developing amyloidosis and should undergo lifelong monitoring for symptomatic myeloma11 ) have, additionally, NT-proBNP and urine albumin assessed at each visit. Unexplained positive results should promptly trigger procedures to diagnose amyloidosis.
Diagnosis, amyloid typing, and risk stratification
Diagnosing AL amyloidosis involves 4 steps: proving systemic amyloid deposition, typing the deposits, assessing the monoclonal disease, and defining the extent of systemic damage including risk stratification/staging. Localized AL amyloidosis, resulting from in situ (eg, skin, airways, and urinary tract) production of LC, usually does not necessitate systemic therapy and should be differentiated from systemic amyloidosis, characterized by visceral involvement.12
Demonstration of amyloid deposition in a tissue biopsy by Congo red staining remains the gold standard, although novel methods have been proposed.13,14 The most accessible site is periumbilical fat that can be easily and innocuously aspirated. Labial salivary gland biopsy is positive in >50% of patients with negative fat biopsy.15 If needed, target organs can be biopsied after careful assessment of hemostasis. Tissue deposits should be typed using mass spectrometry (the current gold standard),16,17 immuno–electron microscopy,18 or immunohistochemistry in specialized laboratories.19 Confirmation of fibril type is critical because a dozen proteins can cause systemic amyloidosis,13 each requiring distinct therapy. Even in patients with MGUS, cardiomyopathy caused by V122I mutant transthyretin (carrier rate of 4% in Afro-Caribbeans) or by wild-type transthyretin in elderly men with senile systemic amyloidosis, and amyloidosis reactive to chronic inflammation (AA) should be carefully considered in differential diagnosis.20,21 Gene sequencing is needed when familial amyloidosis is possible: such as in those with isolated neuropathic or cardiac disease (transthyretin amyloidosis), isolated renal involvement (fibrinogen amyloidosis), corneal lattice dystrophy, progressive bilateral facial paralysis and cutis laxa (gelsolin amyloidosis), dry mouth/gastrointestinal/kidney/liver involvement (lysozyme amyloidosis), and renal/liver/cardiac involvement in relatively asymptomatic patients (apolipoprotein-A1 amyloidosis). Amyloid typing and gene sequencing are available at all major referral centers.
The identification of amyloidogenic monoclonal proteins requires serum and urine immunofixation combined with FLC quantification.22 Half of all amyloidogenic PC clones produce LC only, with typically modest bone marrow infiltrate (median 5% to 10%). The λ clones dominate κ ones by 4:1, unlike the 2:3 ratio in myeloma. Fluorescence in situ hybridization of bone marrow PC and investigations to rule out symptomatic myeloma, including skeletal survey, should be done at baseline.11
Assessment of amyloid-related organ damage is the next step. Echocardiography, including tissue Doppler and strain imaging, defines baseline cardiac function, in addition to widely available and well-standardized cardiac biomarkers.23,24 Cardiac magnetic resonance imaging is useful in diagnosing and possibly monitoring amyloid deposits.25 Renal involvement is best evaluated by eGFR and albuminuria. Liver function tests and size document involvement. Whole body imaging techniques like serum amyloid P component scintigraphy are useful, where available, for diagnosis and monitoring.26 99mTc-3,3-diphosphono-1,2-propanodicarboxylic acid or pyrophosphate scans help in differentiating cardiac AL from transthyretin amyloidosis (both mutated and wild type) and may spare cardiac biopsy.27 Simple parameters like poor performance status, New York Heart Association class ≥3, and low systolic blood pressure (<100 mm Hg) are useful bedside indicators of poor outcome.28 Because cardiac damage determines survival and treatment tolerability, the Mayo staging system29 using NT-proBNP (>332 ng/L) and cardiac troponin-T (cTnT)/troponin-I (cTnT, >0.035 ng/mL; cardiac troponin-I, >0.1 ng/mL), or more recently high-sensitivity cTnT (>0.077 ng/mL),30 is the most robust method for risk stratification. Stage III patients have a median survival of 3.5 to 8 months.28-31 High FLC levels predict poor outcome and early mortality32,33 and have been incorporated in the Mayo staging system.34 Renal function (especially when eGFR <30 mL/min) affects cardiac biomarkers concentration, and the Mayo staging is not directly applicable for patients in renal failure. BNP is more useful than NT-proBNP in these patients.35
Treatment of AL amyloidosis
Preliminary cytogenetic data suggest that the amyloidogenic clone may represent an early stage in PC dyscrasias progression.36 The recent observation that after ASCT patients with AL amyloidosis survive longer than subjects with multiple myeloma, especially those achieving complete response (CR),37 may indicate that the AL clone is more susceptible to chemotherapy than the myeloma clone or, alternatively, that it takes longer to resurface due to its slower proliferative rate.
Treating AL amyloidosis needs to be adapted to the heterogeneous manifestations and seriousness of the disease, resulting from different patterns and severity of organ involvement. Low-risk patients may be candidates for aggressive therapy, and high-risk subjects need gentle yet rapidly acting regimens (Figure 1). Clinical benefit depends on profound hematologic response translating into organ, especially cardiac or renal, response. Treatment is aimed at rapidly eliminating the toxic LC, ideally achieving CR (negative serum and urine immunofixation and normal FLC ratio) or at least very good partial response (VGPR; difference between involved and uninvolved FLC <40 mg/L). Partial response (decrease in difference between involved and uninvolved FLC >50%) is now considered an unsatisfactory end point.38 Therapy can be continued for 1 to 2 cycles beyond best response for consolidation. Hematologic and cardiac response should be assessed frequently, every 2 cycles (or 3 months after ASCT), promptly switching therapy, if ineffective, to prevent progression of organ damage. An NT-proBNP decrease of >30% and >300 ng/L (if baseline is ≥650 ng/L)38 guides treatment adequacy, mindful of increases in renal failure35 and immune-modulatory therapy.39 The best renal outcomes are seen in patients who achieve VGPR or better.40 Renal responses can be delayed and, hence, difficult to use as a guide for therapy adequacy. Factors influencing progression to dialysis are lower GFR (<60 mL/min), lower serum albumin, and less than VGPR.40
Most available evidence derives from small uncontrolled trials and retrospective series (Table 1)41 with only 1 randomized trial completed in the past 15 years. It failed to demonstrate an advantage for ASCT over MDex, even accounting for the high (24%) transplant-related mortality,42 but was before the era of cardiac biomarkers. Therefore, it is of paramount importance that patients are entered into controlled trials whenever possible.
Selected therapy regimens in AL amyloidosis
| Therapy regimens . | Dosing schedule . | N (F) . | H (stage III) . | HR/CR (OR) . | Most common grade ≥3 SAE . | 100-d mortality . | Median PFS/OS (y) . |
|---|---|---|---|---|---|---|---|
| Controlled phase 3 trial | |||||||
| MDex | M 10 mg/m2 + Dex 40 mg days 1-4 q28 | 43 (100%) | 46% | 68%/32% (39%) | Overall 16% | 2% | TTP 2.7/OS 4.7 |
| vs ASCT42 | M 140-200 mg/m2 | 37 (100%) | 48% | 67%/41% (45%) | Hemodialysis 22% | 24% | TTP 2.7/OS 1.8 |
| Autologous stem cell transplantation | |||||||
| ASCT45 | M 100-200 mg/m2 | 421 (76%) | 45% | M200 -/43% | — | M200 9% | M200 3.4/8.4 |
| M100-140 -/24% (53%) | M100-140 14% | M100-140 1.8/3.8 | |||||
| ASCT41 | M 100-200 mg/m2 | 434 | 49% (25%) | 76%/39% (47%) | — | 10% | CR -/not reached |
| PR -/8.9 | |||||||
| NR -/2.7 | |||||||
| Risk-adapted ASCT48 | M 100/140/200 mg/m2 | 40 (100%) | 65% (28%) | 79%/58% (70%) | During BDex, Tp 43%, cardiac 20%, anemia 13% | ASCT 10% | At 2 y: 69%/82% |
| B 1.3 mg/m2 (OW first 2 cycles, then TW) | BDex 4% | ||||||
| Dex 20 mg day of B and following day | |||||||
| Conventional chemotherapy | |||||||
| MDex55,56 | M 0.22 mg/kg + Dex 40 mg days 1-4 q28 | 46 (100%) | 70% | 67%/33% (48%) | Overall 11%, infection 6% | 4% | 3.8/5.1 |
| Immune-modulatory–based therapy | |||||||
| CTD57 | C 500 mg on days 1, 8, 15 q21/28 | 75 (41%) | 59% | 74%/21% (27%) | Grade ≥2: sedation 40%, fluid retention 21% | 4% | 1.7/3.4 |
| T 50-200 mg/d | |||||||
| Dex 40 mg on days 1-4, 9-12 q21 | |||||||
| or 20 mg on days 1-4 and 15-18q28 | |||||||
| LDex61 | L up to 25 mg on days 1-21 q28 | 22 (41%) | 64% (23%) | 41%/- (23%) | Overall 86%, neutropenia 45%, Tp 27%, rash 18%, infection 18%, fatigue 18% | 18% | 1.6/- |
| Dex 40 mg on days 1-4, 15-18 q28 | |||||||
| added in nonresponders to L alone | |||||||
| CLD66 | C 300 mg/m2 on days 1, 8, 15 q 28 | 35 (11%) | 63% (43%) | 60%/11% (31%) | Overall 74%, Np 40%, Tp 40%, rash 10%, thrombosis 10% | 9% | 2.4/3.1 |
| L 15 mg on days 1-21 q 28 | |||||||
| Dex 40 mg on days 1, 8, 15, 22 q28 | |||||||
| MLD69 | M 0.18 mg/kg on days 1-4 q28 | 26 (100%) | 58% | 58%/23%, 42% with full-dose L (50%) | Overall 81%, Np 11%, heart failure 11% | — | At 2 y: 54%/81% |
| L 5-15 mg on days 1-21 q28 | |||||||
| Dex 40 mg on days 1-4 q28 | |||||||
| PDex63 | Pomalidomide 2 mg/d | 33 (0) | 82% (25%) | 48%/3% (15%) | Np 30%, infection 27%, arrhythmia 21%, fatigue 18% | 3% | 1.2/2.3 |
| Dex 40 mg OW | |||||||
| Proteasome inhibitor–based therapy | |||||||
| Bortezomib60 | 1.6 mg/m2 OW | 70 (0) | 56% | OW: 69%/38% | Overall OW 50%, TW 79% | 3% | At 1 y: OW: 72%/93% |
| 1.3 mg/m2 TW | TW: 67%/24% (29%) | Tp 18% (TW), vomiting 12% (TW) | TW: 75%/84% | ||||
| CyBorD50 | C 350 mg/m2 on days 1, 8, 15 B 1.0-1.3 mg/m2 TW | 43 (47%) | 74% (46%) | 81%/65% frontline, 22% pretreated (46%) | 19% discontinued (due to neuropathy in 14%) | 0 | At 2 y: 53%/98% |
| Dex 20 mg TW | |||||||
| Ixazomib71 | 4.0 mg on days 1, 8, 15 q28 (MTD) | 16 (0) | 69% (6%) | 42%/8%, | (Any grade) nausea 31%, diarrhea 25%, thrombocytopenia 25% | 0 | — |
| Dex 40 mg on days 1-4 (added in nonresponders) | VGPR 25% (-) |
| Therapy regimens . | Dosing schedule . | N (F) . | H (stage III) . | HR/CR (OR) . | Most common grade ≥3 SAE . | 100-d mortality . | Median PFS/OS (y) . |
|---|---|---|---|---|---|---|---|
| Controlled phase 3 trial | |||||||
| MDex | M 10 mg/m2 + Dex 40 mg days 1-4 q28 | 43 (100%) | 46% | 68%/32% (39%) | Overall 16% | 2% | TTP 2.7/OS 4.7 |
| vs ASCT42 | M 140-200 mg/m2 | 37 (100%) | 48% | 67%/41% (45%) | Hemodialysis 22% | 24% | TTP 2.7/OS 1.8 |
| Autologous stem cell transplantation | |||||||
| ASCT45 | M 100-200 mg/m2 | 421 (76%) | 45% | M200 -/43% | — | M200 9% | M200 3.4/8.4 |
| M100-140 -/24% (53%) | M100-140 14% | M100-140 1.8/3.8 | |||||
| ASCT41 | M 100-200 mg/m2 | 434 | 49% (25%) | 76%/39% (47%) | — | 10% | CR -/not reached |
| PR -/8.9 | |||||||
| NR -/2.7 | |||||||
| Risk-adapted ASCT48 | M 100/140/200 mg/m2 | 40 (100%) | 65% (28%) | 79%/58% (70%) | During BDex, Tp 43%, cardiac 20%, anemia 13% | ASCT 10% | At 2 y: 69%/82% |
| B 1.3 mg/m2 (OW first 2 cycles, then TW) | BDex 4% | ||||||
| Dex 20 mg day of B and following day | |||||||
| Conventional chemotherapy | |||||||
| MDex55,56 | M 0.22 mg/kg + Dex 40 mg days 1-4 q28 | 46 (100%) | 70% | 67%/33% (48%) | Overall 11%, infection 6% | 4% | 3.8/5.1 |
| Immune-modulatory–based therapy | |||||||
| CTD57 | C 500 mg on days 1, 8, 15 q21/28 | 75 (41%) | 59% | 74%/21% (27%) | Grade ≥2: sedation 40%, fluid retention 21% | 4% | 1.7/3.4 |
| T 50-200 mg/d | |||||||
| Dex 40 mg on days 1-4, 9-12 q21 | |||||||
| or 20 mg on days 1-4 and 15-18q28 | |||||||
| LDex61 | L up to 25 mg on days 1-21 q28 | 22 (41%) | 64% (23%) | 41%/- (23%) | Overall 86%, neutropenia 45%, Tp 27%, rash 18%, infection 18%, fatigue 18% | 18% | 1.6/- |
| Dex 40 mg on days 1-4, 15-18 q28 | |||||||
| added in nonresponders to L alone | |||||||
| CLD66 | C 300 mg/m2 on days 1, 8, 15 q 28 | 35 (11%) | 63% (43%) | 60%/11% (31%) | Overall 74%, Np 40%, Tp 40%, rash 10%, thrombosis 10% | 9% | 2.4/3.1 |
| L 15 mg on days 1-21 q 28 | |||||||
| Dex 40 mg on days 1, 8, 15, 22 q28 | |||||||
| MLD69 | M 0.18 mg/kg on days 1-4 q28 | 26 (100%) | 58% | 58%/23%, 42% with full-dose L (50%) | Overall 81%, Np 11%, heart failure 11% | — | At 2 y: 54%/81% |
| L 5-15 mg on days 1-21 q28 | |||||||
| Dex 40 mg on days 1-4 q28 | |||||||
| PDex63 | Pomalidomide 2 mg/d | 33 (0) | 82% (25%) | 48%/3% (15%) | Np 30%, infection 27%, arrhythmia 21%, fatigue 18% | 3% | 1.2/2.3 |
| Dex 40 mg OW | |||||||
| Proteasome inhibitor–based therapy | |||||||
| Bortezomib60 | 1.6 mg/m2 OW | 70 (0) | 56% | OW: 69%/38% | Overall OW 50%, TW 79% | 3% | At 1 y: OW: 72%/93% |
| 1.3 mg/m2 TW | TW: 67%/24% (29%) | Tp 18% (TW), vomiting 12% (TW) | TW: 75%/84% | ||||
| CyBorD50 | C 350 mg/m2 on days 1, 8, 15 B 1.0-1.3 mg/m2 TW | 43 (47%) | 74% (46%) | 81%/65% frontline, 22% pretreated (46%) | 19% discontinued (due to neuropathy in 14%) | 0 | At 2 y: 53%/98% |
| Dex 20 mg TW | |||||||
| Ixazomib71 | 4.0 mg on days 1, 8, 15 q28 (MTD) | 16 (0) | 69% (6%) | 42%/8%, | (Any grade) nausea 31%, diarrhea 25%, thrombocytopenia 25% | 0 | — |
| Dex 40 mg on days 1-4 (added in nonresponders) | VGPR 25% (-) |
B, bortezomib; BDex, bortezomib dexamethasone; C, cyclophosphamide; CLD, cyclophosphamide, lenalidomide, dexamethasone; Dex, dexamethasone; F, frontline therapy; H, heart involvement; HR, overall hematologic response; L, lenalidomide; LDex, lenalidomide plus dexamethasone; M, melphalan; MLD, melphalan, lenalidomide, dexamethasone; MTD, maximum tolerated dose; N, number of patients; Np, neutropenia; NR, nonresponders; OR, organ response; OS, overall survival; OW, once weekly; PDex, pomalidomide plus dexamethasone; PFS, progression-free survival; PR, partial response; q, every; SAE, severe adverse events; T, thalidomide; Tp, thrombocytopenia; TTP, time to hematologic progression; TW, twice weekly.
Intention-to-treat response rates are reported.
Frontline therapy
ASCT should be considered in low-risk patients (∼15% to 20%) given the long-term survival in responders. Improvements in patient selection, and particularly the exclusion of subjects with elevated cardiac biomarkers, can reduce transplant-related mortality to <5%.43-45 Current eligibility criteria include the following: cTnT <0.06 ng/mL, NT-proBNP <5000 ng/L,43 age <65 years, performance status 0 to 2, ejection fraction >45%, systolic blood pressure >90 mm Hg (standing), and CO diffusion capacity >50%.46 Melphalan dose may be risk adapted (100, 140, or 200 mg/m2) based on age and renal and cardiac function. After risk-adapted melphalan, consolidation with bortezomib (or thalidomide) increases CR rate to ∼50%, with median survival approaching 8 years.47-49 Alternatively, patients not willing to undergo ASCT can receive stem cell–sparing regimens, such as CyBorD,50,51 as “induction therapy,” transplanting only subjects not attaining CR. However, the definition of the durability of responses with CyBorD requires longer follow-up.
Stage III patients with NT-proBNP >8500 ng/L are at high-risk of death within a few weeks28 and need rapidly active treatment. They are extremely fragile and sensitive to treatment toxicity, particularly high-dose dexamethasone-induced fluid retention and arrhythmias. Standard regimens cannot overcome their dismal outcome.52 Bortezomib combinations, particularly CyBorD, given the rapid action and the vulnerability of amyloidogenic PC to bortezomib,53,54 seem ideal (long-term survivors reported), but prospective upfront studies are still lacking.50 Reduced doses of dexamethasone (10-20 mg) and bortezomib (0.7-1.0 mg/m2) are recommended in high-risk patients with cautious week-by-week dose increase.
Most patients (∼60%) are intermediate risk and are best treated with combination chemotherapy, MDex, although some may still be suitable for ASCT in centers of experience. There is a trend to treat these patients with bortezomib-containing regimens, but evidence of superiority, tolerability (particularly in the elderly), and comparable documented long-lasting responses of standard regimen MDex remain lacking.42,55,56 An ongoing randomized trial comparing MDex with MDex plus bortezomib aims to establish the standard of care in these subjects. If contraindications to ASCT are potentially reversible, a stem cell–sparing regimen is preferable, and CyBorD or CTD57 may be considered.
Patients with overt myeloma and symptomatic AL amyloidosis have a poor prognosis58 and require risk-adapted chemotherapy. Asymptomatic amyloid deposits will not alter the prognosis in myeloma. AL amyloidosis caused by an underlying (usually lymphoplasmacytic) lymphoma poses a particular therapeutic challenge especially due to the low achievable CR/VGPR rates. Suitable patients should be considered upfront for ASCT; otherwise, a rapidly acting combination regimen including rituximab and bortezomib should be used.
Relapsing-refractory patients
The choice of drugs depends on previous treatment and clinical presentation. An attempt to restore response with the regimen used frontline can be made if first remission was prolonged. A bortezomib combination is the first choice in subjects, without severe neuropathy, unexposed to this drug.59,60 Lenalidomide and pomalidomide are good options for overcoming resistance to alkylators, bortezomib, and thalidomide.61-64 Lenalidomide requires careful monitoring of renal function.65 Combination of lenalidomide and alkylators may improve the response rate, but at the cost of significant myelosuppression.66-70 New drugs (carfilzomib, ixazomib,71 and bendamustine72 ) are under evaluation and may widen the therapeutic options. At present, there are no data to support maintenance treatment.
Supportive therapy
Supportive therapy, recently reviewed in Merlini et al3 and Palladini et al,73 is vital. Patient education with daily weights, judicious diuretic use, salt-poor albumin, cautious angiotensin converting enzyme-inhibitors, and close multidisciplinary monitoring makes lifesaving differences. Renal and cardiac transplantation may prolong survival and improve quality of life in subjects with irreversible organ damage. Young patients with isolated advanced cardiac involvement should be considered for heart transplant followed by ASCT.74-77 The use of left ventricular assist devices needs further study.78
Conclusion
Biomarker-based staging and frequent response assessment has helped to improve the survival of patients with AL amyloidosis. When the diagnosis is made early, before advanced organ dysfunction ensues, recovery of organ function and prolonged survival are possible. Changing the approach to monitoring MGUS may reduce late diagnoses, preventing early deaths, but this remains a major challenge. A new framework for randomized clinical trials with novel agents will establish contemporary standards of care.79 Novel agents and therapeutic approaches, such as those targeting amyloid deposits,80 are now under development and, hopefully in the near future, will be used synergistically to give a concrete hope of curing AL amyloidosis.
Acknowledgments
The authors thank Dr Helen J. Lachmann and Dr Laura Obici for critical reading of the manuscript.
This work was supported by grants from “Associazione Italiana per la Ricerca sul Cancro” Special Program Molecular Clinical Oncology 5 per mille n. 9965 “Harnessing tumor cell/microenvironment cross talk to treat mature B cell tumors.”
Authorship
Contribution: G.M., A.D.W., and G.P. designed the review and wrote the manuscript.
Conflict-of-interest disclosure: G.M. received honoraria from Neotope, Pfizer, and Millennium. A.D.W. received honoraria from Janssen-Cilag. The remaining author declares no competing financial interests.
Correspondence: Giampaolo Merlini, Amyloidosis Research and Treatment Center, Fondazione IRCCS Policlinico San Matteo, Viale Golgi, 19 – 27100 Pavia, Italy; e-mail: gmerlini@unipv.it.