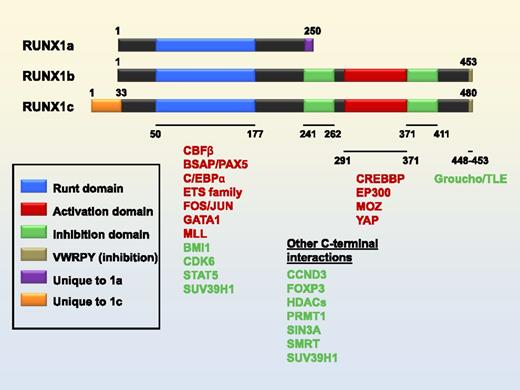
We reported in Ran et al that ectopic expression of RUNX1a, a naturally occurring isoform of RUNX1 that contains a DNA binding domain but lacks both transactivation and inhibitory domains of RUNX1b/c (Figure 1), enhanced the production of CD34+CD45+ hematopoietic stem/progenitor cells (HSPCs) from human embryonic stem cells and induced pluripotent stem cells (ES/iPS).1 HSPCs produced from ES/iPS cells transduced with lentiviruses overexpressing RUNX1a engrafted NSG mice and produced both myeloid and lymphoid cells. In the “Letter to the Editor” by Real et al,2 a valid point was raised that the transformation potential of RUNX1a could partially contribute to the enhancement of HSPC formation by RUNX1a, although no overexpansion or any other clear signs of transformation were observed throughout the 9 weeks post-transplantation in our studies. It should be noted that in addition to the dominant negative effect mentioned by Real et al, RUNX1a can be an activator or repressor in gene expression, but loses certain regulatory functions due to its lack of interaction with some positive and negative cofactors (Figure 1).3,4 In mouse models, overexpression of RUNX1a results in expansion of hematopoietic cells,5 lymphoid leukemia,6 and enhanced engraftment upon transplantation.5,7 In contrast, overexpression of RUNX1b/c promotes p53-dependent senescence,8,9 hematopoietic cell differentiation,10 and the loss of transplanted blood cells.5,11 Using RUNX1a, but not RUNX1c, in our studies is based on these previous discoveries.
Three isoforms of RUNX1 and interacting proteins. Selected domains and isoform-specific regions indicated in key. Coactivator interaction partners are in red. Co-repressor interaction partners are in green. Numbering of domains refers to the RUNX1b isoform.
Three isoforms of RUNX1 and interacting proteins. Selected domains and isoform-specific regions indicated in key. Coactivator interaction partners are in red. Co-repressor interaction partners are in green. Numbering of domains refers to the RUNX1b isoform.
We agree that the potential for hematopoietic cell transformation due to long-term overexpression of RUNX1a is a concern. However, regulated transient expression of RUNX1a during hematopoietic development of ES/iPS cells could be very useful for expanding a rare population of HSPCs. This same principle is illustrated by the use of the very potent proto-oncogene c-Myc to generate iPS cells. In the “Discussion” section, we suggested the use of cell-permeable transcription factors as an alternative to lentiviral transduction and expression of RUNX1a.1 Although we suggested this strategy to avoid the inappropriate expression of endogenous genes via lentiviral integration, transient expression strategies would also eliminate the potentially negative impact of long-term overexpression of RUNX1a on HSPCs. We thank Real et al for raising this important issue, and giving us the opportunity to clarify our argument.
Regarding the expression of 3 isoforms of RUNX1, our data agree with the finding of Real et al that the expression of RUNX1a and RUNX1b/c is increased during the hematopoietic differentiation of human ES/iPS cells, and that RUNX1b/c expression is always higher than RUNX1a expression. This was illustrated in Ran et al,1 Figure 1A-B, and supplemental Figure 1.
Finally, Real et al2 questioned whether the engraftment we observed by CD45+ CD34+ HSPCs derived from RUNX1a-expressing human ES cells was due to an intrinsic feature of the HSPCs, or simply because we transplanted an unusually large number of HSPCs. At present, we cannot distinguish between those 2 possibilities. However, regardless of the mechanism, overexpression of RUNX1a permitted engraftment, either by promoting expansion of HSPCs in vitro, or by altering the properties of HSPCs in vivo; determining which is the case will be a focus of future studies.
In short, we demonstrate a positive effect of RUNX1a on promoting hematopoiesis from human pluripotent stem cells, which provides a potential novel avenue for generating therapeutic HSCs. Additional studies are necessary to examine its possible transforming ability and to create inducible expression systems for using RUNX1a in regenerative medicine.
Authorship
Acknowledgments: The authors thank Dr Nancy Speck for valuable discussion and critical suggestions.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dong-Er Zhang, Moores UCSD Cancer Center, University of California San Diego, La Jolla, CA 92093; e-mail: d7zhang@ucsd.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal