Abstract
Polycythemia vera (PV) is characterized by an increased RBC mass, spontaneous erythroid colony formation, and the JAK2V617F mutation. PV is associated with a high risk of mesenteric and cerebral thrombosis. PV RBC adhesion to endothelial laminin is increased and mediated by phosphorylated erythroid Lu/BCAM. In the present work, we investigated the mechanism responsible for Lu/BCAM phosphorylation in the presence of JAK2V617F using HEL and BaF3 cell lines as well as RBCs from patients with PV. High levels of Rap1-GTP were found in HEL and BaF3 cells expressing JAK2V617F compared with BaF3 cells with wild-type JAK2. This finding was associated with increased Akt activity, Lu/BCAM phosphorylation, and cell adhesion to laminin that were inhibited by the dominant-negative Rap1S17N or by the specific Rap1 inhibitor GGTI-298. Surprisingly, knocking-down EpoR in HEL cells did not alter Akt activity or cell adhesion to laminin. Our findings reveal a novel EpoR-independent Rap1/Akt signaling pathway that is activated by JAK2V617F in circulating PV RBCs and responsible for Lu/BCAM activation. This new characteristic of JAK2V617F could play a critical role in initiating abnormal interactions among circulating and endothelial cells in patients with PV.
Key Points
In polycythemia vera RBCs, JAK2V617F is able to activate an adhesion molecule through a Rap1/Akt pathway, despite the absence of EpoR.
In polycythemia vera, the abnormal adhesion of RBCs to laminin is due to the phosphorylation of Lu/BCAM by a JAK2V617F/Rap1/Akt pathway.
Introduction
Polycythemia vera (PV) is the most common myeloproliferative neoplasm.1 It is characterized by erythropoietin-independent erythroid colony formation in vitro, which is associated with somatic gain-of-function mutations in the gene encoding the tyrosine kinase JAK2.2-5 The most frequent mutation is a substitution from valine to phenylalanine at position 617, which renders JAK2 constitutively active, leading to uncontrolled cell proliferation in the erythroid lineage and resulting in increased red cell mass. Patients with PV exhibit increased platelet and leukocyte counts and have a high risk of thrombosis, which is considered a major cause of mortality and morbidity in this disease.6
In a previous work, we showed that PV RBCs were abnormally adherent to endothelial cells because of the interaction between erythroid Lutheran/basal cell-adhesion molecule (Lu/BCAM) and endothelial laminin.7 Lu/BCAM is an adhesion protein of the immunoglobulin superfamily with a large tissue distribution.8,9 It is the unique erythroid receptor of laminin α5 chain, constituent of laminin 511/521 isoforms, and a major component of the extracellular matrix.10,11 Lu/BCAM is expressed as 2 isoforms of 85 and 78 kDa, named Lu and Lu(v13), respectively.9,10 The 2 isoforms differ only by the length of their cytoplasmic domain, which is composed of 19 a.a. for Lu(v13) and 59 a.a. for Lu. The extra 40 a.a. of Lu comprise phosphorylated serines,12 which have been shown to play a critical role in Lu/BCAM-mediated RBC adhesion to laminin in sickle cell disease.13,14 We showed in our previous work that PV RBC adhesion to laminin was associated with Lu/BCAM hyperphosphorylation. Lu/BCAM phosphorylation also was strongly increased in a K562 cell line coexpressing Lu and JAK2V617F, suggesting that erythroid activation of Lu/BCAM could result from the presence of JAK2V617F in PV RBCs.7 The aim of the present work was to explore the relationship between JAK2V617F signaling and activated Lu/BCAM.
JAK2 signaling in the erythroid lineage is tightly linked to the erythropoietin receptor (EpoR), both proteins being extensively studied in healthy tissues and cancer tissues.15,16 Under physiologic conditions, Epo binding to EpoR triggers the phosphorylation and activation of EpoR-bound JAK2, which in turn activates several downstream signaling pathways that include STAT5, PI3K/Akt, and ERK/MAPK.17 Although the V617F substitution results in a constitutively active JAK2, its activation of the downstream signaling pathways inducing cell proliferation requires the presence of cytokine receptors, such as EpoR or the thrombopoietin receptor TpoR.18 The activity of JAK2V617F in mature RBCs has never been investigated because it is commonly assumed that EpoR down-regulation during terminal differentiation would shut down the JAK2V617F-induced pathways. Thus, our finding that Lu/BCAM was phosphorylated in circulating PV versus healthy RBCs7 strongly suggested that JAK2V617F would still be active in PV RBCs, raising the question about the triggered signaling pathway(s) in the absence of EpoR.
In this study, we investigated the role of JAK2V617F in activating Lu/BCAM-mediated cell adhesion to laminin using HEL and BaF3 cell lines, as well as RBCs from patients with PV. Our findings revealed a novel erythroid role of JAK2V617F that is different from its well-documented oncogenic role in myeloproliferative neoplasms.
Methods
Patients and blood samples
The study was approved by the internal review boards from participating institutions; blood samples were obtained with informed consent in accordance with the Declaration of Helsinki. Six patients with PV, 3 females and 3 males, were included in this study, and they had an average age of 68.8 ± 12.2 years. All 6 patients were positive for JAK2V617F mutation and were seen as outpatients in the Cell Biology Department of the Hôpital Saint-Louis, Paris, France. Patients were treated with phlebotomy; in addition, 4 patients were treated with low-dose aspirin. Two of the 4 had a history of thrombosis; blood samples were drawn at > 3 months after their last thrombotic event. No cytotoxic antiproliferative treatment was administered to these patients. Blood samples from 6 healthy control patients also were analyzed.
Cell culture, transfection, and viral transduction
Parental, JAK2 wt-, or JAK2V617F-expressing BaF3-EpoR cells (a generous gift of Dr W. Vainchenker and Dr J. L. Villeval, Inserm UMR 1009, Institut Gustave Roussy, Villejuif, France)3 were grown in RPMI 1640 medium (Invitrogen) complemented with 10% FCS, 10% of WEHI-3B cell supernatant as a source of IL-3, and 1% penicillin/streptomycin. They were transfected with the pcDNA3 plasmid encoding Lu (BaF3-Lu), Lu(v13) [BaF3-Lu(v13)], or the LuS621A (BaF3-LuS621A) mutant, using Amaxa Nucleofector Technology (Lonza). Stable cell lines expressing each of these proteins were established as described.12
HEL cells were grown in RPMI 1640 medium complemented with 10% FCS and 1% penicillin/streptomycin. Cells were transduced with TRIPizi lentiviral vector expressing Lu (HEL-Lu), LuS621A (HEL-LuS621A), Lu6A (HEL-Lu6A), or Lu5A-S621 (HEL-Lu5A-S621). HEL-Lu cells were transduced with ZEFIR-GFP lentiviral vector expressing Rap1 wt or Rap1S17N (kindly provided by Dr Alfred Wittinghofer, Max-Plank-Institut, Dortmund, Germany). Lentiviral shRNAs targeting EpoR (Sigma-Aldrich) were used according to the manufacturer's instructions.
To summarize in brief, vector particles were produced by transient cotransfection of 293T cells with each of the lentiviral constructs described previously, an encapsidation plasmid lacking all accessory HIV-1 proteins, and a G protein of the vesicular somatitis virus envelope expression-plasmid, as previously described by Sirven et al.19 After 72 hours, the culture medium (RPMI, 10% FCS) was purified and concentrated by ultracentrifugation (22 000g, 4°C, 1 hour, 30 minutes). The pellet was suspended in PBS, aliquoted, and stored at −80°C before use. Cells were transduced with 10 μL of lentiviral vector productions at 2 × 105 cells/mL in normal growing medium. The different BaF3 and HEL cell lines expressed equivalent amounts of recombinant Lu proteins, as determined by flow cytometry (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Adhesion assays
Cell adhesion to laminin 511/521 was measured under flow conditions with the use of a capillary flow chamber. Laminin 511/521 from human placenta (Sigma-Aldrich) was immobilized (1 μg/cm2) in uncoated μI Luer0.2 microslides (Sigma-Aldrich; internal channel dimensions: length 50 mm, width 5 mm, height 0.2 mm) at 4°C overnight. Serum-deprived cells were washed 3 times with 10 mL of PBS and suspended in Hanks balanced salt solution without calcium chloride and magnesium sulfate (Sigma-Aldrich), supplemented with 0.4% BSA, at 107 cells/mL. Cell suspensions were perfused through the microslide at a shear stress of 0.1 dyn/cm2 for 10 minutes and washouts used the Hanks/0.4% BSA buffer at 1, 1.5, 2, and 4 dyn/cm2 for 5 minutes each. After each wash, adherent cells were counted in 11 representative areas along the centerline of the microslide using the AxioObserver Z1 microscope and AxioVision 4 analysis software (Carl Zeiss). Images of the same 11 areas were obtained throughout each experiment using the “Mark and Find” module of AxioVision analysis software.
RBC adhesion assays were performed with laminin-coated Vena8 Endothelial+ biochips (internal channel dimensions: length 20 mm, width 0.8 mm, height 0.12 mm) and Mirus Nanopump (Cellix). RBCs were perfused at hematocrit 0.5% for 10 minutes at 0.2 dyn/cm2, and 5-minute washouts were performed at 1, 2, 3, 4, and 5 dyn/cm2. Adhesion was quantified as described previously.
Adhesion assays were performed in presence of inhibitors after overnight incubation in Hanks/0.4% BSA buffer at 37°C: JAK2 Inhibitor II (HBCH), 100μM (Merck Millipore); JAK2 Inhibitor V (Z3), 50μM; PKA inhibitor 6-22 Amide (PKAi),10μM (Merck Millipore); H89 Dihydrochloride (H89), 10μM (Merck Millipore); InSolution LY294002 (LY), 25μM (Merck Millipore); Akt 1/2 kinase Inhibitor (Akti), 25μM (Sigma-Aldrich); and GGTI-298 trifluoroacetate salt hydrate (GGTI), 100μM (Sigma-Aldrich). Adhesion assays in presence of Epo were performed by incubating PV RBCs with 10 U/mL of Epo during 30 minutes at 37°C.
Phosphorylation assays
Phosphorylation of Lu/BCAM isoforms and mutants was assessed in BaF3 and HEL cell lines, and PV RBCs, as described.12,13 Inhibitors were used under the conditions described for the adhesion assays. In vitro phosphorylation of Lu cytoplasmic domain by purified Akt (Sigma-Aldrich) was performed as described12 by the use of 5 μg of glutathione-S-transferase (GST) fusion proteins in which a serine of the linker region, potentially targeted by Akt, was replaced by alanine.
Flow cytometry
Akt activity quantification
Akt activity was measured in cell lysates of HEL cells or RBCs using the Akt kinase activity kit (Enzo Life Sciences). Protein quantification after cell lysis was performed with the BCA Protein Assay Kit (Pierce-Thermo Scientific). Active Akt was measured after overnight cell incubation with indicated inhibitors as described previously.
Rap1-GTP quantification assay
RBCs or serum-deprived cells were lysed on ice in 50mM Tris-HCl, pH 7.5; 200mM NaCl; 2.5mM MgCl2; 10% glycerol; 1% Nonidet P-40; 1mM phenylmethylsulfonyl fluoride; and 1mM Na3VO4, supplemented with protease inhibitor cocktail (Roche). After centrifugation, the protein concentration was measured with the BCA Protein Assay Kit (Thermo Scientific Pierce). Equal protein amounts were incubated for 1 hour at 4°C with 50 μg of the GST-RalGDS-RBD fusion protein (a generous gift of Dr Johannes L. Bos, Molecular Cancer Research, UMC, Utrecht, The Netherlands) bound to glutathione-Sepharose 4B beads. After several washes, Rap1-GTP was eluted in Laemmli buffer and quantified by Western blot with an anti-Rap1 mAb (Becton Dickinson).20
Statistical analyses
Results are presented as means ± SEM, numbers, or medians, as appropriate. Statistical significance was determined using unpaired t test, paired t test, or Mann-Whitney test, as indicated in the figure legends.
Results
JAK2V617F increases Lu/BCAM-mediated adhesion of BaF3 and HEL cells to laminin
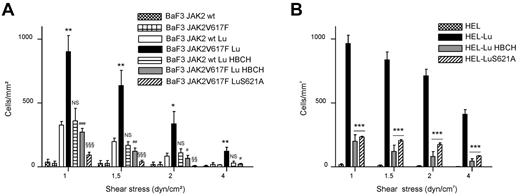
We tested the effect of JAK2V617F expression on Lu/BCAM adhesion function. BaF3 JAK2 wt and BaF3 JAK2V617F cells3 were transfected to express the Lu/BCAM long isoform Lu then used for adhesion assays onto immobilized laminin under flow conditions. These 2 murine cell lines were chosen because they expressed recombinant human JAK2 wt or JAK2V617F, which enabled us to compare the effects of both forms in the same cellular context. Cells expressing JAK2V617F adhered more than those expressing JAK2 wt (Figure 1A). Adding the specific JAK2 inhibitor HBCH decreased the adhesion of BaF3 JAK2V617F cells but not of BaF3 JAK2 wt. Nontransfected BaF3 JAK2 wt and BaF3 JAK2V617F cells were used as negative controls (Figure 1A). HEL cells, which express endogenous JAK2V617F, were transduced by a lentiviral vector encoding the Lu isoform. HEL-Lu cells adhered to laminin, whereas HEL wt did not (Figure 1B). Similarly to BaF3 JAK2V617F cells, incubating HEL-Lu cells with HBCH dramatically decreased their adhesion level (Figure 1B). All these data indicated that Lu-mediated cell adhesion to laminin was activated in the presence of JAK2V617F.
JAK2V617F increases BaF3-Lu and HEL-Lu adhesion to laminin via LuS621. Cell adhesion to laminin was measured under flow conditions using (A) BaF3 JAK2 wt or BaF3 JAK2V617F and (B) HEL cells expressing Lu or the LuS621A mutant. Cells were incubated or not incubated with the JAK2 inhibitor HBCH. Histograms indicate the mean number ± SEM of adherent cells per mm2 at each shear stress. Unpaired t test, (A) *P < .05, **P < .01, compared with BaF3 JAK2 wt Lu; #P < .05, ##P < .01, ###P < .001, compared with BaF3 JAK2V617F Lu; §§P < .01, §§§P < .001, compared with BaF3 JAK2V617F Lu; NS, nonsignificant, compared with BaF3 JAK2 wt Lu, (n = 6); (B) ***P < .001, compared with HEL-Lu (n = 3).
JAK2V617F increases BaF3-Lu and HEL-Lu adhesion to laminin via LuS621. Cell adhesion to laminin was measured under flow conditions using (A) BaF3 JAK2 wt or BaF3 JAK2V617F and (B) HEL cells expressing Lu or the LuS621A mutant. Cells were incubated or not incubated with the JAK2 inhibitor HBCH. Histograms indicate the mean number ± SEM of adherent cells per mm2 at each shear stress. Unpaired t test, (A) *P < .05, **P < .01, compared with BaF3 JAK2 wt Lu; #P < .05, ##P < .01, ###P < .001, compared with BaF3 JAK2V617F Lu; §§P < .01, §§§P < .001, compared with BaF3 JAK2V617F Lu; NS, nonsignificant, compared with BaF3 JAK2 wt Lu, (n = 6); (B) ***P < .001, compared with HEL-Lu (n = 3).
Phosphorylation of serine 621 is required for Lu/BCAM activation by JAK2V617F
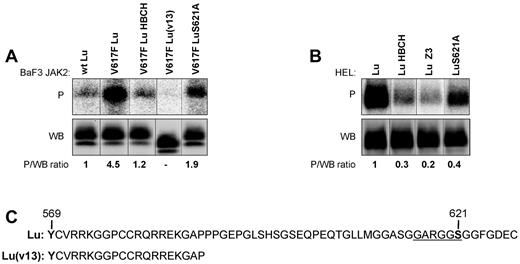
Lu-mediated cell adhesion to laminin in sickle cell disease is activated by the phosphorylation of Lu cytoplasmic domain.12 Phosphorylation assays were performed with BaF3 and HEL cells expressing Lu. Lu was highly phosphorylated in BaF3 JAK2V617F cells, 4.9-fold more than in BaF3 JAK2 wt cells (4.9 ± 0.29, n = 5, P < .01; Figure 2A). Lu phosphorylation was strongly inhibited by the JAK2 inhibitor HBCH (1.1 ± 0.2 vs 4.9 ± 0.29, n = 5, P < .01; Figure 2A). Similar high phosphorylation also was measured in HEL-Lu cells (Figure 2B). This phosphorylation also was strongly decreased in the presence of JAK2 inhibitors HBCH and Z3 (0.3 ± 0.07, P < .01 and 0.2 ± 0.04, P < .001 vs 1, respectively, n = 4; Figure 2B).
Effect of JAK2V617F on Lu phosphorylation in BaF3 and HEL cells. Quantification of Lu, Lu(v13), and LuS621A phosphorylation in (A) BaF3 and (B) HEL cell lines in the presence or absence of JAK2 inhibitors HBCH or Z3. The top (P) and bottom (WB) panels show the phosphorylation and the total amount of the immunopurified proteins, respectively. The phosphorylated fraction is determined by the P/WB ratio, with the ratio of the first column being the reference value. The minor band in the WB panel A is believed to be the nonglycosylated precursor form of Lu/BCAM. It is also detected in HEL-Lu cells (B) but to a lesser extent, as in Figures 3A and 5C. Typical results of 5 (A) and 4 (B) independent experiments. (C) The cytoplasmic domain of Lu and Lu(v13). Lu(v13) cytoplasmic sequence is identical for both isoforms and includes a single tyrosine residue (Y569) predicted to be at the border of the membrane and the cytoplasmic domains. The Lu 40 extra a.a. comprise the GARGGS motif (underlined) that is similar to the Akt consensus motif RXRXXS/T.
Effect of JAK2V617F on Lu phosphorylation in BaF3 and HEL cells. Quantification of Lu, Lu(v13), and LuS621A phosphorylation in (A) BaF3 and (B) HEL cell lines in the presence or absence of JAK2 inhibitors HBCH or Z3. The top (P) and bottom (WB) panels show the phosphorylation and the total amount of the immunopurified proteins, respectively. The phosphorylated fraction is determined by the P/WB ratio, with the ratio of the first column being the reference value. The minor band in the WB panel A is believed to be the nonglycosylated precursor form of Lu/BCAM. It is also detected in HEL-Lu cells (B) but to a lesser extent, as in Figures 3A and 5C. Typical results of 5 (A) and 4 (B) independent experiments. (C) The cytoplasmic domain of Lu and Lu(v13). Lu(v13) cytoplasmic sequence is identical for both isoforms and includes a single tyrosine residue (Y569) predicted to be at the border of the membrane and the cytoplasmic domains. The Lu 40 extra a.a. comprise the GARGGS motif (underlined) that is similar to the Akt consensus motif RXRXXS/T.
To test whether JAK2 could directly phosphorylate Lu on its single tyrosine residue Y569, predicted to be at the border of its membrane and cytoplasmic domains, the short isoform Lu(v13), exhibiting the Y569 residue but lacking all cytoplasmic serines and threonines (Figure 2C), was expressed in BaF3 JAK2V617F cells. Lu(v13) was not phosphorylated in these cells (Figure 2A), indicating that the JAK2-mediated Lu phosphorylation was taking place on serine/threonine residues but not on Y569.
We previously showed that Lu-mediated cell adhesion could be activated by the phosphorylation of its serine 621.12 We investigated the role of this serine in JAK2V617F-induced cell adhesion to laminin. S621 was substituted by alanine and LuS621A mutant was expressed in BaF3 JAK2V617F and HEL cells. Compared with Lu wt, LuS621A was less phosphorylated in both cell lines (2.1 ± 0.32 vs 4.9 ± 0.29, n = 5, P < .05 and 0.44 ± 0.12 vs 1, n = 4, P < .05, respectively; Figure 2A-B) indicating that S621 was a target of a JAK2V617F-activated signaling pathway. Both cell lines expressing the LuS621A mutant had a dramatic decrease of cell adhesion to laminin (Figure 1A-B), indicating that phosphorylation of the S621 residue was required for Lu activation by JAK2V617F.
JAK2V617F triggers Lu/BCAM adhesion function through Akt
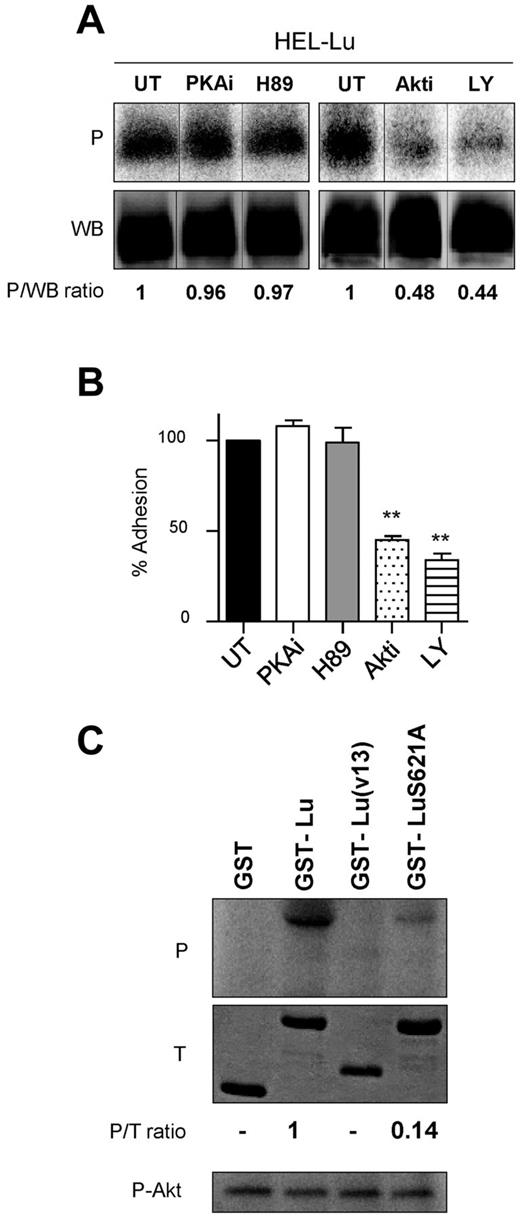
Because PKA was reported to activate Lu/BCAM adhesion function in sickle RBCs,14 we investigated its potential role in phosphorylating Lu isoform and in activating cell adhesion to laminin in the presence of JAK2V617F. PKA inhibitors PKAi and H89 failed to inhibit Lu isoform phosphorylation in HEL cells (1.02 ± 0.12 and 1.07 ± 0.1 vs 1, respectively, n = 3; Figure 3A). Moreover, HEL-Lu cell adhesion to laminin was not affected by PKAi or H89 (Figure 3B), excluding PKA from the JAK2V617F signaling cascade that activates Lu/BCAM. Sequence analyses showed that the S621 residue was comprised into a sequence (GARGGS) similar to the Akt consensus motif (RXRXXS/T; Figure 2C). We investigated the potential role of Akt, a serine/threonine kinase that is activated by JAK2V617F, in Lu phosphorylation. In vitro phosphorylation assays were performed with purified Akt and a GST fusion protein comprising the 59 aa of Lu cytoplasmic domain, GST-Lu. GST-Lu was phosphorylated and GST was not (Figure 3C). A GST fusion protein comprising the 19 aa of the short isoform Lu(v13) was used as negative control and showed no phosphorylation (Figure 3C).
Akt phosphorylates Lu and is responsible for JAK2V617F-induced cell adhesion to laminin. (A) Quantification of Lu phosphorylation in the presence or absence (untreated; UT) of PKA (PKAi or H89) or Akt (Akti or LY) inhibitors in HEL-Lu cells. See Figure 2 legend for the quantification method. Typical results of 3 experiments. (B) Effect of the same inhibitors on HEL-Lu cell adhesion to laminin at 2 dyn/cm2. Data are normalized to values from the UT HEL-Lu cell adhesion. Paired t test, **P < .01 compared with UT, (n = 3). (C) In vitro phosphorylation of purified recombinant GST, GST-Lu, GST-Lu(v13), and GST-LuS621A by purified Akt. The top (P) and middle (T) panels show the phosphorylation and the total amounts of the proteins, respectively. The P/T ratio indicates the relative phosphorylation level. The bottom panel (P-Akt) shows autophosphorylated Akt as positive control.
Akt phosphorylates Lu and is responsible for JAK2V617F-induced cell adhesion to laminin. (A) Quantification of Lu phosphorylation in the presence or absence (untreated; UT) of PKA (PKAi or H89) or Akt (Akti or LY) inhibitors in HEL-Lu cells. See Figure 2 legend for the quantification method. Typical results of 3 experiments. (B) Effect of the same inhibitors on HEL-Lu cell adhesion to laminin at 2 dyn/cm2. Data are normalized to values from the UT HEL-Lu cell adhesion. Paired t test, **P < .01 compared with UT, (n = 3). (C) In vitro phosphorylation of purified recombinant GST, GST-Lu, GST-Lu(v13), and GST-LuS621A by purified Akt. The top (P) and middle (T) panels show the phosphorylation and the total amounts of the proteins, respectively. The P/T ratio indicates the relative phosphorylation level. The bottom panel (P-Akt) shows autophosphorylated Akt as positive control.
To determine the specificity of Akt phosphorylation, the S621 residue was mutated into alanine. The S621A substitution almost abrogated the phosphorylation of the GST-LuS621A protein, with a 7-fold decrease of the phosphorylation level compared with GST-Lu (Figure 3C), clearly indicating that S621 was the main target of Akt in Lu cytoplasmic domain. Akt was autophosphorylated in all assays (Figure 3C), indicating that the absence of GST, GST-Lu(v13), and GST-LuS621A phosphorylation was not because of a defect in Akt enzymatic activity. We then tested whether Akt could phosphorylate Lu in a cellular context by performing phosphorylation assays using HEL-Lu cells in the presence of the Akt inhibitors Akti or LY. Lu phosphorylation was severely inhibited by both reagents (Akti: 0.52 ± 0.09 vs 1, n = 3, P < .05; LY: 0.58 ± 0.12 vs 1, n = 3, P < .05; Figure 3A), indicating that Akt was the primary kinase responsible for Lu phosphorylation in these cells. Finally, inhibiting Akt by Akti or LY significantly decreased HEL-Lu adhesion to laminin (Figure 3B).
EpoR is not essential for Lu/BCAM activation by JAK2V617F
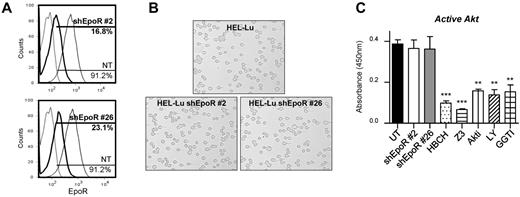
Association with EpoR is known to be essential for JAK2V617F-mediated cellular transformation. Particularly, phosphorylation of EpoR tyrosine 479 was shown to be required for the PI3K/Akt pathway activation by JAK2V617F.21 Yet, EpoR expression is lost in mature RBCs, and we wanted to investigate the impact of this loss on JAK2V617F-mediated activation of Lu/BCAM. EpoR was knocked down in HEL-Lu cells by the use of shRNAs. Despite the high efficiency of EpoR silencing (84% and 77% of EpoR-negative cells in clones #2 and #26, respectively; Figure 4A), cell adhesion to laminin was not altered (Figure 4B). Surprisingly, Akt activity was not modified in these cells, whereas it was severely inhibited by JAK2 or Akt inhibitors (Figure 4C), strongly suggesting that JAK2V617F could activate Akt in the absence of EpoR.
EpoR silencing in HEL-Lu cells does not affect JAK2/Akt signaling. (A) Flow cytometry analysis of EpoR expression in 2 HEL-Lu shEpoR clones. The percentage of EpoR-positive cells is indicated for both clones (black bold curves) and for nontransduced cells (NT; gray thin curves). Dotted lines represent the IgG-isotype control. (B) Typical microscopy images showing HEL-Lu and HEL-Lu shEpoR adhesion to laminin at 2 dyn/cm2. (C) Quantification of active Akt in HEL-Lu shEpoR cells (clones #2 and #26) and HEL-Lu cells in the absence (UT) or presence of different inhibitors. Unpaired t test, **P < .01 and ***P < .001, compared with UT (n = 4).
EpoR silencing in HEL-Lu cells does not affect JAK2/Akt signaling. (A) Flow cytometry analysis of EpoR expression in 2 HEL-Lu shEpoR clones. The percentage of EpoR-positive cells is indicated for both clones (black bold curves) and for nontransduced cells (NT; gray thin curves). Dotted lines represent the IgG-isotype control. (B) Typical microscopy images showing HEL-Lu and HEL-Lu shEpoR adhesion to laminin at 2 dyn/cm2. (C) Quantification of active Akt in HEL-Lu shEpoR cells (clones #2 and #26) and HEL-Lu cells in the absence (UT) or presence of different inhibitors. Unpaired t test, **P < .01 and ***P < .001, compared with UT (n = 4).
Rap1 is the key effector for Lu/BCAM activation by JAK2V617F
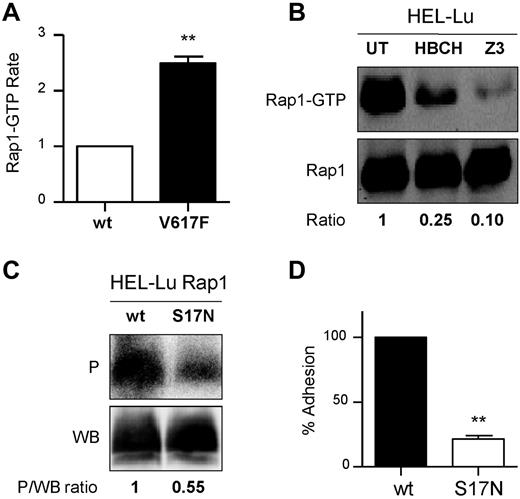
The Epac/Rap1 pathway has been reported to play a role in Lu/BCAM-mediated sickle RBC adhesion.22 We explored the activation of Rap1 in the presence of JAK2V617F using a GST pull-down assay with the fusion protein GST-RalGDS that binds the GTP-bound, active form of Rap1. Compared with BaF3 JAK2 wt, BaF3 JAK2V617F cells exhibited 2.5-fold more Rap1-GTP (2.5 ± 0.24, n = 4; Figure 5A). High levels of Rap1-GTP also were measured in HEL cells that were severely decreased in the presence of JAK2 inhibitors, HBCH and Z3 (0.27 ± 0.07, P < .01 and 0.23 ± 0.09, P < .01 vs 1, respectively, n = 3; Figure 5B). The results of the pull-down assays in BaF3 and HEL cells indicated that JAK2V617F was an upstream activator of Rap1 in these cells.
Rap1 mediates Lu/BCAM activation by JAK2V617F. (A) Quantification of Rap1-GTP in BaF3 JAK2 wt (wt) and BaF3 JAK2V617F (V127F) cells, paired t test, **P < .01 compared with wt, (n = 4). (B) Quantification of Rap1-GTP in the presence or absence of JAK2 inhibitors in HEL-Lu cells. The top and bottom panels show the amounts of Rap1-GTP and total Rap1, respectively. The GTP-bound fraction is determined by the Rap1-GTP/Rap1 ratio, with the ratio of the UT column being the reference value. Typical results of 3 experiments. (C) Quantification of Lu phosphorylation in the presence of Rap1 wt or Rap1 dominant-negative mutant S17N. The phosphorylated fraction is determined by the P/WB ratio, with the ratio of the wt column being the reference value. Results are representative of 3 experiments. (D) Cell adhesion to laminin at 2 dyn/cm2 of HEL-Lu cells expressing Rap1S17N normalized to values from cells expressing Rap1 wt; paired t test, **P < .01 compared with wt, (n = 3).
Rap1 mediates Lu/BCAM activation by JAK2V617F. (A) Quantification of Rap1-GTP in BaF3 JAK2 wt (wt) and BaF3 JAK2V617F (V127F) cells, paired t test, **P < .01 compared with wt, (n = 4). (B) Quantification of Rap1-GTP in the presence or absence of JAK2 inhibitors in HEL-Lu cells. The top and bottom panels show the amounts of Rap1-GTP and total Rap1, respectively. The GTP-bound fraction is determined by the Rap1-GTP/Rap1 ratio, with the ratio of the UT column being the reference value. Typical results of 3 experiments. (C) Quantification of Lu phosphorylation in the presence of Rap1 wt or Rap1 dominant-negative mutant S17N. The phosphorylated fraction is determined by the P/WB ratio, with the ratio of the wt column being the reference value. Results are representative of 3 experiments. (D) Cell adhesion to laminin at 2 dyn/cm2 of HEL-Lu cells expressing Rap1S17N normalized to values from cells expressing Rap1 wt; paired t test, **P < .01 compared with wt, (n = 3).
The potential role of Rap1 in activating Lu was explored by expressing the dominant negative form of Rap1, Rap1S17N, in HEL-Lu cells. Lu phosphorylation was inhibited in the presence of Rap1S17N compared with Rap1 wt (0.53 ± 0.1 vs 1, n = 4, P < .05; Figure 5C). Rap1S17N also significantly inhibited Lu/BCAM-mediated cell adhesion to laminin (Figure 5D), clearly indicating that Rap1 was required for Lu/BCAM activation in the presence of JAK2V617F.
Lu/BCAM is activated by a JAK2V617F/Rap1/Akt pathway in PV RBCs
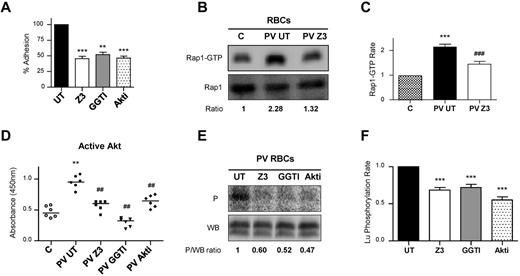
We investigated the newly characterized JAK2V617F/Rap1/Akt signaling pathway in RBCs from 6 patients with PV. First, we tested for EpoR expression on circulating PV RBCs using flow cytometry and found all blood samples negative for EpoR (not shown). Because flow cytometry might not detect a low copy number of EpoR, we performed adhesion assays to test whether stimulation with Epo increases activation of the signaling pathway and subsequently cell adhesion to laminin. Incubation with Epo did not affect PV RBC adhesion to laminin (supplemental Figure 2), indicating the absence of functional EpoR at the cell membrane. As expected, incubating RBCs with JAK2 inhibitor, Z3, significantly inhibited their adhesion to laminin (Figure 6A). Rap1 inhibitor, GGTI, and Akt inhibitor, Akti, also inhibited RBC adhesion to laminin, indicating that Rap1 and Akt were involved in Lu/BCAM-mediated adhesion in mature PV RBCs (Figure 6A). Rap1-GTP was quantified with the GST-RalGDS pull-down assay. High Rap1-GTP levels were measured in PV RBCs compared with control (Figure 6B-C). Incubating the cells with Z3 severely diminished Rap1-GTP levels (Figure 6B-C), indicating that Rap1 was activated in a JAK2V617F-dependent manner. We measured Akt activity in PV RBCs and found that it was significantly greater than in control blood samples (Figure 6D). Adding Z3, GGTI, or Akti as control markedly inhibited Akt activity in PV RBCs. Finally, we investigated Lu/BCAM phosphorylation in all PV RBC samples and found that it also was inhibited in the presence of Z3, GGTI or Akti (Figure 6E-F). Altogether, these experiments showed that JAK2V617F could activate Akt through Rap1 in the absence of EpoR, leading to Lu/BCAM phosphorylation and to the activation of its adhesion function in mature PV RBCs.
The JAK2V617F/Rap1/Akt pathway is involved in increased PV RBC adhesion to laminin. All results shown in this figure were obtained with blood samples from the same 6 patients with PV. (A) Effect of JAK2, Rap1, or Akt inhibitors (Z3, GGTI, or Akti, respectively) on RBC adhesion to laminin at 2 dyn/cm2, determined with blood samples obtained from 6 patients with PV. Paired t test, **P < .01 and ***P < .001, compared with untreated RBCs (UT). (B) Quantification of Rap1-GTP in a control “C” and a PV patient blood samples, in the absence (PV UT) or presence (PV Z3) of Z3. The top and bottom panels show the amounts of Rap1-GTP and total Rap1, respectively. The GTP-bound fraction is determined by the Rap1-GTP/Rap1 ratio, with the ratio of the C column being the reference value. (C) Quantification of Rap1-GTP in blood samples from 6 controls “C” and 6 PV patients, in the absence (PV UT) or presence (PV Z3) of Z3, paired t test, ***P < .001 compared with C, ###P < .001 compared with PV UT (n = 6). (D) Quantification of Akt activity in the same control “C” and PV blood samples in the absence (UT) or presence of Z3, GGTI, or Akti. Horizontal bars indicate medians; Mann-Whitney test, **P < .01, compared with C, ##P < .01 compared with PV UT. (E) Quantification of Lu phosphorylation in a blood sample of a PV patient, in the absence (UT) or presence of Z3, GGTI, or Akti. The phosphorylated fraction is determined by the P/WB ratio, with the ratio of the UT column being the reference value. (F) Quantification of Lu/BCAM phosphorylation in blood samples from the 6 PV patients, in the absence (UT) or presence of the same inhibitors, paired t test, ***P < .001 compared with UT.
The JAK2V617F/Rap1/Akt pathway is involved in increased PV RBC adhesion to laminin. All results shown in this figure were obtained with blood samples from the same 6 patients with PV. (A) Effect of JAK2, Rap1, or Akt inhibitors (Z3, GGTI, or Akti, respectively) on RBC adhesion to laminin at 2 dyn/cm2, determined with blood samples obtained from 6 patients with PV. Paired t test, **P < .01 and ***P < .001, compared with untreated RBCs (UT). (B) Quantification of Rap1-GTP in a control “C” and a PV patient blood samples, in the absence (PV UT) or presence (PV Z3) of Z3. The top and bottom panels show the amounts of Rap1-GTP and total Rap1, respectively. The GTP-bound fraction is determined by the Rap1-GTP/Rap1 ratio, with the ratio of the C column being the reference value. (C) Quantification of Rap1-GTP in blood samples from 6 controls “C” and 6 PV patients, in the absence (PV UT) or presence (PV Z3) of Z3, paired t test, ***P < .001 compared with C, ###P < .001 compared with PV UT (n = 6). (D) Quantification of Akt activity in the same control “C” and PV blood samples in the absence (UT) or presence of Z3, GGTI, or Akti. Horizontal bars indicate medians; Mann-Whitney test, **P < .01, compared with C, ##P < .01 compared with PV UT. (E) Quantification of Lu phosphorylation in a blood sample of a PV patient, in the absence (UT) or presence of Z3, GGTI, or Akti. The phosphorylated fraction is determined by the P/WB ratio, with the ratio of the UT column being the reference value. (F) Quantification of Lu/BCAM phosphorylation in blood samples from the 6 PV patients, in the absence (UT) or presence of the same inhibitors, paired t test, ***P < .001 compared with UT.
Discussion
We provide evidence that JAK2V617F induces Lu/BCAM phosphorylation and activates its mediated cell adhesion to laminin by stimulating a Rap1/Akt signaling pathway in the absence of EpoR. We have previously found that Lu/BCAM adhesion function was increased in PV RBCs,7 and here we characterize the signaling pathway responsible for this activation. This pathway involves JAK2V617F, Rap1, and Akt because inhibiting 1 of these 3 proteins inhibits the adhesion of PV RBCs to laminin. The JAK2V617F/PI3K/Akt pathway is well described in myeloproliferative neoplasms, but this is the first time Rap1 is identified as a downstream effector of JAK2V617F.
One of our major findings is the persistence of an active JAK2V617F/Akt signaling pathway in mature PV RBCs despite the absence of EpoR. In HEL cells, silencing EpoR did not abrogate Lu/BCAM activation, indicating that it was independent of the well-described EpoR/JAK2/PI3K/Akt pathway.23 JAK2V617F has been extensively studied for its role in cell proliferation, survival, and differentiation during erythropoiesis. Despite the constitutive activation feature of the V617F mutation, the authors of several reports showed that JAK2V617F-mediated transformation requires a homodimeric type I cytokine receptor.24 This receptor plays the role of a scaffold for the JAK2V617F-mediated signal transduction. Indeed, tyrosine phosphorylation of EpoR cytoplasmic domain is important for the activation of the downstream pathways involving STATs and PI3K/Akt.21,25 Hence, we investigated the presence of class I and class II cytokine receptor constituents such as gp130 subunit (class I) and interferon-α/β R1 (IFNAR1, class II) on PV and control RBCs. Flow cytometry analysis showed that gp130 and IFNAR1 were not expressed on either cell types (data not shown). Although no report described the presence of cytokine receptors on RBCs, further experiments are needed to investigate their potential expression on the surface of PV RBCs. Nevertheless, this is not the first time JAK2 is shown to act independently of a receptor tyrosine kinase because Dawson et al reported that human JAK2 was present in the nucleus of hematopoietic cells and directly phosphorylated histone H3.26
Our data clearly showed that Rap1 was specifically activated in a JAK2V617F context because BaF3 JAK2V617F cells had 2.5-fold more Rap1-GTP than BaF3 JAK2 wt cells. Rap1 is a small GTP-bound protein of the Ras superfamily that has been described as an upstream effector of Akt in several reports.27-29 Ras-like proteins couple extracellular signals to various cellular responses and have the specificity of activating proteins at the inner surface of cell membranes. Rap1 is mainly involved in controlling cell adhesion, cell junction formation, cell secretion, and cell polarity.30 Rap1 cycles between an inactive GDP-bound state and an active GTP-bound state. The GDP-GTP cycle is regulated by guanine nucleotide-exchange factors, which allow the GTP to bind to Rap1 after the GDP is released. Rap1 can be activated by several guanine nucleotide-exchange factors, such as C3G,31,32 Epac1, and Epac2.33 C3G is activated after the phosphorylation of its tyrosine residue 504 after its recruitment to the membrane by the adaptor protein c-Crk.34,35 C3G is phosphorylated by the combined action of JAK2 and c-Src in NIH-3T3 cells, driving the activation of Rap1 in response to cellular stimulation with growth hormone.20 Our hypothesis is that Rap1 could be activated in a similar manner in PV RBCs, HEL, and BaF3 JAK2V617F cells. Consistent with this hypothesis, Feller et al and Chin et al showed that Rap1 was activated by C3G and a member of the Crk family, CrkL, that is predominantly expressed in hematopoietic cells36 and phosphorylated in response to stimulation with Epo or IL-3.37 In their study, Arai et al showed that adhesion of the 32D hematopoietic cell line was activated by Epo or IL-3 through the activation of the C3G/CrkL complex, Rap1, and β1 integrins.38
Lu/BCAM adhesion function could be activated by the phosphorylation of its serine 62112 or by the dissociation of its cytoplasmic domain from the spectrin-based skeleton.39,40 In sickle cell disease, RBCs exhibit an abnormal cAMP-dependent Lu/BCAM phosphorylation12,13 associated with high adhesion to laminin.13,14 This adhesion seems to involve protein kinase A14 or Rap1.22 Our findings indicate that Lu/BCAM activation could occur through another serine/threonine kinase, Akt (or protein kinase B). The in vitro phosphorylation experiments revealed that Akt could directly phosphorylate Lu cytoplasmic domain, and this was strongly supported by our experiments in HEL cells in which Akti inhibited Lu phosphorylation. Inhibiting PKA activity by PKAi or H89 did not impact HEL-Lu cell adhesion or Lu phosphorylation, clearly indicating that it was not involved in Lu activation in the presence of a JAK2V617F background. Because increased Lu/BCAM phosphorylation could also result from serine phosphatase inhibition, we investigated the enzymatic activity of protein phosphatase 2 (PP2A), a ubiquitous serine/threonine phosphatase with broad substrate specificity. JAK2 was shown to bind to PP2A in 32D myeloid progenitor cells and to phosphorylate its catalytic subunit resulting in inhibition of phosphatase activity.41,42 We measured the PP2A enzymatic activity in BaF3 JAK2 wt and BAF3 JAK2V617F cells and found no difference between these cell lines (not shown), indicating that JAK2V617F did not affect PP2A activity.
The S621A substitution almost abrogated cell adhesion to laminin, but the LuS621A mutant was still phosphorylated in BaF3 and HEL cells. Lu cytoplasmic domain includes 4 other serines (596, 598, 600, 614) and one threonine (606; Figure 2C). We have previously shown that serines 596 and 598 were substrates of glycogen synthase kinase 3 β and casein kinase 2, and were phosphorylated in transfected K562 cells.12 Serines 596, 598, and 621 seemed to be the only phosphorylated residues in Lu cytoplasmic domain, as the triple mutant in which the 3 serines were substituted by alanine was not phosphorylated in K562 and MDCK cells.12 To selectively address the role of S621, we expressed 2 Lu mutants in HEL cells for which the threonine and all 5 serines (Lu6A), or the threonine and all serines but S621 (Lu5A-S621), were substituted by alanine. As expected, the Lu6A mutant was not phosphorylated, and HEL-Lu6A cells showed no significant adhesion to laminin (supplemental Figure 3). The Lu5A-S621 mutant was phosphorylated, and the level of HEL-Lu5A-S621 cell adhesion to laminin was similar to HEL-Lu cells, indicating that S621 was the main residue mediating the activating effect of JAK2V617F on cell adhesion. The function of the other phosphorylated residues is still unknown.
Thrombosis is the main clinical complication in PV. Growing evidence supports the hypothesis that endothelial dysfunction might contribute to thrombotic events by orchestrating the recruitment of blood cell elements to sites of injury.43,44 In PV patients with Budd-Chiari syndrome and portal vein thrombosis, Sozer et al. showed that cells with endothelial characteristics lining liver sinusoids and venules were JAK2V617F positive.45,46 Using an immunodeficient mouse transplant assay system, the same group showed that JAK2V617F-positive CD34+ cells were able to generate endothelial like cells in vivo expressing human JAK2V617F.47 Endothelial cells express a wide range of adhesion proteins, including integrins and CAMs (ie, cell adhesion molecules). We show that JAK2V617F is able to activate Lu/BCAM through Rap1 and Akt that are both ubiquitously expressed proteins regulating cell adhesion and cell-cell interactions. Hence, it would be challenging to test the adhesive properties of endothelial cells expressing JAK2V617F and their recruitment potential of blood components under flow conditions.
In conclusion, our study shows that JAK2V617F activates an erythroid adhesion protein through a novel EpoR-independent pathway. Considering the ubiquitous feature of this signaling pathway, we assume that this activation could occur in most cell types expressing JAK2V617F and could affect other adhesion proteins than Lu/BCAM. Our work opens new perspectives in understanding cell adhesion and cell-cell interactions in human pathologies characterized by the JAK2V617F mutation.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Anne Dubart-Kupperschmitt from Inserm Unit 804 (Hôpital Kremlin Bicêtre, France) for access to the lentiviral production facility. They are grateful to Dr William Vainchenker and Dr Jean-Luc Villeval from UMR 1009 (Institut Gustave Roussy, Villejuif, France) for sharing the JAK2 wt- and JAK2V617F-expressing BaF3-EpoR cells. They thank Dr Alfred Wittinghofer from Max-Plank-Institut (Dortmund, Germany) for providing the plasmids encoding the wild-type and mutant forms of Rap1, and also thank Dr Johannes L. Bos (Molecular Cancer Research, UMC, Utrecht, The Netherlands) for sending the plasmid encoding the GST-RalGDS-RBD fusion protein for Rap1-GTP pull-down assays. Finally, they thank Dr Patrick Mboungou for PV blood samples.
This work was funded by Inserm, the Institut National de la Transfusion Sanguine (INTS), and a grant from Région Île-de-France (SESAME 2007 no. F-08-1104/R). The PhD student, M.D.G., was funded by the Ministère de l'Enseignement Supérieur et de la Recherche at the Ecole Doctorale B3MI.
Authorship
Contribution: M.D.G designed and performed research, analyzed data, and wrote the paper; M.C. performed all transduction experiments and commented on the paper; B.C and C.C. provided patient blood samples and clinical information; M.-P.W., Y.C., J.-L.W., and C.L.V.K. discussed the results and commented on the paper; and W.E.N. designed and performed research, analyzed data, and wrote and edited the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Wassim El Nemer, Inserm, UMR_S665, INTS, 6 rue Alexandre Cabanel, 75015 Paris, France; e-mail: wassim.el-nemer@inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal