In this issue of Blood, Rafii et al present an elegant study of human embryonic stem cell (ESC)–derived hematopoiesis incorporating live imaging at the single-cell level to track hematopoietic lineage potential during the endothelial to hematopoietic transition.1
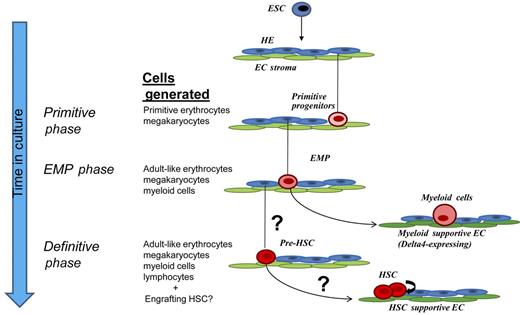
Derivation of therapeutically useful hematopoietic stem and progenitor cells (HSPCs) from embryonic and induced pluripotent stem cells by ex vivo culture will require recapitulation of the developmental hematopoietic ontogeny leading to the adult-like or “definitive” hematopoietic stem cell program. To achieve this, detailed studies in ESC and animal models are needed to elucidate the clonal evolution of HSPCs from their developmental precursors and the signals that regulate sequential developmental phases. Recent studies in animal models and murine ESCs have directly visualized the process by which hemogenic endothelium give rise to hematopoietic progenitors (reviewed in Swiers et al2 ). To advance such efforts in human ESCs, Rafii and colleagues present a platform in which ESCs engineered to express dual fluorescence markers under endothelial and hematopoietic-specific promoters are differentiated using a novel endothelial co-culture method to promote successive generation of hemogenic endothelium (HE) and their hematopoietic progeny. By live imaging of single cells, tracking the endothelial to hematopoietic transition, they demonstrate that hematopoietic ontogeny arise from HE with distinct stage-specific hematopoietic potential (see figure). Importantly, this parallels embryonic development, in which sequential generation of distinct hematopoietic programs occurs (reviewed in Medvinsky et al3 ); the first hematopoietic progenitors arise in the extra-embryonic yolk sac, and include a “primitive” wave characterized by progenitors giving rise to megakaryocytes and erythrocytes expressing embryonic globins; this is followed shortly thereafter by a distinct wave of erythroid/myeloid progenitors (EMPs) with expanded myeloid potential as well as potential for more “adult-like” erythrocytes expressing adult (β) globins.4 These early phases generally provide rapidly differentiating, transient hematopoietic cells to support the developing embryo before definitive HSC emergence. Thus, the hematopoietic progenitor cells described in the study by Rafii et al seemingly reflect primitive and EMP waves based on the distinct hematopoietic potential of early verses later hemogenic endothelium, transition of expression from embryonic to adult forms of globins, and lack of hematopoietic engraftment in transplantation assays.
Platform described in Rafii et al for differentiation of human embryonic stem cells (ESCs) to hemogenic endothelium (HE) and subsequent waves of hematopoietic progenitor cells using endothelial cell (EC) co-culture. Sequential waves of transient primitive progenitors and erythroid/myeloid progenitors (EMPs) unique to embryonic development arise from populations of hemogenic endothelium with distinct hematopoietic potential, as revealed by single cell–lineage tracking described in their study. Dll4 expression in some types of vascular stroma was shown to specifically promote myelopoiesis from the more multipotent EMPs identified in the study. The capacity to generate precursors to the definitive hematopoietic stem cell program (pre-HSC) remains to be determined, but use of appropriate stage-/organ-specific vascular stroma could play a role in differentially promoting this phase of hematopoiesis and subsequently supporting HSC expansion. Adapted from Figure 5I in the article by Rafii et al on page 770.
Platform described in Rafii et al for differentiation of human embryonic stem cells (ESCs) to hemogenic endothelium (HE) and subsequent waves of hematopoietic progenitor cells using endothelial cell (EC) co-culture. Sequential waves of transient primitive progenitors and erythroid/myeloid progenitors (EMPs) unique to embryonic development arise from populations of hemogenic endothelium with distinct hematopoietic potential, as revealed by single cell–lineage tracking described in their study. Dll4 expression in some types of vascular stroma was shown to specifically promote myelopoiesis from the more multipotent EMPs identified in the study. The capacity to generate precursors to the definitive hematopoietic stem cell program (pre-HSC) remains to be determined, but use of appropriate stage-/organ-specific vascular stroma could play a role in differentially promoting this phase of hematopoiesis and subsequently supporting HSC expansion. Adapted from Figure 5I in the article by Rafii et al on page 770.
This raises the question as to the presence of HE capable of generating definitive HSPCs during ESC differentiation in the current study and those of others. In the embryo, definitive hematopoietic precursors that are multipotent (including lymphoid potential) and give rise to transplantable long-term HSCs have been shown to arise later in development than the primitive and EMP waves, from hemogenic endothelium associated with arterial vessels in multiple anatomic locations including the intra-embryonic AGM (aorta-gonad-mesonephros) region, yolk sac, and placenta (reviewed in Medvinsky et al3 ). Although the ability to detect lymphoid potential from murine and human ESCs in some studies suggests the capacity to specify a definitive HSPC program, with the exception of approaches in murine ESCs relying on overexpression of hematopoietic transcription factors, the ability to derive efficiently engrafting, multilineage hematopoietic stem cells from pluripotent stem cells, particularly human, remains elusive (reviewed in Lengerke and Daley5 ). This may result from failure to fully recapitulate the developmental ontogeny leading to HE capable of the definitive hematopoietic stem cell program. Alternatively, precursors with HSC potential may be derived, but either lack appropriate niche signals that prevent their premature differentiation and facilitate self-renewal and/or promote the acquisition of properties such as the ability to home and allow for efficient long-term repopulation. In this regard, co-culture with stage- and organ-specific niche stroma is a promising method to facilitate expansion of ESC-derived progenitors, supported by a recent study showing that organ-matched mesenchymal cells provide appropriate signals to enhance the self-renewal of pancreatic β-cell precursors in relatively large numbers.6 Similarly, Rafii's group has previously shown that organ- and activation state–specific instructive vascular niches, via production of defined growth factors such as Notch-ligands, are essential for organ regeneration in vivo and bone marrow–derived HSC expansion in vitro.7 Thus, by defining critical signal pathways that differentially regulate sequential phases of hematopoietic development, the platform described by Rafii et al should allow for further detailed studies to elucidate microenvironmental contributions to hematopoietic ontogeny.
A key mechanism of microenvironmental regulation of development highlighted by Rafii et al is the endothelial niche–mediated activation of the Notch pathway. Differential activation of this pathway by Notch ligand/receptor interactions regulates lineage determination versus maintenance of progenitor/stem cell state in a context-dependent fashion. Whereas Notch signaling generally has been shown to inhibit myeloid differentiation in the context of adult hematopoiesis, Rafii and colleagues identify a novel function for Dll4/Notch-mediated enhancement of myelopoiesis from EMP-stage human ESC-derived progenitors by particular vascular stroma in which Dll4 is highly expressed. Previous in vivo studies have shown the Notch pathway essential in the definitive HSC-producing phase of embryonic hematopoiesis (reviewed in Bigas and Espinosa8 ). Notch1 seems to be the critical receptor for this phase, but this is complicated by the fact that Notch1 is required for arterial endothelial determination, presumably a prerequisite for HSC-generating HE that are localized to the arteries. In contrast, Jagged1 is required for the definitive HSCs but not for arterial fate. Thus, it remains to be determined whether other Notch receptors could also be involved in promoting transition from HE to HSC, and subsequently, in embryonic- and fetal liver–stage HSC expansion by limiting their differentiation as they self-renew. In the adult context, Notch2 activation has been shown to induce expansion of murine marrow stem and precursor cells in vivo during stress hematopoiesis, and expansion of murine marrow and human cord blood precursor cells ex vivo (reviewed in Dahlberg et al9 ). Thus, further understanding of the Notch receptor/ligand interactions that promote HSC generation and expansion, and identification of the unique organ-/stage-specific vascular niches that recapitulate this ligand-mediated activation of Notch in the context of the developing HSC, will be essential. Such strategies, extending the platform presented in the current study by Rafii et al to elucidate the key stromal niche signal pathways that differentially regulate the definitive HSC phase of hematopoiesis, could play a critical role in finally achieving the goal of generating and expanding HSCs from ESCs and iPSCs sufficient for therapeutic purposes.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal