Key Points
Five cases of EBV− PTLD in pediatric recipients of combined liver and small bowel allografts are reported.
The lesions were plasma cell neoplasms that resolved completely after minimal treatment.
Abstract
EBV− posttransplantation lymphoproliferative disorders (PTLDs) are rare compared with EBV+ PTLDs, occur later after transplantation, and have a poor response to treatment. Few studies have reported EBV− PTLD in pediatric solid-organ transplantation recipients. We describe 5 cases of EBV− PTLD in recipients of combined liver and small bowel allografts ranging in age from 16 months to 7 years. EBV− PTLD developed 9-22 months (median, 15) after transplantation. Morphologically, the lesions ranged from atypical plasma cell hyperplasia (a term not currently included in the World Health Organization classification) to plasmacytoma like. In all cases, in situ hybridization for EBV was negative, and molecular studies demonstrated clonal IgH gene rearrangements. Protein electrophoresis showed multiple clonal paraproteins in 4 of 5 cases. In 2 cases with a donor-recipient sex mismatch, FISH cytogenetics demonstrated that the plasma cells were of mixed donor/recipient origin. One patient died before therapy. Four patients were treated with high-dose dexamethasone, and 1 patient subsequently required thalidomide. All 4 remain in remission 75-128 months (median, 86) after diagnosis. In contrast to reports of EBV− PTLD in adults, these plasma cell lesions occurred early after transplantation and resolved completely after minimal treatment.
Introduction
Posttransplantation lymphoproliferative disorders (PTLDs) are lymphoid or plasmacytic proliferations that develop in recipients of solid organ (SOT) and hematopoietic stem cell transplantations. According to the 2008 World Health Organization (WHO) classification,1 PTLDs are divided into early, polymorphic, monomorphic, and classic Hodgkin lymphoma-type lesions. The incidence of PTLDs among SOT recipients is correlated with the type of allograft and the intensity of the associated immunosuppressive regimen. Patients receiving intestinal allografts have a much higher incidence of PTLD compared with recipients of kidney, heart, or liver allografts.1 Large studies of recipients of small bowel allografts report an incidence of PTLDs ranging from 12% to more than 20%.2,3 Children have a higher incidence of PTLDs compared with adult recipients.4,5 Most PTLDs in the pediatric age group are EBV+ after the development of primary EBV infection in the posttransplantation period.1 EBV seronegativity at the time of transplantation is one of the main risk factors for PTLD development, and a large number of children undergoing transplantation are EBV naive.6
Although most PTLDs are EBV+, reports of EBV− cases are increasing.1 EBV− PTLD is more common in adults than children, tends to occur late after transplantation, and is more commonly monomorphic.1,7-11 The proportion of EBV− PTLDs varies widely in different series, ranging from 0%-50%, with an overall estimate of approximately 30%.1,7-12 This variability may be due in part to differences in the methodology used to identify EBV in the tissue. Human herpesvirus 6 (HHV-6) and HHV-8 have also been reported in association with cases of PTLD, suggesting that other viruses may account for some EBV− cases.1,13,14
Among the various types of PTLD, plasma cell neoplasms are rare after SOT, and fewer than 100 cases have been reported in the literature.12,15-25 Most patients have been adults, with only a few well-described pediatric cases reported.17,23 Plasma cell PTLDs are very heterogeneous neoplasms with a histologic spectrum ranging from plasma cell hyperplasia to monomorphic plasmacytoma-like lesions and plasma cell myelomas, and are variably positive for EBV.18,21,23-25 In the present study, we report a unique group of EBV− plasma cell PTLDs that developed in pediatric patients early after combined liver and small bowel (L/SB) transplantation and describe their clinical, histologic, molecular, and cytogenetic features.
Methods
Case selection
From 1991 to 2008, more than 700 SOTs were performed at the University of Nebraska Medical Center (UNMC) in pediatric patients (< 19 years of age), including 253 isolated or combined small bowel transplantations. In the same interval, 66 cases of PTLD in pediatric SOT recipients were identified retrospectively in the files of the Nebraska Lymphoma Study Group. Of these cases, 60 were EBV+ and 5 were EBV−. In a sixth case, a latent membrane protein (LMP) stain was reported as negative, but in situ hybridization for EBV-encoded RNAs (EBERs) could not be performed because of the lack of adequate material and the case was excluded from further study. The 60 EBV+ PTLD cases had various morphologies classified as early, polymorphous, or monomorphic PTLD. Thirty-two of 60 EBV+ cases were in recipients of isolated small bowel or L/SB transplantations. All 5 EBV− PTLD cases occurred in recipients of L/SB transplantations. This study was approved by the institutional review board of the UNMC.
Morphologic and immunophenotypic studies
H&E-stained sections from routinely fixed, paraffin-embedded lymph nodes, tonsils, adenoids, small bowel, lungs, and bone marrow (BM) specimens were examined. Wright-Giemsa–stained BM aspirate smears and touch imprints were also available for morphologic examination. Immunohistochemical stains were performed using a Ventana ES automated immunostainer (Ventana Biotek) with a streptavidin-biotin peroxidase detection system and antibodies against CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD20, CD21, CD45RO, CD45 (LCA), CD56, CD68, CD79a, TIA-1, Ki-67, IgA, IgM, IgG, kappa, lambda, LMP-1, CMV, and HHV-8. Antigen retrieval was performed as described previously or as appropriate for the antibodies.26 In situ hybridization for κ and λ immunoglobulin light chain mRNA and EBERs was performed on a Ventana ES automated immunostainer using a streptavidin-biotin peroxidase detection system with Neat-Probe standard kits (Ventana Biotek).
For flow cytometry analysis, BM aspirate was collected in sodium heparin and processed according to a standard protocol as described previously.26 Cells were labeled using monoclonal antibodies to CD2, CD3, CD4, CD5, CD8, CD10, CD11b, CD11c, CD14, CD16, CD19, CD20, CD23, CD24, CD25, CD38, CD45, CD56, CD103, CD138, HLA-DR, and κ and λ light chains as appropriate. Antibodies were used according to the manufacturer's recommendations. Cytoplasmic staining was performed using FIX AND PERM Cell Permeabilization reagents (Caltag). Multicolor flow cytometry analysis was performed using an Epics XL or FC500 flow cytometer (Beckman Coulter).
Cytogenetic studies
Standard G-banded analysis was performed on 24- to 48-hour–unstimulated suspension cultures as described previously.27,28 Karyotypes were described according to the International System for Human Cytogenetic Nomenclature (ISCN).29 Interphase FISH analysis was performed on formalin-fixed, paraffin-embedded tissue sections according to a previously described procedure.30 The following probes were used in the multiple myeloma FISH panel: LSI D13S319 (13q14) and LSI 13q34 DNA probes with CEP 9; IgH (14q32) dual-color break-apart probe; LSI p53 (17p13.1)/CEP 17 probes with CEP 11; Cytocell SNRPN (15q11.2)/15qtel DNA probe set with LSI MYB (6q23); and LSI c-MYC (8q24) dual-color break-apart probe (Vysis). The specimens were analyzed with an Olympus BX-51 or Leica DM 6000B microscopes. When possible, the signals were scored in 200 well-delineated cells. The abnormal ranges for the probes used in this study were established by the Human Genetics Laboratory at the UNMC. For reference, the abnormal range for del 13q14 and del 13q34 is 25%-100%. FISH studies using the CEP X/Y DNA probe specific for the X (DXZ1) and the Y (CYZ1) centromeres were also performed on paraffin-embedded tissue sections from lymph nodes in cases 2 and 3, both of whom had received sex-mismatched allografts. Areas with prominent plasma cell infiltration were scored.
Assessment of clonality by PCR analysis
Case 1 was analyzed for the presence of clonal IgH gene rearrangements by a PCR targeting the complementarity-determining region III (CDRIII). A semi-nested procedure was used with a consensus primer complementary to the conserved sequences in framework region III of the IgH variable gene segments and external (5′-ACCTGAGGAGACGGTGACC-3′) and nested internal (5′-ACCAGGGTCCCTTGGCCCCA-3′) primers complementary to framework region IV segments of the IgH joining gene segments, as described previously.31 Products were visible in the 70- to 140-bp range after electrophoresis on 8% polyacrylamide gels. The 4 remaining cases were analyzed using the InVivoScribe IgH framework III/heavy-chain joining assay according to the manufacturer's protocol (InVivoScribe Technologies). Framework II/heavy-chain joining segments were amplified using forward (5′ TGG RTC CGM CAG SCY YCN GG 3′) and reverse (5′ 6-FAM ACC TGA GGA GAC GGT GAC C 3′) primers as described previously.32 The PCR products were analyzed using capillary electrophoresis as described previously.33
Results
Clinical features
Clinical features of the patients are summarized in Table 1. The patients included 2 males and 3 females between the ages of 16 months and 7 years (median, 3 years). The underlying diseases that led to transplantation in these 5 children included small bowel lymphangiectasia, gastroschisis, intrauterine bowel volvulus, intestinal atresia, and microvillous inclusion disease. All patients received a combined L/SB transplantation and were on an identical immunosuppressive regimen consisting of tacrolimus and prednisone. None of the patients developed graft failure. The interval from transplantation to the development of PTLD ranged from 9-22 months (median, 15). Sites of involvement by PTLD included lymph nodes, tonsils and adenoids, BM, allograft small bowel (case 4), and lungs. Four children had more than 1 site involved. All patients had normal skeletal surveys.
Clinical features of pediatric liver/small bowel transplantation patients with EBV− PTLD
| Case . | Sex/age . | Underlying disease . | Immunosuppressive regimen . | Interval to PTLD, mo* . | Sites of involvement by PTLD . | Treatment . | Follow-up, mo† . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| 1 | F/7 y | Small bowel lymphangiectasia | TAC + prednisone | 9 | TA, LN, BM | Dexamethasone, thalidomide | 128 | ANED |
| 2 | M/16 mo | Gastroschisis | TAC + prednisone | 9 | TA, LN, L, BM | NT | 0.5 | DOC |
| 3 | F/3 y | Intrauterine bowel volvulus | TAC + prednisone | 15 | LN | Dexamethasone | 91 | ANED |
| 4 | F/5 y | Intestinal atresia | TAC + prednisone | 15 | LN, ASB, BM | Dexamethasone | 82 | ANED |
| 5 | M/ 3y | Microvillous inclusion disease | TAC + prednisone | 22 | TA, LN | Dexamethasone, prednisone | 75 | ANED |
| Case . | Sex/age . | Underlying disease . | Immunosuppressive regimen . | Interval to PTLD, mo* . | Sites of involvement by PTLD . | Treatment . | Follow-up, mo† . | Outcome . |
|---|---|---|---|---|---|---|---|---|
| 1 | F/7 y | Small bowel lymphangiectasia | TAC + prednisone | 9 | TA, LN, BM | Dexamethasone, thalidomide | 128 | ANED |
| 2 | M/16 mo | Gastroschisis | TAC + prednisone | 9 | TA, LN, L, BM | NT | 0.5 | DOC |
| 3 | F/3 y | Intrauterine bowel volvulus | TAC + prednisone | 15 | LN | Dexamethasone | 91 | ANED |
| 4 | F/5 y | Intestinal atresia | TAC + prednisone | 15 | LN, ASB, BM | Dexamethasone | 82 | ANED |
| 5 | M/ 3y | Microvillous inclusion disease | TAC + prednisone | 22 | TA, LN | Dexamethasone, prednisone | 75 | ANED |
ANED indicates alive, no evidence of disease; ASB, allograft small bowel; DOC, died of other cause; F, female; L, lungs; LN, lymph nodes; M, male; NT, no therapy; TA, tonsils and adenoids; and TAC, tacrolimus.
Interval between organ transplantation and development of PTLD.
Interval between PTLD diagnosis and last follow-up.
Laboratory findings
Laboratory findings are summarized in Table 2. Quantitative immunoglobulins, serum protein electrophoresis (SPEP), and immunofixation electrophoresis (IFE) were performed at diagnosis of PTLD and subsequently for disease monitoring. In cases 1 to 4, multiple monoclonal proteins were identified. Case 5 had normal immunoglobulins and no identifiable monoclonal protein. The patient in case 1 was hypoproteinemic secondary to lymphangiectasia. At diagnosis, the patient had IgG-λ and IgM-λ in the serum and clonal free κ and λ light chains in the urine. Subsequently, 3 IgG-κ monoclonal proteins, an IgG-lambda, and an IgM-κ were identified at various times over the next 2 years. In case 2, the patient had a β fraction of 2.93 g/dL and a γ-globulin fraction of 1.55 gm/dL on SPEP. The SPEP revealed a major monoclonal paraprotein migrating in the β region and 2 additional paraproteins migrating in the γ region; immunofixation studies were not performed to characterize these further. Case 3 had a serum IgA of 1340 mg/dL and IgG of 1240 mg/dL, with large IgA-λ and small IgG-κ monoclonal proteins on IFE. Case 4 had 2 IgA-λ monoclonal proteins quantified at 4820 mg/dL and 650 mg/dL, respectively, on electrophoresis at diagnosis and a decreased total IgG of 270 mg/dL.
Pathologic, laboratory, and cytogenetic features of EBV− PTLD
| Case . | PTLD morphology . | Kappa/lambda ISH . | SPE/UPE/IFE . | Genetic findings . |
|---|---|---|---|---|
| 1 | TA: atypical PC hyperplasia | NA | 3 IgG-κ, IgG-λ, IgM-κ, IgM-λ, κ and λ light chains | 47,XX/+3(7)/46,XX(9) |
| LN: plasmacytoma-like | NA | |||
| BM: atypical PC hyperplasia | NA | |||
| 2 | TA, LN, L: atypical PC hyperplasia | −/+ | MP* | 47,XXY or 47,XY, +X(4)/46,XY(4) |
| BM: plasma cells, lymphocytes, and histiocytes | ||||
| 3 | LN: atypical PC hyperplasia | +/+ | IgA-λ, IgG-κ | 46,XX |
| 4 | ASB: atypical PC hyperplasia | −/+ | 2 IgA-λ | 46,XX |
| LN: plasmacytoma-like | −/+ | del 13q14, del 13q34† | ||
| BM: plasma cell myeloma | NA | |||
| 5 | TA: plasmacytoma-like | +/− | NMPD | NA |
| Case . | PTLD morphology . | Kappa/lambda ISH . | SPE/UPE/IFE . | Genetic findings . |
|---|---|---|---|---|
| 1 | TA: atypical PC hyperplasia | NA | 3 IgG-κ, IgG-λ, IgM-κ, IgM-λ, κ and λ light chains | 47,XX/+3(7)/46,XX(9) |
| LN: plasmacytoma-like | NA | |||
| BM: atypical PC hyperplasia | NA | |||
| 2 | TA, LN, L: atypical PC hyperplasia | −/+ | MP* | 47,XXY or 47,XY, +X(4)/46,XY(4) |
| BM: plasma cells, lymphocytes, and histiocytes | ||||
| 3 | LN: atypical PC hyperplasia | +/+ | IgA-λ, IgG-κ | 46,XX |
| 4 | ASB: atypical PC hyperplasia | −/+ | 2 IgA-λ | 46,XX |
| LN: plasmacytoma-like | −/+ | del 13q14, del 13q34† | ||
| BM: plasma cell myeloma | NA | |||
| 5 | TA: plasmacytoma-like | +/− | NMPD | NA |
ASB indicates allograft small bowel; ISH, in situ hybridization; L, lungs; LN, lymph nodes; MP, monoclonal protein; NA, not available; NMPD, no monoclonal proteins detected; PC, plasma cell; TA, tonsils and adenoids; and UPE, urine protein electrophoresis.
One major monoclonal protein in beta and 2 additional monoclonal proteins in gamma region, not further characterized.
Detected by FISH.
Molecular studies (PCR) for EBV and HHV-6 were performed on blood samples of all the patients, and PCR studies for HHV-8 and CMV were performed on 4 patients and all were negative. Donor EBV serology revealed that 2 of 5 donors (cases 3 and 4) showed evidence of past EBV infection. The donor serologic pattern in case 5 was interpreted as possibly consistent with persistent maternal antibodies.
Clinical management and follow-up
One patient (case 2) died of pneumonitis 15 days after diagnosis and before treatment for PTLD could be initiated. For the remaining 4 children, immunosuppression was initially decreased after the diagnosis of PTLD. After no significant response, the 4 patients were treated with intravenous or oral dexamethasone (10 mg/m2 twice daily) on days 1-4, 9-12, 17-20, and 25-28. Patients were then evaluated for disease response by imaging studies and SPEP/IFE. On resolution of clinical disease, patients received dexamethasone (10 mg/m2 twice daily) orally for 4 days every 28 days until monoclonal proteins were not detectable in the serum (approximately 6 months in all cases). This treatment plan was derived from established treatment protocols for adult patients with multiple myeloma.34 Three patients (cases 3, 4, and 5) had a complete response to steroid treatment alone with no detectable monoclonal proteins by SPEP/IFE. In case 1, SPEP/IFE detected circulating monoclonal proteins 6 months after completion of therapy without evidence of recurrent disease. This patient was treated with daily oral thalidomide (200 mg/d) with 4 days of dexamethasone every 28 days for 14 months until the SPEP/IFE was again negative.35
The 4 treated children all remained disease free 75-128 months (median, 86) from PTLD diagnosis without significant comorbidities and with a Lansky performance status of 8-10 on minimal immunosuppression.
Histologic findings, immunohistochemistry, and in situ hybridization
The histologic features are summarized in Table 2. Histologically, all lesions were plasma cell neoplasms, with the morphologic spectrum ranging from atypical plasma cell hyperplasia to plasmacytoma- and myeloma-like lesions. Two patients (cases 2 and 3) were diagnosed with atypical plasma cell hyperplasia and 1 patient (case 5) with plasmacytoma-like PTLD. Two patients (cases 1 and 4) had histologically different lesions at different biopsy sites. BM biopsies were performed in 4 patients, and 3 (cases 1, 2, and 4) had BM involvement by PTLD.
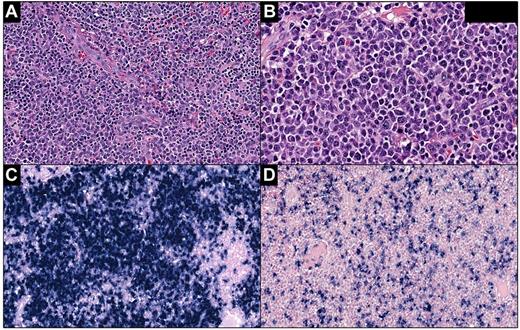
Lesions characterized as “atypical plasma cell hyperplasia” were composed of sheets of plasma cells that ranged cytologically from mature to atypical with enlarged forms containing irregular nuclei and prominent nucleoli. In the tonsils, adenoids, and lymph nodes, the plasma cells expanded the interfollicular areas with focal architectural effacement (Figure 1A-B). In the lungs, plasma cell infiltration was interstitial, peribronchial, and perivascular in distribution. In the small bowel, the infiltrate was in the lamina propria and submucosa without significant distortion of the bowel architecture. Variable numbers of small lymphocytes, plasmacytoid lymphocytes, immunoblasts, and histiocytes were admixed with plasma cells. In situ hybridization for κ and λ light chains showed light-chain restriction in cases 2 and 4. Case 3 showed a polyclonal pattern of κ and λ staining that was consistent with the IgA-λ and IgG-κ proteins detected in the serum (Figure 1C-D). To emphasize the range of cytologic atypia present in the plasma cells of these lesions, we described these lesions as atypical plasma cell hyperplasia, a descriptive term that is not currently included in the WHO classification.
Atypical plasma cell hyperplasia in the lymph nodes (case 3). (A) The lesion is characterized by sheets of plasma cells expanding the interfollicular areas admixed with small lymphocytes, plasmacytoid lymphocytes, immunoblasts, and histiocytes. (B) Plasma cells range from morphologically mature to atypical, enlarged forms with nucleoli. In situ hybridization for κ (C) and λ (D) shows similar numbers of positive cells. Panel A, H&E staining; original magnification, 200×. Panel B, H&E staining; original magnification, 400×. Panels C and D, in situ hybridization; original magnification, 200×. Slides were scanned using Ventana iScan Coreo scanner (Ventana Medical Systems Inc), and Image Viewer Version 3.1.1. program was used to capture the images. Final image preparation was performed with Adobe Photoshop CS5 Extended Version 12.1x64.
Atypical plasma cell hyperplasia in the lymph nodes (case 3). (A) The lesion is characterized by sheets of plasma cells expanding the interfollicular areas admixed with small lymphocytes, plasmacytoid lymphocytes, immunoblasts, and histiocytes. (B) Plasma cells range from morphologically mature to atypical, enlarged forms with nucleoli. In situ hybridization for κ (C) and λ (D) shows similar numbers of positive cells. Panel A, H&E staining; original magnification, 200×. Panel B, H&E staining; original magnification, 400×. Panels C and D, in situ hybridization; original magnification, 200×. Slides were scanned using Ventana iScan Coreo scanner (Ventana Medical Systems Inc), and Image Viewer Version 3.1.1. program was used to capture the images. Final image preparation was performed with Adobe Photoshop CS5 Extended Version 12.1x64.
The plasmacytoma-like lesions partially or completely effaced lymph node and tonsillar architecture with a diffuse infiltrate of atypical plasma cells with large nuclei and prominent nucleoli (Figure 2A-B). Compared with plasma cell hyperplasia, this infiltrate was more confluent and destructive, as well as more atypical, lacking mature plasma cells. Admixed with atypical plasma cells were rare lymphocytes. In situ hybridization for κ and λ showed light-chain restriction in both tested specimens (cases 4 and 5; Figure 2C-D).
Plasmacytoma-like lesion in the tonsil (case 5). (A) Architecture of the tonsil is completely effaced by sheets of atypical plasma cells. (B) The majority of plasma cells are large with prominent nucleoli. In situ hybridization for κ (C) and λ (D) shows κ light-chain restriction. Panel A, H&E staining; original magnification, 200×. Panel B, H&E staining; original magnification, 400×. Panels C and D, in situ hybridization; original magnification, 200×. Slides were scanned using Ventana iScan Coreo scanner (Ventana Medical Systems Inc), and Image Viewer Version 3.1.1. program was used to capture the images. Final image preparation was performed with Adobe Photoshop CS5 Extended Version 12.1x64.
Plasmacytoma-like lesion in the tonsil (case 5). (A) Architecture of the tonsil is completely effaced by sheets of atypical plasma cells. (B) The majority of plasma cells are large with prominent nucleoli. In situ hybridization for κ (C) and λ (D) shows κ light-chain restriction. Panel A, H&E staining; original magnification, 200×. Panel B, H&E staining; original magnification, 400×. Panels C and D, in situ hybridization; original magnification, 200×. Slides were scanned using Ventana iScan Coreo scanner (Ventana Medical Systems Inc), and Image Viewer Version 3.1.1. program was used to capture the images. Final image preparation was performed with Adobe Photoshop CS5 Extended Version 12.1x64.
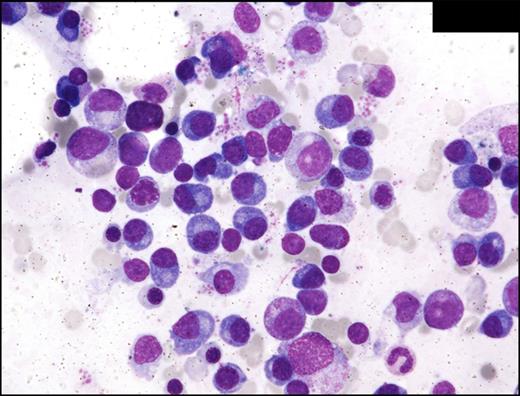
BM involvement by PTLD was diagnosed morphologically and by flow cytometry in 2 cases. In the most extensively involved case (case 4), atypical plasma cells comprised 38% of all nucleated cells in the BM and sheets of atypical plasma cells were present on the aspirate smears, core biopsy, and clot sections, giving the appearance of plasma cell myeloma (Figure 3). In case 1, the burden of BM involvement was smaller, with 5.6% plasma cells noted by differential count. In both of these cases, the plasma cells exhibited a morphologic spectrum ranging from well differentiated to enlarged dysplastic forms and flow cytometry confirmed clonality. In case 2, the BM involvement was diagnosed at autopsy and aspirate smears were not obtained. The BM biopsy in case 2 showed infiltration of mature plasma cells (less than 10%) admixed with lymphocytes and histiocytes. In cases 1 and 2, there was no evidence of end organ damage (ie, hypercalcemia, renal insufficiency, anemia, or bone lesions) that could be attributed to the plasma cell proliferation and the WHO criteria for plasma cell myeloma were not met. EBERs in situ hybridization and immunohistochemical studies for CMV and HHV-8 were performed in all 5 cases and were negative.
Multiple myeloma (case 4). BM aspirate smear shows numerous atypical, enlarged plasma cells. Wright-Giemsa staining, oil; original magnification, 600×. Photomicrographic images were acquired with a Nikon Eclipse E600 microscope equipped with Nikon DS-Fi1 camera and software. Final image preparation was performed with Adobe Photoshop CS5 Extended Version 12.1x64.
Multiple myeloma (case 4). BM aspirate smear shows numerous atypical, enlarged plasma cells. Wright-Giemsa staining, oil; original magnification, 600×. Photomicrographic images were acquired with a Nikon Eclipse E600 microscope equipped with Nikon DS-Fi1 camera and software. Final image preparation was performed with Adobe Photoshop CS5 Extended Version 12.1x64.
Cytogenetic and molecular studies
Conventional cytogenetic studies were performed in 4 cases and demonstrated clonal abnormalities including trisomy 3 (7 of 16 metaphases) in case 1 and an additional chromosome X (4 of 8 metaphases) in case 2. Two patients (cases 3 and 4) had normal karyotype (Table 2). Molecular cytogenetic studies (FISH) were performed in 4 cases; case 4 showed del 13q14 and del 13q34 in 35% of interphase nuclei. All cases were negative for rearrangements of IgH, P53, MYB, and c-MYC and negative for loss or gain of chromosomes 9, 11, and 15.
Two patients (cases 2 and 3) received sex-mismatched allografts. In both cases, FISH cytogenetic studies using probes for the X and Y chromosome centromeres were performed on paraffin-embedded sections of involved axillary (case 2) or cervical (case 3) lymph nodes and scored in areas where the plasma cells were most prominent. In case 2, FISH studies detected female cells of donor origin in 38% and male cells of recipient origin in 62% of the cells analyzed. In case 3, FISH studies detected male cells of donor origin in 13% and female cells of host origin in 87% of cells analyzed. Molecular studies by PCR demonstrated clonal IgH gene rearrangements in all 5 cases.
Discussion
The incidence of EBV− PTLDs appears to be increasing but is much less prevalent than EBV+ PTLDs, especially among pediatric SOT recipients.1,5,6 Polyclonal plasma cell hyperplasia is a relatively common presentation of early PTLD, whereas frank plasmacytomas and multiple myelomas are rarely described in pediatric SOT recipients.1 We describe a unique group of pediatric patients with EBV− plasma cell PTLDs with morphology ranging from atypical plasma cell hyperplasia to plasmacytoma-like lesions. Clinically, the 5 patients reported herein represent a uniform group. All were young (16 months to 7 years; median, 3 years) and all developed PTLDs early (9-22 months; median, 15 months) after transplantation. They all received combined L/SB transplantations and tacrolimus and prednisone for posttransplantation immunosuppression. This clinical presentation is in contrast to the majority of previously reported EBV− PTLD cases, which typically occurred in adults late after transplantation (median of more than 50 months).7,8,10,24,25 A review of the literature identified only 1 other reported pediatric case of EBV− PTLD that was similar to atypical plasma cell hyperplasia in our patients.36 Quintini et al reported on 119 children who received isolated or combined small bowel transplantations between 1994 and 2005.2 They reported 14 (11.8%) cases of PTLD; 13 were EBV+ and 1 was EBV− polymorphic T-cell PTLD. We report herein 37 (14.6%) cases in 253 isolated or combined small bowel transplantations between 1991 and 2008, with 32 EBV+ and 5 EBV− cases. Quintini et al reported that 6 of 14 cases occurred < 4 months after transplantation, whereas 8 cases occurred > 20 months after transplantation.2 All of our EBV− plasma cell neoplasms occurred between 9 and 22 months after transplantation.
The causative role of EBV in the development of PTLD is well known; however, in some PTLD cases, EBV is not detected.1,7-12 EBER in situ hybridization is one of the most reliable markers of EBV detection because it detects a high-copy polyadenylated RNA transcribed in all types of EBV latency.12,37 EBER in situ hybridization studies established EBV-negativity in all of our cases and all patients also tested negative for EBV by blood PCR. EBV− PTLD has also been described in association with other viruses such as HHV-81,13,14 and, rarely, HHV-6.38 Immunohistochemical staining for HHV-8 and CMV and blood PCR studies for HHV-8, HHV-6, and CMV were also negative in our cases.
EBV− PTLDs are reported as aggressive neoplasms that require multiagent chemotherapy and have a poor prognosis.7-9,11 However, EBV− low-grade MALT-type lymphomas have also been reported to occur in the posttransplantation setting as an indolent disease.39 Two recent reports suggest that a decrease in immunosuppression with the addition of rituximab significantly improves the outcome of adult EBV− PTLD patients.12,40 Reshef et al reported similar cure rates in EBV− and EBV+ PTLDs.40 Our EBV− PTLD patients did not require aggressive chemotherapy regimens and remained in remission 75-128 months (median, 86) after the PTLD diagnosis. None of our cases received rituximab because none expressed CD20.
Histologically, all of the lesions were plasma cell neoplasms, with the morphologic spectrum ranging from atypical plasma cell hyperplasia to plasmacytoma- and myeloma-like lesions. The lesions diagnosed as atypical plasma cell hyperplasia had morphologic features overlapping with early plasmacytic PTLD as defined by the WHO classification.1 However, in addition to mature plasma cells, there was a substantial number of atypical plasma cells with abnormal nuclear and cytoplasmic features. We have used the descriptive term atypical plasma cell hyperplasia to emphasize the range of cytologic atypia exhibited by the plasma cells in these lesions. Recognition of the range of cytologic atypia that may be present in lesions that are not frankly plasmacytomas or plasma cell myeloma may improve their recognition, particularly when the lesions present early after transplantation and are EBV−. Additional studies are needed to determine whether atypical plasma cell hyperplasia constitutes a separate category of PTLDs that should be included in future WHO classification schemes.
The diagnosis of plasmacytoma-like lesions was made in 3 cases. Despite the aggressive clinical and histologic features, these 3 patients had favorable outcomes with minimal therapy. Richendollar et al24 and Trappe et al25 reported cases of EBV+ and EBV− plasmacytoma-like PTLDs in adult SOT recipients (kidney, lung, liver, heart, and small intestine). All except 2 cases occurred late after transplantation (median 8.3 and 7 years, respectively) and required treatment with different modalities, including decreased immunosuppression, surgery, irradiation, and chemotherapy, with variable responses.
To our knowledge, this is the first report of cytogenetic findings in EBV− plasma cell PTLD. Even though genetic abnormalities are extremely common in plasma cell neoplasms and are detected in more than 90% of multiple myeloma patients by FISH,41 our patients showed remarkably few cytogenetic abnormalities. This finding may in part explain the excellent response to minimal therapy. Deletion of chromosome 13 portends an unfavorable risk in patients with multiple myeloma.41 One patient (case 4) in our group who had del 13q14 and del 13q34 showed the most extensive BM involvement by atypical plasma cells. However, she required only a short course of dexamethasone to achieve and maintain complete remission.
In the 2 cases with donor/recipient sex mismatch, we demonstrated by FISH cytogenetics that the plasma cell proliferations were a mixture of donor and recipient plasma cells. If the donor plasma cells were part of the malignant proliferation, recovery of host immunity could potentially promote a host versus tumor response. It will be important in future studies of both EBV+ and EBV− PTLD to determine whether the neoplasms are of recipient, donor, or mixed origin. Further, as in 1 of our 5 cases, it will be important to determine whether the donor allograft is involved by PTLD. Insights into these issues will help in understanding the pathogenesis and may influence therapeutic approaches to PTLDs occurring after L/SB transplantation.
In conclusion, in the present study, we report a distinct group of pediatric L/SB recipients who developed early onset, EBV− plasma cell PTLD. The clinical and histologic similarities among these 5 patients suggest that they represent a distinct group of pediatric PTLDs that do not require myelosuppressive chemotherapy and achieve long-lasting complete remission with minimal interventions.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: A.M.P. performed the immunohistochemical stains, analyzed the data, and wrote the manuscript; P.A. designed the research, performed the immunohistochemical stains, analyzed the data, and wrote the manuscript; D.W.C. collected and analyzed the data; W.G.S. performed the conventional cytogenetic and FISH studies; W.J.G. collected the data; P.F.C. designed the research, collected and analyzed the data, and wrote the manuscript; and all authors read and reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for A.M.P. is the Department of Pathology, University of Manitoba, Winnipeg, MB. The current affiliation for P.A. is the Department of Pathology, City of Hope National Medical Center, Duarte, CA.
Correspondence: Patricia Aoun, City of Hope National Medical Center, 1500 E Duarte Rd, Duarte, CA 91010; e-mail: paaoun@coh.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal