The mechanisms that regulate 3-dimensional (3D) neutrophil chemotaxis are poorly understood. In this issue of Blood, Afonso et al demonstrate that the collagen receptor Discoidin domain receptor 2 (DDR2) promotes neutrophil chemotaxis in 3D by triggering matrix metalloproteinase (MMP) activity and the generation of chemotactic collagen peptides.1
Neutrophil-directed migration through interstitial tissues is critical for host defense. However, there is a limited understanding of how the extracellular matrix (ECM) regulates neutrophil motility within interstitial 3D tissues. Integrins are well established as essential regulators of cell adhesion during migration, in particular for mesenchymal cells. In contrast, substantial recent evidence suggests that although integrins are critical for 2D leukocyte adhesion and motility, they can be dispensable for interstitial leukocyte motility. Indeed, neutrophils lacking all integrins can still migrate directionally in 3D matrices.2 However, it is important to note that it is not clear how integrins modulate the efficiency of neutrophil chemotaxis within interstitial tissues.
Afonso et al now show that the non-integrin collagen receptor DDR2 mediates persistence of 3D interstitial neutrophil motility independently of its adhesive function (see figure).1 The ECM commonly functions as a physical barrier to cell motility, which includes the basement membrane where MMPs are needed to degrade the matrix to facilitate cell movement. However, in general, neutrophil motility in interstitial tissues is thought to be independent of matrix degradation due to the ability of neutrophils to squeeze through small spaces. Accordingly, neutrophils are generally thought to lack matrix-degrading adhesive structures known as podosomes that are seen in other leukocytes like dendritic cells or macrophages or invadopodia of cancer cells. Intriguingly, ECM can also be a source of chemotactic peptides through the action of MMPs, as is the case for collagen exposed to the MMPs MMP-8 or MMP-9.
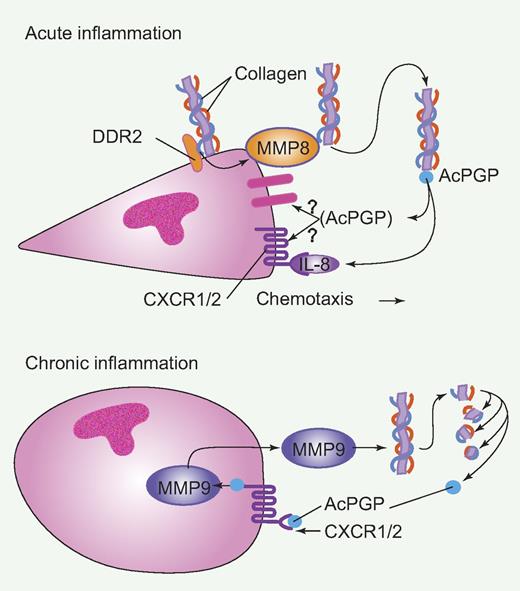
Schematic of Discoidin domain receptor 2 (DDR2) and matrix metalloproteinase (MMP) function during acute and chronic inflammation. During acute inflammation, DDR2 on the surface of neutrophils binds to collagen resulting in the secretion of MMP-8, that processes collagen into N-acetyl Pro-Gly-Pro (Ac-PGP). Ac-PGP binds surface receptors and stabilizes chemoattracant (IL-8)–mediated chemotaxis. During chronic inflammation, neutrophils produce MMP-9 that generates Ac-PGP from collagen and Ac-PGP binds to surface receptors, including CXCR1/2 receptors, leading to more MMP-9 and chronic inflammation. Professional illustration by Paulette Dennis.
Schematic of Discoidin domain receptor 2 (DDR2) and matrix metalloproteinase (MMP) function during acute and chronic inflammation. During acute inflammation, DDR2 on the surface of neutrophils binds to collagen resulting in the secretion of MMP-8, that processes collagen into N-acetyl Pro-Gly-Pro (Ac-PGP). Ac-PGP binds surface receptors and stabilizes chemoattracant (IL-8)–mediated chemotaxis. During chronic inflammation, neutrophils produce MMP-9 that generates Ac-PGP from collagen and Ac-PGP binds to surface receptors, including CXCR1/2 receptors, leading to more MMP-9 and chronic inflammation. Professional illustration by Paulette Dennis.
It is well known that neutrophils are an abundant source of MMP-9 and that MMP-9 levels are increased in inflamed tissues. In fact, MMP-9 has been reported to generate the collagen fragments N-acetyl Pro-Gly-Pro (Ac-PGP) that can mediate neutrophil chemotaxis.3 Moreover, it has been shown that these collagen fragments can perpetuate a cycle of chronic inflammation through the chemokine receptors CXCR1 and CXCR2 leading to the production of more MMPs and neutrophil recruitment.4 Afonso et al now show that DDR2 binding to collagen in circulating neutrophils mediates the production of MMP-8, which in turn can also lead to the generation of collagen fragments that mediate efficient chemokine-induced motility. It is not clear, however, if these fragments mediate the stability of neutrophil chemotaxis through CXCR1/CXCR2 or alternatively through the binding to a PGP receptor, which remains to be determined.5
There is substantial evidence to support the idea that these MMP-mediated pathways are likely critical in disease processes. For example, MMP-9 is important for host defense to bacterial infection at least in part due to of impaired recruitment of neutrophils to sites of infection in MMP-9–deficient mice, despite high chemokine levels.6 The study by Afonso et al now raises the intriguing question of how DDR2 and collagen peptide fragments may be involved in this regulation.1 Moreover, MMP-8 promotes neutrophil migration through collagen barriers in mouse models of obliterative bronchiolitis.7 However, it is not clear if MMP-8 mediates neutrophil motility through its effect on matrix degradation and/or through the generation of chemotactic peptides that may optimize neutrophil recruitment. Future studies using DDR2-deficient mice or other genetic tools will be necessary to address how DDR2 and the generation of collagen chemotactic peptides modulate neutrophil inflammation in vivo.
It is unclear if there is a polarity in the generation of the collagen peptides through this DDR2–MMP-8 pathway, or if polarized production of the peptides are in fact necessary to stabilize neutrophil directed motility. These issues relate to the larger question of how attractant-mediated signaling integrates with adhesion receptor-ligand signaling to regulate persistent polarized motility. Is DDR2 activation and secretion of MMP-8 localized to the leading edge? The PGP-containing peptides are further processed into PGP by prolyl endopeptidase (PE), raising the question of whether this processing also needs to occur locally at the leading edge to optimize directionality. The finding that this signaling functions at the autocrine rather than the paracrine level supports the idea that localized generation of the product may be important for directionality. It is still possible that the receptor is polarized, although the identity of the receptor through which the collagen peptides bind to promote directionality is not clear, and may be mediated by the chemokine receptors CXCR1 or CXCR2.
What emerges from these recent studies is that under some contexts MMP-mediated generation of collagen peptides may function through autocrine signaling to mediate localized signaling and optimize directed migration. In this case, DDR2/MMP-8/PGP signaling is a likely pathway that mediates this directed response. However, in the context of chronic inflammation and the activation of MMP-9 that leads to Ac-PGP production, which mediates the production of more MMP-9, this directed signal may become obscured by the abundant presence of PGPs. Loss of this polarized signal may impair directional neutrophil migration at the site of inflammation and contribute to neutrophil retention and further inflammation. It is intriguing to speculate that this chronic inflammation induced by MMPs and PGP may in part be due to impaired neutrophil reverse migration back to the vasculature and resolution as has been reported in both zebrafish8 and mice.9,10 These types of questions will be the challenge for future investigation using in vivo models and live imaging to understand how DDR2-MMP-PGP signaling pathways orchestrate the onset and/or resolution of neutrophil-mediated inflammation, and how these may be altered in disease.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal