Key Points
B cells contribute to MHC presentation of DEC-205–targeted antigen.
Activated B cells present DEC-205–targeted antigen efficiently, because they retain it longer.
Abstract
DEC-205 is a type I transmembrane multilectin receptor that is predominantly expressed on dendritic cells (DCs). Therefore, previous studies primarily focused on processing of DEC-205–targeted antigens by this potent antigen presenting cell type. Here we show that Epstein-Barr virus (EBV) transformed lymphoblastoid B-cell lines (LCLs) not only express DEC-205 at similar levels to DCs, but also efficiently present targeted EBV nuclear antigen 1 (EBNA1) and EBV-latent membrane protein 1 (LMP1) to EBNA1- and LMP1-specific CD4+ and CD8+ T-cell clones in vitro. Targeting of antigens to DEC-205 on B cells led to more efficient MHC class II than I loading, and stimulated T cells more efficiently than targeting to DEC-205 on DCs. Although LCLs internalized DEC-205–targeted antigens less efficiently than DCs, they retained them for longer time periods and delivered them to endosomal compartments that receive also B-cell receptor targeted proteins. This could facilitate prolonged T-cell stimulation and efficient MHC class II loading, and, indeed, CD4+ T-cell expansion by DEC-205–targeted vaccination was significantly compromised in B-cell deficient mice. These studies suggest that B cells, activated by virus transformation or other means, can contribute to T-cell stimulation after DEC-205 targeting of antigens during vaccination.
Introduction
Dendritic cells (DCs) are sentinels of the immune system that populate nearly all peripheral organs in their immature form.1 On infection or encountering pathogen-associated molecular patterns (PAMPs), DCs mature and migrate at enhanced frequency to secondary lymphoid tissues. They transmit 2 types of information to these immunologic decision centers. Firstly, they transfer antigens from the site of activation and process these antigens for presentation on major histocompatibility complex (MHC) molecules to T cells. Secondly, they communicate the conditions, under which they have encountered these antigens via their maturation pattern, which consists of up-regulated costimulatory molecules and secretion of cytokines and chemokines. These 2 types of transmitted information allow them to initiate the appropriate immune response to the encountered pathogenic challenge, orchestrating both innate and adaptive immunity.2,3 These potent antigen presenting and immune stimulating functions make DCs an attractive tool for vaccination. However, adoptive DC therapy has only provided limited success.4 Therefore, vaccination strategies are currently being developed that target antigens to DCs in vivo. For this purpose antibodies to endocytic, possibly antigen-uptake receptors on DCs are coupled with antigen for injection together with suitable immune activating adjuvants. Several C-type lectin receptors, such as DEC-205, langerin, and Clec9a, have been successfully used for immune response induction in mouse models5,6 and induce efficient human T-cell expansions in vitro.7-9 However, which other cell types, besides DCs, might contribute to the immune response induction via C-type lectin-targeted antigens remains largely unexplored.
Activated B cells are such antigen presenting cells that could amplify DC-induced immune responses. One pathway for human B-cell activation is transformation with the oncogenic γ-herpesvirus Epstein-Barr virus (EBV).10 In EBV transformed B-cell lines, so-called lymphoblastoid cell lines (LCLs), 8 latent EBV gene products are expressed, including the 2 latent membrane proteins, LMP2 and LMP1, which mimic constitutive signaling through the B-cell receptor (BCR) and CD40 for B-cell activation.11 LMP1, in particular, confers efficient antigen processing for MHC presentation and high surface levels of MHC molecules to LCLs.12,13 Because of this good antigen presenting function, LCLs have been explored for purification of MHC ligands.14,15 Although LCLs have a potent proteasome and TAP transporter associated MHC class I ligand processing machinery, it remains largely unknown which endocytic receptors are used to deliver extracellular antigens for efficient MHC class II loading of LCLs. Apart from the BCR, only the complement receptor 2 (CR2 or CD21) and the Fcγ receptor II have been suggested to lead to efficient antigen processing for MHC class II presentation.16-18 Thus, it remains unclear whether antigen targeting to certain endocytic receptors could harness both DC priming and amplification of T-cell responses by virus or otherwise activated B cells at the same time.
Here we show that LCLs efficiently present DEC-205–targeted antigens to CD4+ T cells of multiple specificities and HLA restrictions. They are superior in this capacity to monocyte-derived DCs, possibly because of their prolonged antigen retention and efficient DEC-205–mediated transport to MHC class II loading compartments, which also receive input from cross-linked BCR. In addition, CD4+ T-cell expansion by DEC-205–targeted vaccination was significantly reduced in B-cell deficient mice. These data suggest that activated B cells efficiently present antigens after DEC-205–mediated uptake, and could amplify immune responses, which are induced by DEC-205–targeted vaccination.
Methods
αDEC-205-EBNA1, αDEC-205-LMP1, and α-mouse DEC-205-p24 monoclonal antibodies
The αDEC-205 monoclonal antibodies (mAbs) used are described in supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The presented studies were approved under institutional review board protocol KEK-StV-Nr. 19/08 and ACUC protocol 148/2011. This study was conducted in accordance with the Declaration of Helsinki.
T-cell assay
T-cell clones were generated and characterized as described.19 T-cell assays were performed in triplicates by coculturing LCL cells (5 × 103/well) or DCs (5 × 103/well) overnight with T cells (5 × 103/well) in 96-well V-bottom plates. IFNγ released into the supernatant was measured by IFNγ ELISA19 and the means plus SD are shown.
Flow cytometry
The details of flow cytometry analysis are described in supplemental Methods.
Western blot
The preparation of protein extracts and Western blotting are outlined in supplemental Methods.
Immunofluorescence
Immunofluorescence staining and imaging procedures are described in supplemental Methods.
In vivo immunization
In vivo immunization of mice and intracellular cytokine staining were performed according to the description in supplemental Methods.
Statistical analysis
Plotted data represent means plus SD, unless otherwise stated. P values were calculated with the Mann-Whitney or Student t test.
Results
LCLs express DEC-205 and present DEC-205–targeted antigens efficiently to T-cell clones of different peptide specificities and HLA restrictions
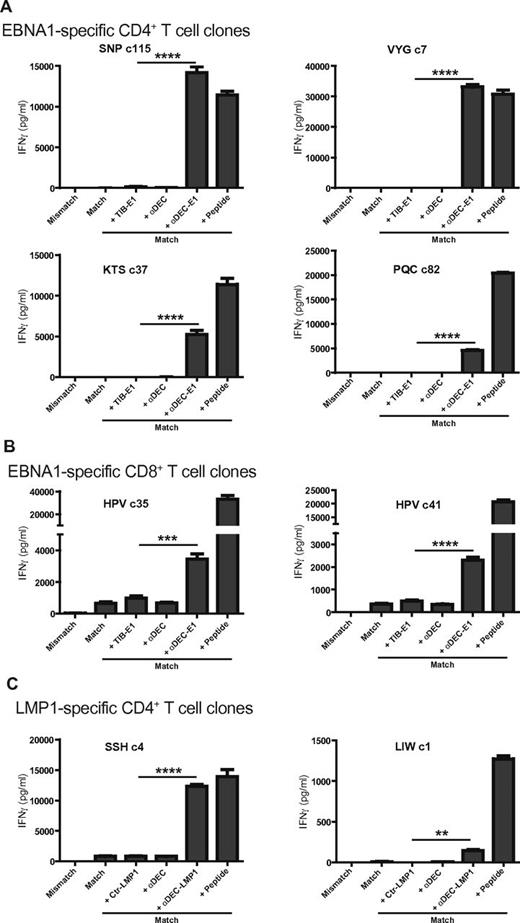
To explore whether other immune cells could contribute to DEC-205–targeted vaccination and because this receptor was previously described to be expressed at moderate level on human B cells20 and mouse B cells,21,22 we tested the DEC-205 expression on human B-cell lines by staining with the 2 monoclonal DEC-205–specific antibodies MG38.2 and 3G9 (supplemental Figure 1A; data not shown).23,24 Surprisingly, DEC-205 was expressed at high level on LCLs, the EBV positive Burkitt lymphoma B-cell line RAJI and also on the EBV negative Burkitt lymphoma B-cell line RAMOS. The expression level of DEC-205 on the B-cell lines was even higher than on both mature and immature monocyte-derived DCs. To investigate whether this high expression level can be used for antigen processing, the mAb MG38.2 against human DEC-205 was fused with EBNA1 (aa 400-641) or LMP1 (aa 180-386). These hybrid as well as isotype control fusion antibodies were produced, characterized by silver staining and by Western blotting using anti-EBNA1 or anti-LMP1 antibodies (supplemental Figure 1B). To assess how well antigens can be presented after targeting to DEC-205 on LCLs, we used EBNA1- or LMP1-specific CD4+ or CD8+ T-cell clones raised from healthy EBV-positive donors. The previously established CD4+ T-cell clones against 4 EBNA1 epitopes, designated SNP restricted through HLA-DR51, VYG, and PQC, both restricted through HLA-DR11, KTS restricted through HLA-DR1, were incubated with HLA mismatched or matched LCLs loaded with mAb fusion proteins or peptides, and the respective T-cell responses were monitored by IFNγ production. αDEC-205-EBNA1–loaded LCLs induced strong T-cell responses, whereas the control isotype fusion antibody or αDEC-205–loaded matched LCLs did not. The T-cell responses of SNP- or VYG-specific T-cell clones against LCLs loaded with αDEC-205-EBNA1 were even higher than LCLs loaded with the same concentration of the cognate peptide epitopes (Figure 1A). PQC presentation was least efficient, and this might reflect the partial susceptibility of the PQC epitope sequence to destructive processing by lysosomal proteases.19 Low production of IFNγ was also observed for the KTS epitope, so we assessed the susceptibility of these 2 epitopes to lysosomal degradation by cathepsins with the program SitePrediction (www.dmbr.ugent.be/prx/bioit2-public/SitePrediction/).25 PQC contained 6 predicted cuts (by cathepsins B, G, K, and L), and might therefore be more susceptible to lysosomal degradation than KTS with 3 predicted cuts (by cathepsins G, K, and L). αDEC-205-EBNA1–loaded LCLs also induced a higher CD8+ T-cell response as seen with 2 EBNA1-specifc T-cell clones against the HPV epitope restricted through HLA-B35 (Figure 1B), although the level of recognition was not as high as observed for the CD4+ T-cell responses in comparison with targets, which were pulsed with the cognate peptide. The observed CD4+ T-cell responses were especially remarkable considering the stoichiometry of the used antigenic formulations. Because of the small molecular weight of the used peptide epitopes, DEC-205–targeted antigen elicited similar T-cell responses at more than 50-fold lower antigen molecule numbers. Apart from EBNA1, we also investigated the targeting of another EBV antigen, LMP1, to DEC-205 using LMP1-specific CD4+ T-cell clones against 2 different epitopes, SSH restricted through HLA-DQ6 and LIW restricted through HLA-DR16. αDEC-205-LMP1–loaded LCLs induced strong T-cell response, whereas the control isotype fusion antibody or αDEC-205–loaded matched LCLs did not (Figure 1C). Taken together, these results indicate that LCLs express DEC-205 and present DEC-205–targeted antigens efficiently to T-cell clones of different peptide specificities and HLA restrictions.
LCLs present DEC-205–targeted EBV antigens efficiently to T-cell clones of different peptide specificities and HLA restrictions. (A) HLA-matched (Match) LCLs for EBNA1-specific CD4+ T-cell clones of 4 different epitopes (SNP, VYG, KTS, and PQC) were incubated with medium, 1 μg/mL control Ig-EBNA1 (+ TIB-E1), αDEC-205 without EBNA1 fusion (+ αDEC), αDEC-205-EBNA1 (+ αDEC-E1) for 24 hours, or for 1 hour with epitope-specific peptide (+ Peptide). An HLA mismatched target LCL was also included into the analysis (Mismatch). T cells were incubated with these targets at an E/T ratio of 1:1. T-cell activity was determined after 18 hours by measuring IFNγ released into the supernatant. (B) As in panel A, T-cell responses of EBNA1-specific CD8+ T-cell clones, HPVc35 and HPVc41, were tested against LCLs incubated without or with αDEC-EBNA1. (C) HLA-matched (Match) LCLs for 2 LMP1-specific CD4+ T-cell clones were incubated with medium, 1 μg/mL control Ig-LMP1 (+ Ctr-LMP1), αDEC-205 without LMP1 fusion (+ αDEC), αDEC-205-LMP1 (+ αDEC-LMP1) for 24 hours, or for 1 hour with epitope-specific peptide (+ Peptide). T-cell activity was determined as in panel A. One representative experiment of 4 per T-cell clone is shown. Statistical analysis of all available data from 4 independent experiments was performed by Mann-Whitney test and P values are represented as **P < .01, ***P < .005, and ****P < .0001.
LCLs present DEC-205–targeted EBV antigens efficiently to T-cell clones of different peptide specificities and HLA restrictions. (A) HLA-matched (Match) LCLs for EBNA1-specific CD4+ T-cell clones of 4 different epitopes (SNP, VYG, KTS, and PQC) were incubated with medium, 1 μg/mL control Ig-EBNA1 (+ TIB-E1), αDEC-205 without EBNA1 fusion (+ αDEC), αDEC-205-EBNA1 (+ αDEC-E1) for 24 hours, or for 1 hour with epitope-specific peptide (+ Peptide). An HLA mismatched target LCL was also included into the analysis (Mismatch). T cells were incubated with these targets at an E/T ratio of 1:1. T-cell activity was determined after 18 hours by measuring IFNγ released into the supernatant. (B) As in panel A, T-cell responses of EBNA1-specific CD8+ T-cell clones, HPVc35 and HPVc41, were tested against LCLs incubated without or with αDEC-EBNA1. (C) HLA-matched (Match) LCLs for 2 LMP1-specific CD4+ T-cell clones were incubated with medium, 1 μg/mL control Ig-LMP1 (+ Ctr-LMP1), αDEC-205 without LMP1 fusion (+ αDEC), αDEC-205-LMP1 (+ αDEC-LMP1) for 24 hours, or for 1 hour with epitope-specific peptide (+ Peptide). T-cell activity was determined as in panel A. One representative experiment of 4 per T-cell clone is shown. Statistical analysis of all available data from 4 independent experiments was performed by Mann-Whitney test and P values are represented as **P < .01, ***P < .005, and ****P < .0001.
LCLs are more efficient in processing DEC-205–targeted antigens for CD4+ T-cell stimulation than mature monocyte-derived DCs
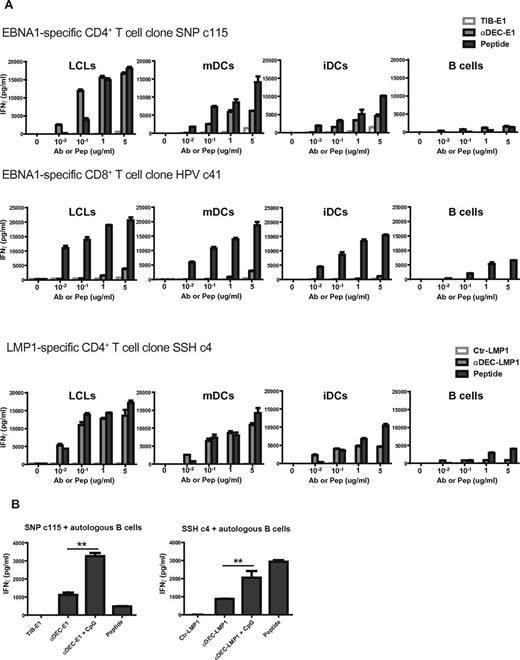
As LCLs present DEC-205–targeted antigens efficiently to T cells and previous reports have shown that antigens can be presented efficiently after targeting to DEC-205 on DCs, we next compared the targeting of antigens on LCLs and DCs. HLA-matched LCLs, mDCs, iDCs, or B cells from the same donor were used as targets for CD4+ and CD8+ T-cell clones specific for the SNP and SSH epitopes and the HPV epitope, respectively. DEC-205 targeting of the antigen caused a dose-dependent increase in MHC presentation of all 3 epitopes on LCLs, mDCs, and iDCs (Figure 2A). With low concentration of Ab or peptides, cells incubated with αDEC-205-EBNA1 or LMP1 fusion could stimulate stronger CD4+ T-cell responses than cells pulsed with the cognate peptides, this is obvious for the SNP epitope presentation by LCLs. To our surprise, LCLs were more efficient in processing DEC-205–targeted EBNA1 or LMP1 for CD4+ T-cell stimulation at the same cell number and antigen concentration than mature monocyte-derived DCs, this was seen with the EBNA1 derived SNP, VYG, and PQC epitopes and the LMP1-derived SSH epitope, as DEC-205 targeting to LCLs stimulated higher INFγ release by T cells than DEC-205 targeting to mDCs. As absolute levels of INFγ release are dependent on the numbers of T cells and target cells in the assay, peptide pulsed target cells can be used as internal assay controls, and the efficiency of DEC-205 targeting can be evaluated in comparison to the IFNγ release resulting from the same target cells pulsed with the cognate peptide. By this measure, LCLs are more efficient than DCs in presenting the CD4+ T-cell epitopes SNP, VYG, and PQC after DEC-205 targeting, whereas mDCs are comparable with LCLs in presenting the SSH epitope (Figure 2A, supplemental Figure 2). On the other hand, we observed similar levels of EBNA1-specific CD8+ T-cell responses after DEC-205 targeting to LCLs and mDCs. Despite the low levels of IFNγ release from the T-cell clones stimulated with primary B cells incubated with αDEC-205-EBNA1 or LMP1 fusion, the responses were dose dependent and even better than responses to peptide-pulsed targets at low antigen doses (Figure 2A, supplemental Figure 2). In addition to enhanced antigen presentation by B cells activated through EBV infection, primary B cells, which were activated with the toll-like receptor (TLR) 9 agonist CpG, presented DEC-205–targeted antigen better to EBNA1- and LMP1-specific CD4+ T cells than resting B cells (Figure 2B). This correlates with the increase in DEC-205 expression on CpG activated B cells. Further analysis of DEC-205 expression on B-cell subpopulations revealed that there is no difference in DEC-205 expression between CD27−IgD+ naive B cells and CD27+IgD− memory B cells. Both subsets up-regulated DEC-205 to similar levels upon CpG activation (supplemental Figure 3A). Moreover, the addition of αDEC-205-EBNA1 did not alter surface expression of HLA-DR nor the costimulatory molecules (CD80, CD86, and PD-L1) on resting (data not shown) or activated naive and memory B cells (supplemental Figure 3B). These data suggest that αDEC-205 does not stimulate normal B cells, but activated B cells can efficiently present antigens targeted to DEC-205.
DEC-205 targeting of EBV antigens to LCLs stimulates better antigen-specific CD4+ T-cell responses than targeting to DCs. (A) HLA-matched LCLs, mDCs, iDCs, or B cells from the same donor were treated with the indicated concentration of control antibody, or αDEC-205 with EBNA1 or LMP1 fusion, or peptide, then incubated with the corresponding matched EBNA1- or LMP1-specific T-cell clones. T-cell responses were measured as IFNγ release after 18 hours of coculture at an E/T ratio of 1:1. (B) HLA-matched CD19+ B cells were incubated with 1 μg/mL control antibody with EBNA1 or LMP1 fusion, αDEC-205-EBNA1 or LMP1 fusion, αDEC-205-EBNA1 or LMP1 fusion with or without 2.5 μg/mL CpG for 24 hours, or for 1 hour with epitope-specific peptide, then incubated with EBNA1- or LMP1-specific CD4+ T cells. T-cell activity was determined as in panel A. One of 2 experiments per T-cell clone is shown. P values were calculated from the data of 2 independent experiments with Mann-Whitney test and are represented as **P < .01.
DEC-205 targeting of EBV antigens to LCLs stimulates better antigen-specific CD4+ T-cell responses than targeting to DCs. (A) HLA-matched LCLs, mDCs, iDCs, or B cells from the same donor were treated with the indicated concentration of control antibody, or αDEC-205 with EBNA1 or LMP1 fusion, or peptide, then incubated with the corresponding matched EBNA1- or LMP1-specific T-cell clones. T-cell responses were measured as IFNγ release after 18 hours of coculture at an E/T ratio of 1:1. (B) HLA-matched CD19+ B cells were incubated with 1 μg/mL control antibody with EBNA1 or LMP1 fusion, αDEC-205-EBNA1 or LMP1 fusion, αDEC-205-EBNA1 or LMP1 fusion with or without 2.5 μg/mL CpG for 24 hours, or for 1 hour with epitope-specific peptide, then incubated with EBNA1- or LMP1-specific CD4+ T cells. T-cell activity was determined as in panel A. One of 2 experiments per T-cell clone is shown. P values were calculated from the data of 2 independent experiments with Mann-Whitney test and are represented as **P < .01.
Because LCLs were more efficient in processing DEC-205–targeted antigens for T-cell stimulations than DCs, we investigated whether, in addition to DEC-205–mediated antigen processing, differential expression of costimulatory molecules could account for the stimulating capacity of LCLs compared with mDCs. For this, we examined the costimulatory molecules CD80, CD86, and PD-L1 on both cell types (supplemental Figure 3C). LCLs express higher level of CD80, whereas mDCs have higher CD86 and PD-L1 expression, and both express comparable levels of HLA-DR. These slight differences of costimulatory molecule expression can probably not account for the difference in presentation of DEC-205–targeted antigen. This notion is also supported by the similar CD4+ T-cell responses to peptide pulsed LCLs and mDCs, whereas DEC-205–targeted antigen was much better presented to CD4+ T cells by LCLs than mDCs, especially at low antigen concentrations (Figure 2A, supplemental Figure 2). We also observed that DEC-205 cross-linking did not change costimulatory molecule expression (supplemental Figure 3D). However, DCs are well known for their potent priming of T-cell mediated immunity, so we assessed the ability of the LCLs, DCs, and primary B cells in stimulating T cells by allogeneic mixed lymphocyte reaction (MLR). Mature DCs were superior to all other antigen-presenting cell populations in eliciting proliferation of CD45RO−CD62L+ naive T cells. However, LCLs stimulated memory T cells (CD45RO+CD62L+, CD45RO+CD62L− or CD45RO−CD62L−) almost as efficiently as DCs. This indicated that DCs confer potent priming of T-cell immunity, but LCLs are capable of amplifying memory T-cell responses (supplemental Figure 3E).
LCLs retain DEC-205–targeted antigens longer than DCs
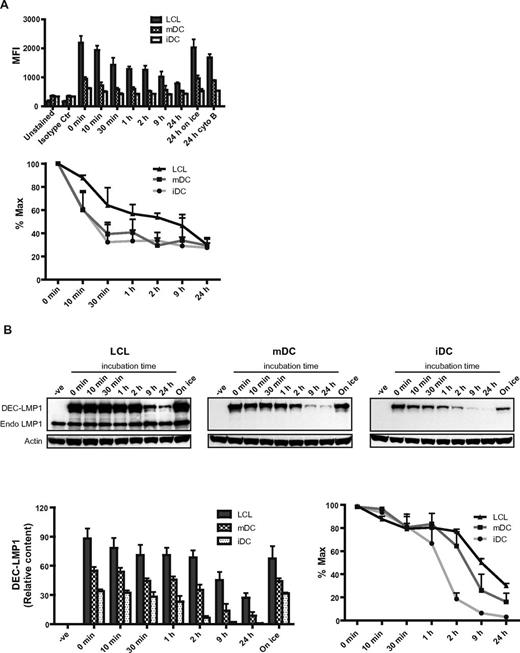
To characterize why LCLs present DEC-205–targeted antigens so efficiently, we studied the internalization of αDEC-205-EBNA1 or αDEC-205-LMP1 fusion Abs on LCLs and DCs. LCLs internalized the αDEC-205 fusion protein slower than DCs, but because of higher density of DEC-205 on LCLs, larger amounts of targeted antigen were internalized by LCLs than by DCs after 24 hours incubation (Figure 3A). Cells kept on ice or treated with the endocytosis inhibitor cytochalasin B retained surface levels of DEC-205–targeted antigen similar to those observed at time point 0 minutes. This indicated that loss in staining resulted from internalization of the αDEC-205 fusion protein into the cells. Consistent with LCLs internalizing DEC-205 slower, we found incubation of αDEC-205 fusion protein for 24 hours with LCLs stimulated specific CD4+ T-cell responses more efficiently than incubation for only 1 hour (data not shown). Because DEC-205–targeted antigen internalization was slower for LCLs than DCs, we wondered whether the degradation of the αDEC-205 fusion proteins differed between LCLs and DCs. For this purpose the respective antigen presenting cells were incubated with αDEC-205-LMP1, washed, and then αDEC-205-LMP1 protein levels were assayed by immunoblotting with mAb specific for LMP1. LCLs retained more αDEC-205-LMP1 protein than mDCs and iDCs, the targeted antigen was most rapidly degraded by iDCs, and slowest by LCLs. As the antigen is longer retained by LCLs, they might be able to continuously load MHC molecules for T-cell stimulation, long after the antigen has been entirely degraded in DCs.
LCLs retain DEC-205–targeted antigen longer than DCs. (A) LCLs, mDCs, or iDCs were incubated with 1 μg/mL control Ig-EBNA1 or αDEC-205-EBNA1 on ice for 30 minutes. Cells were then washed and incubated at 37°C for the indicated time periods. As a control for internalization, cells were left on ice or incubated with 10μM cytochalasin B (cyto B) in addition to αDEC-205-EBNA1 pulsing. Cells were then stained with PE-conjugated anti-mouse antibody. Data are mean values plus SD from 3 independent experiments and relative internalization is shown as percent maximum. (B) LCLs, mDCs, or iDCs were incubated without (−ve) or with 4 μg/mL αDEC-205-LMP1 on ice for 30 minutes. Cells were then washed and incubated at 37°C or left on ice as a control. Cell samples were lysed at the indicated time points and frozen. Protein lysates were separated by SDS/PAGE, transferred to PVDF membranes for Western blotting, and probed with a LMP1-specific mAb. Blots were also probed for actin as a loading control. One representative blot of 3 experiments is shown; mean values plus SD of relative protein content and percentage maximum from 3 independent experiments are also shown.
LCLs retain DEC-205–targeted antigen longer than DCs. (A) LCLs, mDCs, or iDCs were incubated with 1 μg/mL control Ig-EBNA1 or αDEC-205-EBNA1 on ice for 30 minutes. Cells were then washed and incubated at 37°C for the indicated time periods. As a control for internalization, cells were left on ice or incubated with 10μM cytochalasin B (cyto B) in addition to αDEC-205-EBNA1 pulsing. Cells were then stained with PE-conjugated anti-mouse antibody. Data are mean values plus SD from 3 independent experiments and relative internalization is shown as percent maximum. (B) LCLs, mDCs, or iDCs were incubated without (−ve) or with 4 μg/mL αDEC-205-LMP1 on ice for 30 minutes. Cells were then washed and incubated at 37°C or left on ice as a control. Cell samples were lysed at the indicated time points and frozen. Protein lysates were separated by SDS/PAGE, transferred to PVDF membranes for Western blotting, and probed with a LMP1-specific mAb. Blots were also probed for actin as a loading control. One representative blot of 3 experiments is shown; mean values plus SD of relative protein content and percentage maximum from 3 independent experiments are also shown.
DEC-205 and B-cell receptor both deliver antigens to endolysosomal compartments in LCLs
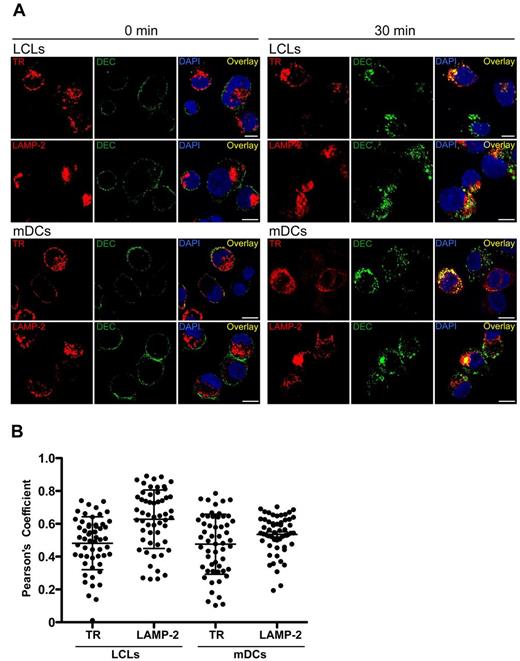
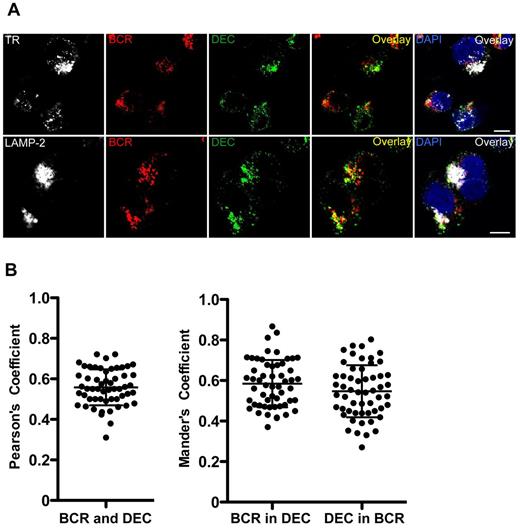
Because the BCR is considered the main antigen uptake receptor for efficient antigen processing by B lymphocytes, we wondered whether it targeted antigens to endosomes similar to those reached by DEC-205–targeted antigens, which could explain the efficient MHC presentation of targeted antigens. For this, we performed confocal immunofluorescence microscopy to analyze the intracellular location of αDEC-205 after internalization by LCLs or mDCs. Colocalization with transferrin receptor or lysosomal-associated membrane protein 2 (LAMP-2), as markers for early and late endosomes, respectively, was investigated. Internalized αDEC-205 was found in both early and late endosomes; control cells fixed immediately after exposure with αDEC-205 only showed surface staining as expected (Figure 4A). The colocalization was quantified and Pearson coefficients were determined. αDEC-205 was enriched in LAMP-2–positive endosomes in both LCLs and mDCs, and αDEC-205 demonstrated slightly higher colocalization with LAMP-2 in LCLs than mDCs (Figure 4B), possibly because of more rapid degradation of DEC-205–targeted protein in mDCs. These data confirm preferential DEC-205 targeting to late endosomes in human cells.26 Moreover, αDEC-205 partially colocalized with BCRs internalized after cross-linking, primarily in late endosomes (Figure 5). These findings suggest that DEC-205–targeted antigens are delivered to late endosomal compartments for efficient processing and loading onto MHC class II molecules, similar to antigens cross-linking the BCRs.
Intracellular localization of αDEC-205 in LCLs and mDCs. (A) LCLs and mDCs were incubated with αDEC-205, washed and incubated for 0 or 30 minutes at 37°C. Cells were then permeabilized, fixed, and stained with antibodies to transferrin receptor (TR) or lysosomal-associated membrane protein 2 (LAMP-2), αDEC-205 (DEC), and DAPI. Scale bars represent 5 μm. Representative cells from 1 experiment of 3 are shown. (B) Quantitative analysis for colocalization of αDEC-205 with TR and LAMP-2 in LCLs and mDCs loaded with αDEC-205 and incubated for 30 minutes at 37°C. Fifteen to 20 cells were analyzed from 1 independent experiment, data represent means from 50 to 60 cells from 3 experiments. Error bars indicate SD.
Intracellular localization of αDEC-205 in LCLs and mDCs. (A) LCLs and mDCs were incubated with αDEC-205, washed and incubated for 0 or 30 minutes at 37°C. Cells were then permeabilized, fixed, and stained with antibodies to transferrin receptor (TR) or lysosomal-associated membrane protein 2 (LAMP-2), αDEC-205 (DEC), and DAPI. Scale bars represent 5 μm. Representative cells from 1 experiment of 3 are shown. (B) Quantitative analysis for colocalization of αDEC-205 with TR and LAMP-2 in LCLs and mDCs loaded with αDEC-205 and incubated for 30 minutes at 37°C. Fifteen to 20 cells were analyzed from 1 independent experiment, data represent means from 50 to 60 cells from 3 experiments. Error bars indicate SD.
DEC-205 and B-cell receptor deliver antigens in LCLs with partial colocalization. LCLs were incubated with αDEC-205 and biotinylated F(ab′)2 anti-IgM Ab for 2 hours, washed, permeabilized, fixed, and stained with antibodies to transferrin receptor (TR) or lysosomal-associated membrane protein 2 (LAMP-2), αDEC-205 (DEC), αIgM (BCR), and DAPI. Scale bars represent 5 μm. Representative cells from 1 experiment of 3 are shown. (B) Quantitative analysis for colocalization of αDEC-205 (DEC) with αIgM (BCR) in LCLs incubated with αDEC-205 and αIgM for 30 minutes at 37°C. Fifteen to 20 cells were analyzed from 1 independent experiment, data represent means from 50 to 60 cells from 3 experiments. Error bars indicate SD.
DEC-205 and B-cell receptor deliver antigens in LCLs with partial colocalization. LCLs were incubated with αDEC-205 and biotinylated F(ab′)2 anti-IgM Ab for 2 hours, washed, permeabilized, fixed, and stained with antibodies to transferrin receptor (TR) or lysosomal-associated membrane protein 2 (LAMP-2), αDEC-205 (DEC), αIgM (BCR), and DAPI. Scale bars represent 5 μm. Representative cells from 1 experiment of 3 are shown. (B) Quantitative analysis for colocalization of αDEC-205 (DEC) with αIgM (BCR) in LCLs incubated with αDEC-205 and αIgM for 30 minutes at 37°C. Fifteen to 20 cells were analyzed from 1 independent experiment, data represent means from 50 to 60 cells from 3 experiments. Error bars indicate SD.
B cells play a role in amplifying antigen-specific CD4+ T-cell response on DEC-205 targeting in vivo
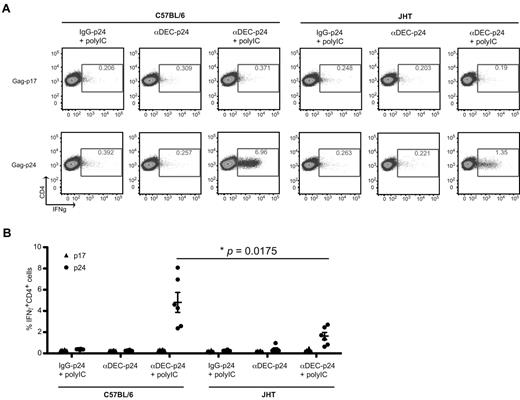
DEC-205 targeting of antigen to activated B cells led to efficient antigen presentation to both CD4+ and CD8+ T cells in vitro. Thus, we investigated whether B cells play a role in stimulating antigen-specific T-cell response on antigen targeting to DEC-205 in vivo. We immunized B-cell deficient JHT−/− (JHT) mice27 and their wild-type C57BL/6 counterparts with engineered mAbs against mouse DEC-205 with a HIV gag p24 fusion protein (αDEC-205-p24).28 The TLR 3 ligand polyIC was used as the adjuvant and an IgG-p24 fusion protein served as the isotype control. Antigen-specific T-cell responses were evaluated by IFNγ production in response to HIV gag p24 peptide mix by multicolor flow cytometry. Mice immunized with αDEC-205-p24 plus polyIC had gag p24-specific IFNγ-producing CD4+ T-cell responses. No significant CD4+ T-cell immunity was observed after immunization with isotype control mAb plus polyIC and αDEC-205-p24 without polyIC (Figure 6). These data confirm previous results.6,29 In addition, no significant CD8+ T-cell immunity could be detected in the immunized mice (data not shown), which could be induced by the addition of αCD40 as adjuvant.6 Interestingly, C57BL/6 mice with an intact B-cell compartment, which were immunized with αDEC-205-p24 with polyIC, exhibited significantly stronger gag p24-specific CD4+ T-cell responses compared with B-cell deficient mice with the same immunization (Figure 6). However, C57BL/6 and JHT mice both had comparable T-cell responses on αCD3 and αCD28 stimulation (data not shown). This data indicated that B cells play a role in amplifying antigen-specific CD4+ T-cell response on antigen targeting to DEC-205 in vivo. Next, we analyzed whether this amplification would lead to a more exhausted HIV gag p24-specific CD4+ T-cell response by C57BL/6 and JHT mice immunized with αDEC-205-p24 plus polyIC, by examining PD1 expression on these T cells. We did not see any difference in PD1 expression on gag p24-specific CD4+ T cells between the wild-type and JHT mice (data not shown). Therefore, targeting antigens to DEC-205 on B cells does not seem to promote the exhaustion of antigen-specific T cells.
B cells play a role in amplifying antigen-specific CD4+ T-cell responses toward DEC-205–targeted antigen in vivo. (A) C57BL/6 mice or the B-cell deficient JHT mice were immunized with 5 μg of isotype control fusion mAb with 50 μg polyIC (IgG-p24 + polyIC), or αDEC-205 mAb conjugated with HIV gag p24 (αDEC-p24) without or with 50 μg polyIC (αDEC-p24 + polyIC) as adjuvant, and boosted 1 month later. The mice were killed a week after boost, bulk splenocytes were harvested, stimulated with either gag p24 peptides or gag p17 peptide mix, IFNγ production was evaluated by intracellular cytokine staining in the CD3+CD4+ gated cells. (B) As in panel A, mean ± SD from 2 independent experiments with 3 mice per group is shown. P value was calculated with 2-tailed Student t test.
B cells play a role in amplifying antigen-specific CD4+ T-cell responses toward DEC-205–targeted antigen in vivo. (A) C57BL/6 mice or the B-cell deficient JHT mice were immunized with 5 μg of isotype control fusion mAb with 50 μg polyIC (IgG-p24 + polyIC), or αDEC-205 mAb conjugated with HIV gag p24 (αDEC-p24) without or with 50 μg polyIC (αDEC-p24 + polyIC) as adjuvant, and boosted 1 month later. The mice were killed a week after boost, bulk splenocytes were harvested, stimulated with either gag p24 peptides or gag p17 peptide mix, IFNγ production was evaluated by intracellular cytokine staining in the CD3+CD4+ gated cells. (B) As in panel A, mean ± SD from 2 independent experiments with 3 mice per group is shown. P value was calculated with 2-tailed Student t test.
Apart from investigating the IFNγ production for evaluating the immunization, we also tested the proliferation of antigen-specific T cells on restimulation with gag p24 peptide mix by CFSE dilution. Because of the limited amount, we had to pool splenocytes of mice from the same immunized group. We depleted the CD19+ B cells from splenocytes of the wild-type mice so that the amount of antigen presenting cells would be similar to splenocytes from JHT mice. Stronger CD4+ T-cell proliferation against gag p24 peptide stimulation was observed in C57BL/6 mice immunized with αDEC-205-p24 plus polyIC than JHT mice with the same immunization (supplemental Figure 4B), which correlates with the results observed in IFNγ production (Figure 6). Taken together, B cells seem to play a role in amplifying antigen-specific CD4+ T-cell response on antigen targeting to DEC-205 in vivo.
Discussion
Targeting antigens to DCs by incorporating specific microbial or tumor antigens into antibodies against the endocytic receptor, DEC-205, on DCs is a promising strategy for therapeutic vaccinations.5,7,24,30 Although this strategy was originally intended to only deliver antigen to DCs, B cells might play an important role in amplifying primed T-cell responses by retaining and loading DEC-205–targeted antigens for MHC presentation. Here, we demonstrated that human B cells, especially activated B cells, such as LCLs, express high levels of DEC-205 and efficiently process DEC-205–targeted antigen for MHC presentation (Figure 1, supplemental Figure 1). Surprisingly, LCLs are more efficient in presenting DEC-205–targeted antigens to CD4+ T cells than mature monocyte-derived DCs (Figure 2). However, mature DCs were superior to all other antigen-presenting cell populations in priming naive T cells (supplemental Figure 3). This suggested that DCs confer potent priming of T-cell immunity, whereas LCLs are capable of amplifying memory T-cell responses. Along these lines we previously demonstrated that EBV-transformed B cells stimulate, and are efficiently controlled by, peripheral blood T cells of EBV-seropositive donors in vitro, whereas cells from EBV-seronegative donors require bystander DCs to cross-prime protective T-cell response.31 As healthy EBV carriers have latency III expressing LCL-like cells in their tonsillar naive B-cell compartment,32 and LCL-like cells are the dominant population of EBV infected B cells in the tonsils of the affected individuals during acute infection,33 EBV infected B cells, such as LCLs, may play a role in augmenting MHC presentation of DEC-205–targeted antigens in vivo.
Extending these findings to an animal model, antigen targeting to DEC-205 led to reduced expansion of CD4+ T cells in B cell– deficient JHT mice (Figure 6), demonstrating the role of B cells in amplifying DC-induced immune responses in vivo. Although protein-based vaccine development targeting DCs has been extended from DEC-205 to other C-type lectin receptors, such as Clec9A34 or langerin,35 which are more restricted to DCs, our data suggest that it may be beneficial to target a receptor that is not only expressed on DCs, but also on other antigen presenting cells, to harness this immune response amplification. Additional targeting to B cells might also improve humoral immune responses. It was recently reported that depletion of CD11c+ cells had no effect on antibody responses but impaired T-cell immunity after targeting antigen to DEC-205 for immunization.36 Furthermore, germinal center B cells in mouse spleen express high level of DEC-205.22 The effect of antigen targeting to DEC-205 on humoral immune response needs further investigation.
In addition, B cells might constitute an antigen reservoir for prolonged antigen presentation after DEC-205 targeting. We were surprised by the observation that LCLs process DEC-205–targeted antigens more efficiently for CD4+ T-cell stimulation than mature monocyte-derived DCs. Our study on internalization and degradation of DEC-205 on LCLs and DCs indicated that LCLs retain DEC-205–targeted antigen longer than DCs (Figure 3). A similar difference was observed between DCs and macrophages. Macrophages were found to degrade injected antigen more rapidly in vivo than DCs, correlating with a more superior and sustained antigen presentation by DCs.37 Interestingly, when antigen was rendered more resistant to lysosomal degradation, it was more efficiently presented to CD4+ T cells.38 In addition, DCs seem to limit lysosomal degradation in phagosomes for their superior antigen presentation by various mechanisms, including attenuated acidification.39 Thus, the enhanced ability of B cells to present DEC-205–targeted antigen may reflect the slower rate of degradation exhibited by these cells.
Our data also suggested that apart from the BCR, the endocytic receptor DEC-205 can mediate efficient antigen processing for MHC class II presentation on B cells. DEC-205–targeted antigens are delivered to late endosomal compartments for efficient processing and loading onto MHC class II molecules, similar to antigens that are cross-linking the BCR (Figure 5) on B cells. In fact, BCR targeted antigen is retained by B cells in late endosomal compartments,40 some of which can even fuse with TLR containing endosomes for both antigen loading onto MHC class II molecules and B-cell activation.41,42 Although this targeting to late endosomal compartments is beneficial for MHC class II loading, it might, however, compromise cross-presentation onto MHC class I molecules via escape of the antigen from endosomes. Indeed, although the cross-presenting vesicular compartment is still ill-defined, early endosomes seem to potently promote cross-presentation of endocytosed antigens.43,44 In contrast, DEC-205–targeted antigen gets efficiently transported to late endosomes in mouse26 and human cells (this study), and therefore might favor antigen processing for MHC class II presentation over class I presentation. Accordingly, the preference of DEC-205–targeted antigen to stimulate CD4+ T cells that we observed in our study, was also noticed with most, particularly pathogen derived antigens in mouse studies in vivo.28,29,45 Thus, the intracellular localization of the targeted antibodies may explain why both DEC-205 and BCR targeting lead to more efficient MHC class II presentation than class I presentation.
In conclusion, antigen targeting to DEC-205 was originally intended to only deliver antigen to DCs, but we have shown here activated B cells might amplify primed T-cell responses by processing DEC-205–targeted antigens for MHC presentation. This dual targeting function of DEC-205 directed antigens might be beneficial for vaccination.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Cancer Institute (R01CA108609), the Sassella Foundation (10/02, 11/02, and 12/02), Cancer Research Switzerland (KFS-02 652-08-2010), the Association for International Cancer Research (11-0516), KFSPMS and KFSPHLD of the University of Zürich, the Vontobel Foundation, the Baugarten Foundation, the EMDO Foundation, the Sobek Foundation, Fondation Acteria, Novartis, and the Swiss National Science Foundation (310030_143979 and CRSII3_136241) to C.M. C.S.L. is a recipient of a postdoctoral fellowship from the Croucher Foundation Hong Kong. C.S.L. and S.M. are supported by a junior research fellowship from the University of Zürich.
National Institutes of Health
Authorship
Contribution: C.S.L. and C.M. designed the experiments; C.S.L., M.A.M., S.M., and A.L. performed the experiments; C.S.L., M.A.M., and C.M. analyzed the data; C.C., J.Z., T.A.H., and G.S.T. contributed new and essential reagents; C.S.L. and C.M. wrote the paper; and all authors approved the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Christian Münz, Department of Viral Immunobiology, Institute of Experimental Immunology, University of Zürich, Winterthurerstrasse 190, CH-8057 Zürich, Switzerland; e-mail: muenzc@immunology.uzh.ch.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal